Abstract
Since premenstrual syndrome (PMS) is one of the most common and debilitating disorders in women, risk factor modification is an urgent health priority. Therefore, this systematic review aimed to summarize and discuss the outcomes of observational and interventional studies in humans regarding the relationship between Calcium and PMS. PubMed, Scopus, ISI web of sciences and Google scholar were searched up to January 2019 to identify relevant studies. The Newcastle-Ottawa and Jadad scales were used for quality assessment. A total of 14 studies (8 interventional and 6 observational) met our inclusion criteria. Majority of the studies showed that not only serum calcium levels are lower in PMS subjects, but also calcium supplementation could significantly improve the incidence of PMS and its related symptoms. This systematic review suggests a beneficial role for calcium in PMS subjects. However, in order to draw a firm link between calcium and PMS, further dose-response clinical trials with larger sample size and better methodological design are warranted.
Keywords: Calcium, premenstrual syndrome, systematic review
Introduction
Women's health is one of the main goals of social and economic development of societies; therefore, problems and diseases compromising women's physical and mental health, such as premenstrual syndrome (PMS), are among health priorities.[1] PMS is characterized with a set of physical and emotional symptoms, including mood swings, tender breasts, food craving, fatigue, irritability, and depression beginning in the luteal phase of the menstrual cycle and resolving with the start of menstruation.[2] A recent meta-analysis estimated a worldwide prevalence of 10%–98%.[3] The syndrome is associated with significant economic burden. Distress and impairment in interpersonal or workplace functioning occur in a regular basis every month which can lead to significant amount of absenteeism at work and increase in medical costs.[4] Therefore, sufficient attention is needed to decrease its undesirable effects.
The etiology of PMS appears to be related to ovarian function, as suppression of ovarian hormone secretion markedly attenuates PMS,[5] although differences in ovarian steroid hormones have not been consistently observed between symptomatic and asymptomatic women. Several biological, social, and behavioral factors have consistently been positively associated with PMS, whereas demographic factors, education, employment, and marital status have shown inconsistent relationships.[6] Younger age, less education, and higher levels of perceived stress have been reported to be risk factors for premenstrual emotional symptoms.[7,8]
Among various pharmacological treatments recommended for women with PMS, selective serotonin reuptake inhibitors (SSRIs) have been evaluated as the first-line of therapy.[9] These therapies may be effective at resolving PMS in many women, but they are also associated with significant side effects and can be expensive. Alternatives to hormone therapy, such as dietary supplementation, are being evaluated.[10] Non-pharmacologic management with some evidence for efficacy includes cognitive-behavioral relaxation therapy, aerobic exercise, as well as magnesium, vitamin B6/D, or L-tryptophan supplementation or intake of complex carbohydrates.[11]
Also, scientific evidence suggests that cyclic fluctuations in calcium levels may help to explain some features of PMS. Changes in extracellular calcium concentration could have stimulatory effects on neuromuscular junctions,[12] and irritability, mania and agitation have been reported in conjunction with hypocalcemia.[13] Calcium supplementation may act by resolving an underlying physiologic deficit, suppressing parathyroid hormone secretion, and, ultimately, reducing neuromuscular irritability and vascular reactivity.[13] The possibility that calcium can be useful in alleviating mood disorders associated with PMS has been evaluated in various studies. Several interventional studies found that calcium supplementation was associated with reductions in the incidence of several symptoms of PMS.[5,11,13] Also, there is cross-sectional evidence indicating an evident association between PMS symptoms and calcium status.[14,15,16] However, the results were inconsistent and several studies found no such an association.[17,18] To address the divergence mentioned above, we carried out this systematic review on the observational and interventional studies to explore the association between calcium and PMS in general populations to reach a firm conclusion in this regard.
Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement and registered on Prospero database (CRD42018114474).[19]
Data source and search strategy
We searched relevant databases including PubMed, Scopus, ISI web of sciences and Google scholar up to January 2020 to identify relevant studies. Furthermore, the reference lists of the eligible papers were also reviewed to discover additional relevant studies. The following search strategy was run in PubMed and tailored to each database when necessary: “calcium” [Title/Abstract] OR “Ca” [Title/Abstract] AND “premenstrual syndrome” [Title/Abstract] OR “premenstrual dysphoric disorder” [Title/Abstract] AND “premenstrual tension” [Title/Abstract] OR “PMS” [Title/Abstract].
Inclusion criteria
Articles were considered for inclusion if they assessed the association between serum Ca level or Ca supplementation and prevalence of PMS or its related symptoms. Studies assessing calcium effect in combination with other elements including vitamin D or the consumption of dairy products as a source of calcium were excluded. Non-human studies, review articles, case reports, editorials and poster abstracts were excluded as well.
Data extraction
Pairs of independent reviewers screened the titles and abstracts of each study prior to full-text screening of nominee studies. Any divergences in terms of the decision on a given study were dealt with via discussion and if necessary, arbitration by a third reviewer. For all included studies, two reviewers independently extracted information, including first author's name, year of publication, location of the study, sample size, participants' age, study design, dietary and PMS assessment method and statistical adjustment.
Study quality
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the quality of each study.[20] Studies with 7–10, 5–6 and 0–4 points were identified as high, moderate, and low quality, respectively.[21]
Jadad scale for reporting randomized clinical trial (RCT) was used to assess the quality of interventional studies. In this scale, studies with 3 points or more were ranked as high quality.[22]
Results
Search results
Our initial search through databases identified 361 papers. After removing duplicates, the remaining 134 articles were reviewed based on the title and abstract by two independent reviewers. Totally, 79 articles were retrieved and reviewed based on full-text availability, and finally, 13 studies met our inclusion criteria and were included in our systematic review. The PRISMA flow diagram summarizes the results of study selection process for this systematic review [Figure 1].
Figure 1.
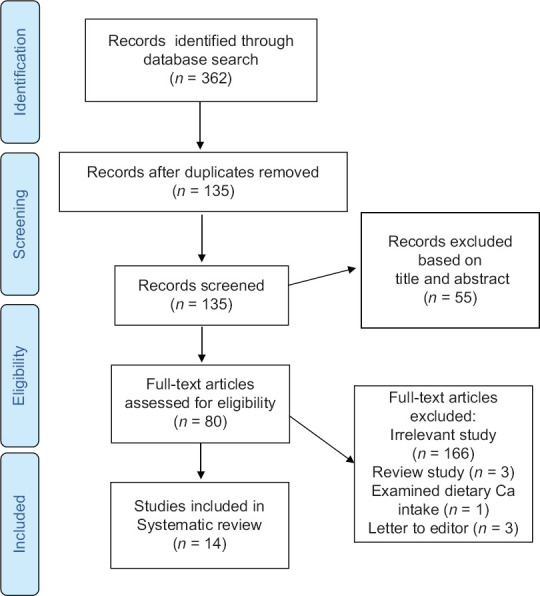
The flow diagram of study selection
Overview of the included studies
Studies included in this systematic review were carried out between 1989 and 2019. In total, 1185 and 1460 participants were recruited in the interventional and observational studies, respectively. Among 14 studies included, 8 (7 RCTs[18,23,24,25,26,27,28] and 1 quasi-experimental[29]) were interventional and 5 (3 case-controls[14,15,16] and 3 cross-sectional[17,30,31]) were observational. In terms of study location, 7 papers were conducted in Iran,[14,17,23,24,25,26,31] 6 in United States[15,16,18,27,28,30] and one in India.[29] Only 3 articles mentioned the dietary assessment method.[14,27,30] The dose of calcium administered in interventional studies, mainly in the form of calcium carbonate, ranged from 500 to 1200 mg daily. Based on the quality assessment scales, 5 interventions[18,25,26,27,28] and 1 observational[17] study ranked as high quality. Characteristics of the included studies are illustrated in Tables 1 and 2.
Table 1.
Characteristics of interventional studies
| Author, Year | Location | Sample size | Age range | Duration | Study Design | DAM | PMSAM | calcium dosage | Calcium type | Adjustment | Result | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thys-Jacobs et al., 1989 | USA | 60 | 35.1±6 | 3 months | RCT | Food record | PDSR | 1000 mg daily | Calcium carbonate | Baseline symptoms score | Negative affect, water retention and pain reduced significantly | High |
| Thys-Jacobs et al., 1998 | USA | 497 | 32.8±6.7 | 3 months | RCT | - | PMS Diary | 1200 mg daily | Calcium carbonate | - | Total symptoms score reduced significantly | High |
| Ghanbari et al., 2009 | Iran | 179 | 21.4±3.6 | 3 months | RCT | - | - | 500 mg twice daily | Calcium carbonate | - | Tiredness, Appetite and Depressive symptoms reduced significantly | Low |
| Yonkers et al., 2013 | USA | 39 | 25-45 | 4 months | RCT | - | Daily Record of Severity of Problems | 600 mg twice daily | Calcium carbonate | - | NS | High |
| Bharati et al., 2016 | India | 58 | 18-22 | 3 months | Quasi-experimental | - | - | 500 mg daily | Calcium carbonate | - | Number and severity of PMS symptoms reduced significantly | Low |
| Samieipour et al., 2016 | Iran | 210 | 20.32±8.92 | 2 months | RCT | - | PMS symptoms questionnaire | 500 mg daily | Calcium carbonate | Physical, psychological and general symptoms of PMS reduced significantly | High | |
| Masoumi et al., 2016 | Iran | 76 | 21±2.06 | 2 months | RCT | - | PDSR | 500 mg twice daily+ 40 mg vitamin B6 | - | - | Physical, psychological and general symptoms of PMS reduced significantly | Low |
| Shobeiri et al., 2017 | Iran | 66 | 20.95±1.19 | 2 months | RCT | - | Daily Record of Severity of Problems | 500 mg daily | - | - | anxiety, depression, emotional changes, water retention, and somatic changes reduced significantly | High |
DAM=Dietary Assessment Method, MSAM=Premenstrual Syndrome Assessment Method, PMS=Premenstrual Syndrome, RCT=Randomized clinical trial, PDSR=PMS Daily Symptom Record, GHQ-28=General Health Questionnaires-28, COPE=Calendar of Premenstrual Experiences, PSST=Premenstrual Syndrome Screening Tool, IU=International Unit
Table 2.
Characteristics of observational studies
| Author, Year | Location | Sample size | Age range | Study Design | DAM | PMSAM | Results | Quality score |
|---|---|---|---|---|---|---|---|---|
| Jacobs et al., 1995 | USA | 12 | 28-45 | Case-control | - | PMS Diary | Ionized calcium but not total calcium is lower in PMS subjects | Moderate |
| Shamberger et al., 2003 | USA | 96 | 19-52 | Case control | NM | NM | RBC total calcium is lower in PMS subjects | Moderate |
| Jacobs et al., 2007 | USA | 115 | 26-31 | Cross-sectional | Food record | PMS Diary | NS | Moderate |
| Saeedian Kia et al., 2015 | Iran | 62 | 20-22 | Case-control | 24-hour recall questionnaire | Utah PMS Calendar II | Total calcium is lower in PMS subjects | Moderate |
| Bahrami et al., 2018 | Iran | 897 | 12-18 | Cross-sectional | - | COPE | There is no association between PMS and calcium except irritability | High |
| Fatemi et al., 2019 | Iran | 278 | 19-21 | Cross-sectional | - | PMS symptoms questionnaire | NS | Moderate |
DAM=Dietary Assessment Method, MSAM=Premenstrual Syndrome Assessment Method, PMS=Premenstrual Syndrome, COPE=Calendar of Premenstrual Experiences, PSST=Premenstrual Syndrome Screening Tool, SRQ=Self-reported questionnaire, GHLQ=General health and lifestyle questionnaire, FFQ=Food Frequency Questionnaire, IU=International Unit, BMI=Body Mass Index
Interventional studies
The efficacy of calcium supplementation in patients with PMS was examined in 7 randomized clinical trials[18,23,24,25,26,27] and 1 quasi-experimental study.[29]
In the earliest study found, Jacobs et al. explored the efficacy of calcium supplementation in thirty-three women with premenstrual syndrome (PMS). Each participant received six months of treatment involving three months of daily calcium supplementation (1,000 mg of calcium carbonate) and three months of placebo. Results demonstrated a reduction in symptoms on calcium treatment during both the luteal and the menstrual phases of the reproductive cycle. Calcium supplementation had no effect during the inter-menstrual phase. 73% of the women reported fewer symptoms during the treatment phase on calcium, 15% preferred placebo, and 12% had no clear preference. Three premenstrual factors (negative affect, water retention, and pain) and one menstrual factor (pain) were significantly alleviated by calcium.[27]
A few years later in 1998, same authors evaluated the effects of Ca carbonate on the luteal and menstrual phases of the menstrual cycle among 466 healthy, premenopausal women with PMS (32.8 ± 6.7 years old). Subjects were randomly assigned to receive 1200 mg of elemental Ca per day in the form of calcium carbonate or placebo for 3 menstrual cycles. During the luteal phase of the treatment cycle, a significantly lower mean symptom complex score was observed in the Ca-treated group for both the second and third treatment cycles (P < 0.05). By the third treatment cycle Ca effectively resulted in an overall 48% reduction in total symptom scores from baseline compared with a 30% reduction in placebo. All 4 symptom factors (negative affect, water retention, food cravings, and pain) were significantly reduced by the third treatment cycle.[27]
Moreover, the efficacy of Ca supplementation in young female college students suffering from PMS was similarly examined in another double-blind clinical trial in 2009. The subjects (179 women; mean age 21.4 ± 3.6 years old) were divided in two groups; one group received placebo and the other received 500 mg of Ca carbonate twice daily for 3 months. Results showed that calcium supplements reduced early fatigability, changes in appetite, and depression in women with PMS (P < 0.05).[23]
Next study was performed in 2016 aiming to attenuate symptoms of PMS by simple lifestyle measures like yoga and/or oral Ca among fifty-eight subjects (18-22 age range) diagnosed with PMS. Twenty girls were given yoga training (45 minutes daily, five days a week, for three months). Another group of 20 was given oral tablets of Ca carbonate daily (500 mg, for three months) and rest 18 girl served as control group. The yoga and Ca groups showed a significant decrease (P < 0.05) in number and severity of premenstrual symptoms whereas in the control group there was not the significant difference.[29]
In another attempt, in 2016, Samieipour et al. investigated the effects of Ca and vitamin B1 on PMS symptoms among 210 female students (20.32 ± 8.92 years old). Participants were assigned in 3 groups of 70 individuals with the following regimen: group 1 received one pill containing 100 milligrams vitamin B1, group 2 received Ca pills and group 3 received placebo. The participants in all groups took medicines for 2 months and then reported the intensity of their symptoms by a questionnaire. Results of this study showed that both vitamin B1 and Ca reduce physical symptoms of PMS, but in terms of reducing psychological symptoms Ca was more effective.[25]
One year later, 66 female students (20.95 ± 1.19 years old) diagnosed with PMS were examined in another study. The participants were randomly assigned into two groups to receive 500 mg of Ca daily or placebo for 2 months. Significant differences were noticed between the two intervention groups in the first and second menstrual cycles after the intervention. The differences were significant (P < 0.05) in subgroups of anxiety, depression, emotional changes, water retention, and somatic changes in Ca group compared with placebo in the menstrual cycle before the intervention and 2 menstrual cycles after the intervention and among menstrual cycles (0, cycle 1, cycle 2) in calcium group.[26]
Recently, Masoumi et al., conducted a double blind randomized controlled trial on 76 students (21 ± 2.06 years old) with PMS. Students in group 1 received Ca tablet (500 mg) and vitamin B6 (40 mg) and subjects in group 2 received only vitamin B6 twice a day for two consecutive months. The results showed that although the severity of symptoms decreased in both groups, this reduction was more significant in the combined Ca and vitamin B6 group (P < 0.05).[24]
Inconsistent with the above-mentioned studies is the evidence conducted by Yonkers et al. in 2013. A pilot study was conducted to compare fluoxetine and Ca to placebo in the treatment of premenstrual symptoms. Thirty-nine women (age range 25–45 years old) with at least 3 moderate to severe premenstrual symptoms and functional impairment received fluoxetine (10 mg twice daily), Ca carbonate (600 mg twice daily), and placebo over the course of 4 menstrual cycles. In the Ca group during study period, there were no significant differences relative to placebo (P > 0.05).[18]
Observational studies
The association between serum calcium and PMS was investigated in 3 cross-sectional[17,30,31] and 3 case-control studies.[14,15,16]
In the first study, Thys-Jacobs et al., observed Ca metabolism across one menstrual cycle in 12 healthy, premenopausal women (age range 28–45 years old). Seven women had documented PMS, and 5 were asymptomatic controls. Multivariate analysis indicated significant variation in total and ionized calcium across the menstrual cycle. Significant between-group differences were found for total calcium (P < 0.05).[16]
A few years later, Shamberger et al. compared the levels of 18 red blood cell and 22 hair elements including calcium in 46 patients diagnosed with PMS and 50 normal subjects. Significantly lower amounts of calcium were screened in the blood of patients with PMS (P < 0.05). The ratios of Mg/Ca were significantly higher in the PMS patients. The highly significant Mg/Ca ratio in blood cells may be indicative of a more complex relationship between PMS and Magnesium (Mg) and Ca than either element alone.[15]
Another evidence conducted by Saeedian Kia et al. which compared the nutritional status of vitamin D, Ca, and Mg in 62 students aged 20-'25 years old (31 PMS cases and 31 controls), found lower serum levels of Ca in PMS participants as compared to healthy controls (P < 0.05).[14]
In another study, Thys-Jacobs et al. measured fluctuations and group differences in Ca-regulating hormones across the menstrual cycle in women with and without premenstrual dysphoric disorder (PMDD). The PMDD group, when compared with controls, had significantly lower ionized calcium at phase 1 and significantly lower urine calcium excretion at three of the five phases (late follicular phase 2, midcycle phase 3, and early luteal phase 4) (P < 0.05).[30]
Bahrami et al. assessed the relationship between the menstrual bleeding pattern, PMS symptoms and Ca levels among 897 high school adolescent girls. It revealed an association between calcium level and menstrual blood loss and irritability but no other PMS symptoms.[17]
The last study examined the association between serum level of Ca and presence of PMS among 278 women (19–21 years old). It failed to show any association between Ca and PMS.[31]
Discussion
To the best of our knowledge, this is the first systematic review evaluating the association between Ca and PMS in general population. Owing to the association between diets rich in dairy products and decreased PMS complications,[32] it is hypothesized that serum Ca level may be associated with the severity of PMS symptoms.
First section of our systematic review belongs to the observational studies with 4 of them showing lower serum Ca levels among PMS subjects.[27,29,30,31] Inconsistent with these, a study by Bahrami et al.[17] suggested a significant association between high corrected serum calcium and the incidence of irritability without any significant differences between the groups with regard to other physical or psychological symptoms of PMS. This discrepancy may be due to the different population samples; cycle regularity may be varying between younger and older women. Also, they found that in the hypercalcemia group, the majority of the participants presented moderate blood flow. The amount of blood loss during menstruation affects the overall quality of life of women,[33] so, this may be clinically relevant.
The other section of our review consists of interventional studies mostly reporting beneficial effects for Ca supplementation in PMS.[23,24,25,26,27,28,29] Only 1 pilot study reported small or no benefit of calcium as compared with placebo as a treatment for PMS.[18] However, the sample size for this pilot may not have been sufficient to draw definitive conclusions about treatment efficacy. Also, given that 1000 women were screened and slightly less than 5% were willing to participate in this pilot protocol, generalizability may be an issue with its results.
The characteristic feature of PMS is its occurrence during the luteal phase of the menstrual cycle with symptomatology unmasked, then remitting with the onset of menses. The most likely explanation for this temporal occurrence is the relationship between the ovarian steroid hormones and the calciotropic hormones. Ovarian steroid hormones, estrogens in particular, are known to influence the actions of the calciotropic hormones, specifically calcium and PTH.[7] Estrogen lowers serum calcium, and in its absence as seen at menopause, serum calcium concentrations rise.[9] Estrogen is believed to lower serum calcium through an inhibition of bone resorption by suppressing the mesenchymal process involved in bone remodeling and promoting bone mineralization.[34] Parathyroid hormone appears to act in an exactly opposite manner. Estrogen treatment in patients with mild primary hyperparathyroidism has been shown to lower serum and urinary calcium levels. Recent evidence suggests that estrogen has calcium antagonistic properties, inhibiting calcium currents and decreasing calcium entry into vascular smooth muscle.[35] During the menstrual cycle, estradiol has two peaks, one immediately before the LH surge and ovulation, and the second during the luteal phase. Increasing estrogen levels would result in falling calcium concentrations with compensatory rises in parathyroid hormone preventing marked degrees of hypocalcemia. Therefore, it may be hypothesized that women with an already underlying calcium disturbance, such as those suffering with PMS (lower calcium concentrations and higher PTH concentrations), would be subjected to further decrements in calcium concentrations on exposure to increasing estrogen levels during the luteal phase of the menstrual cycle. Since extracellular calcium is the ultimate source of intracellular calcium, intracellular calcium may be perturbed resulting in abnormalities of neurotransmitter synthesis and release. As a result, fluctuating estrogen levels in these women could alter serotonin receptor availability, binding and neurotransmission, which in turn can cause premenstrual mood symptoms.[36] It has been hypothesized that Ca supplementation could increase extracellular Ca which in turn may affect monoamine metabolism and reverse serotonin dysregulation.[5]
There are some limitations to this study that should be taken into account. First, most studies included in the systematic review had small sample sizes with short intervention periods. Second, great differences in the studies in terms of statistical analyses or investigated exposures prevented us from doing meta-analysis. Third, except for one study which was established from Egypt, others were carried out in the United States and Iran which makes it difficult to generalize the results of this review to the rest of the world. On the other hand, inadequate calcium intake in the general population, especially among women, is a public health concern in developing countries, including Iran.[37] Therefore, the results need to be further investigated in populations with normal calcium levels. Besides, since PMS symptoms are recalled by the participants, recall bias is another limitation. Moreover, differences in the participants' diets might have affected serum levels of calcium in the studies that did not evaluate and control the women's diet.
According to what was discussed, it may be concluded that not only serum Ca levels are lower in PMS subjects, but also Ca supplementation could significantly improve the incidence of PMS and its related symptoms. However, based upon available evidence, we could not reach the overall results in the case of administered calcium dosage. Overall, in order to draw a firm link between Ca and PMS, further dose-response clinical trials with larger sample size and better methodology are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zendehdel M, Elyasi F. Biopsychosocial etiology of premenstrual syndrome: A narrative review. J Family Med Prim Care. 2018;7:346–56. doi: 10.4103/jfmpc.jfmpc_336_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arslantaş H, Abacigil F, Çinakli Ş. Relationship between premenstrual syndrome and basic personality traits: A cross-sectional study. Sao Paulo Med J. 2018;136:339–45. doi: 10.1590/1516-3180.2018.0061240418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Direkvand-Moghadam A, Sayehmiri K, Delpisheh A, Kaikhavandi S. Epidemiology of premenstrual syndrome (PMS)-A systematic review and meta-analysis study. J Clin Diagn Res. 2014;8:106–9. doi: 10.7860/JCDR/2014/8024.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Mar Fernández M, Saulyte J, Inskip HM, Takkouche B. Premenstrual syndrome and alcohol consumption: A systematic review and meta-analysis. BMJ Open. 2018;8:e019490. doi: 10.1136/bmjopen-2017-019490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thys-Jacobs S. Micronutrients and the premenstrual syndrome: The case for calcium. J Am Coll Nutr. 2000;19:220–7. doi: 10.1080/07315724.2000.10718920. [DOI] [PubMed] [Google Scholar]
- 6.Logue CM, Moos RH. Perimenstrual symptoms: Prevalence and risk factors. Psychosom Med. 1986;48:388–414. doi: 10.1097/00006842-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Halbreich U, Endicott J, Lesser J. The clinical diagnosis and classification of premenstrual changes. Can J Psychiatry. 1985;30:489–97. doi: 10.1177/070674378503000706. [DOI] [PubMed] [Google Scholar]
- 8.Steiner M. Premenstrual syndrome and premenstrual dysphoric disorder: Guidelines for management. J Psychiatry Neurosci. 2000;25:459–68. [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner M, Pearlstein T, Cohen LS, Endicott J, Kornstein SG, Roberts C, et al. Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: The role of SSRIs. J Womens Health. 2006;15:57–69. doi: 10.1089/jwh.2006.15.57. [DOI] [PubMed] [Google Scholar]
- 10.Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246–52. doi: 10.1001/archinte.165.11.1246. [DOI] [PubMed] [Google Scholar]
- 11.Rapkin A. A review of treatment of premenstrual syndrome & premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28:39–53. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 12.Carman J, Crews E, Bancroft A, Wyatt E, Cooper B, Ledbetter J, et al. Calcium and calcium regulating hormones in the biphasic periodic psychoses. J Operat Psychiat. 1980;11:5–17. [Google Scholar]
- 13.Jimerson DC, Post RM, Carman JS, Van Kammen DP, Wood JH, Goodwin FK, et al. CSF calcium: Clinical correlates in affective illness and schizophrenia. Biol Psychiatry. 1979;14:37–51. [PubMed] [Google Scholar]
- 14.Kia AS, Amani R, Cheraghian B. The association between the risk of premenstrual syndrome and vitamin D, calcium, and magnesium status among university students: A case control study. Health Promot Perspect. 2015;5:225–30. doi: 10.15171/hpp.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamberger RJ. Calcium, magnesium, and other elements in the red blood cells and hair of normals and patients with premenstrual syndrome. Biol Trace Elem Res. 2003;94:123–9. doi: 10.1385/BTER:94:2:123. [DOI] [PubMed] [Google Scholar]
- 16.Thys-Jacobs S, Alvir M. Calcium-regulating hormones across the menstrual cycle: Evidence of a secondary hyperparathyroidism in women with PMS. J Clin Endocrinol Metab. 1995;80:2227–32. doi: 10.1210/jcem.80.7.7608284. [DOI] [PubMed] [Google Scholar]
- 17.Bahrami A, Bahrami-Taghanaki H, Afkhamizadeh M, Avan A, Mazloum Khorasani Z, Esmaeili H, et al. Menstrual disorders and premenstrual symptoms in adolescents: Prevalence and relationship to serum calcium and vitamin D concentrations. J Obstet Gynaecol. 2018;38:989–95. doi: 10.1080/01443615.2018.1434764. [DOI] [PubMed] [Google Scholar]
- 18.Yonkers KA, Pearlstein TB, Gotman N. A pilot study to compare fluoxetine, calcium, and placebo in the treatment of premenstrual syndrome. J Clin Psychopharmacol. 2013;33:614–20. doi: 10.1097/JCP.0b013e31829c7697. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Arab A, Rafie N, Mansourian M, Miraghajani M, Hajianfar H. Dietary patterns and semen quality: A systematic review and meta-analysis of observational studies. Andrology. 2018;6:20–8. doi: 10.1111/andr.12430. [DOI] [PubMed] [Google Scholar]
- 22.Arab A, Mehrabani S, Moradi S, Amani R. The association between diet and mood: A systematic review of current literature. Psychiatry Res. 2019;271:428–37. doi: 10.1016/j.psychres.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Ghanbari Z, Haghollahi F, Shariat M, Foroshani AR, Ashrafi M. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124–9. doi: 10.1016/S1028-4559(09)60271-0. [DOI] [PubMed] [Google Scholar]
- 24.Masoumi SZ, Ataollahi M, Oshvandi K. Effect of combined use of calcium and vitamin B6 on premenstrual syndrome symptoms: A randomized clinical trial. J Caring Sci. 2016;5:67–73. doi: 10.15171/jcs.2016.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samieipour S, Kiani F, Babaei Heydarabadi A, Tavassoli E. Comparing the effects of vitamin B1 and calcium on Premenstrual syndrome (PMS) among female students, Ilam-Iran. Int J Pediatr. 2016;4:3519–28. [Google Scholar]
- 26.Shobeiri F, Araste FE, Ebrahimi R, Jenabi E, Nazari M. Effect of calcium on premenstrual syndrome: A double-blind randomized clinical trial. Obstet Gynecol Sci. 2017;60:100–5. doi: 10.5468/ogs.2017.60.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thys-Jacobs S, Ceccarelli S, Bierman A, Weisman H, Cohen MA, Alvir J. Calcium supplementation in premenstrual syndrome. J Gen Intern Med. 1989;4:183–9. doi: 10.1007/BF02599520. [DOI] [PubMed] [Google Scholar]
- 28.Thys-Jacobs S, Starkey P, Bernstein D, Tian J, Group PSS. Calcium carbonate and the premenstrual syndrome: Effects on premenstrual and menstrual symptoms. Am J Obstet Gynecol. 1998;179:444–52. doi: 10.1016/s0002-9378(98)70377-1. [DOI] [PubMed] [Google Scholar]
- 29.Bharati M. Comparing the effects of yoga & oral calcium administration in alleviating symptoms of premenstrual syndrome in medical undergraduates. J Caring Sci. 2016;5:179–85. doi: 10.15171/jcs.2016.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thys-Jacobs S, McMahon D, Bilezikian JP. Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. J Clin Endocrinol Metab. 2007;92:2952–9. doi: 10.1210/jc.2006-2726. [DOI] [PubMed] [Google Scholar]
- 31.Fatemi M, Allahdadian M, Bahadorani M. Comparison of serum level of some trace elements and vitamin D between patients with premenstrual syndrome and normal controls: A cross-sectional study. Int J Reprod BioMed. 2019;17:647–52. doi: 10.18502/ijrm.v17i9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penland JG, Johnson PE. Dietary calcium and manganese effects on menstrual cycle symptoms. Am J Obstet Gynecol. 1993;168:1417–23. doi: 10.1016/s0002-9378(11)90775-3. [DOI] [PubMed] [Google Scholar]
- 33.Kocaoz S, Cirpan R, Degirmencioglu AZ. The prevalence and impacts heavy menstrual bleeding on anemia, fatigue and quality of life in women of reproductive age. Pak J Med Sci. 2019;35:365–70. doi: 10.12669/pjms.35.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaney RP. Estrogens and postmenopausal osteoporosis. Clin Obstet Gynecol. 1976;19:791–803. doi: 10.1097/00003081-197612000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Stice SL, Ford SP, Rosazza JP, Van Orden DE. Role of 4-hydroxylated estradiol in reducing Ca2+uptake of uterine arterial smooth muscle cells through potential-sensitive channels. Biol Reprod. 1987;36:361–8. doi: 10.1095/biolreprod36.2.361. [DOI] [PubMed] [Google Scholar]
- 36.Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. Depression in women: Windows of vulnerability and new insights into the link between estrogen and serotonin. J Clin Psychiatry. 2011;72:e1563–9. doi: 10.4088/JCP.11com07089. [DOI] [PubMed] [Google Scholar]
- 37.Ebrahimi F, Shariff ZM, Rezaeian M, Tabatabaei SZ, Mun CY, Tajik E. Socioeconomic status and intake of energy and sodium are associated with calcium intake among pregnant women in Rafsanjan city, Iran. J Obstet Gynaecol Res. 2013;39:146–53. doi: 10.1111/j.1447-0756.2012.01948.x. [DOI] [PubMed] [Google Scholar]


