Abstract
Background:
Nowadays, the use of green tea supplements has increased. Studies have shown that green tea can have positive effects on anti-inflammatory and antioxidative factors, as well as improve aerobic performance capacity. Therefore, in this study, we investigate the effects of this supplement on inflammatory factors, total antioxidant capacity responses, and maximum oxygen uptake (VO2 Max) of healthy young men in summer.
Methods:
This study is a double-blind randomized controlled trial (RCT) in which 15 young men (age 25.06 ± 2.1) were randomly assigned into the green tea (GT) and placebo groups. Subjects performed maximum aerobic exercises (shuttle run 20 m) in separate workouts (14 days) in summer. They consumed 640 mg green tea extracts or maltodextrin 90 min before exercise in a double-blind design. Blood samples were collected before and after the exercise and then evaluated in the biochemistry laboratory. In this study, one way analysis of variance (ANOVA) tests were used for the statistical analysis.
Results:
The results of this study show that green tea supplement significantly slowed down the increasing tumor necrosis factor alpha (TNF-α) (GT: 15.03 ± 4.31 [pg/ml], placebo: 31.38 ± 7.18 [pg/ml], [P = 0.000]); increased the total antioxidant capacity (GT: 1.04 ± 0.06 [mm], placebo group: 0.72 ± 0.04 [mm], [P = 0.001 VO2]); and Max (GT: 44.43 ± 3.06 [ml/kg/min], placebo group: 34.88 ± 1.30 [ml/kg/min], [P = 0.001]) in the supplement group than placebo. In addition, no significant differences in interleukin 1 beta (IL-1β) was observed between thee groups (GT: 26.86 ± 5.05 [pg/ml], placebo group: 23.47 ± 3.16 [pg/ml], [P = 0.251]).
Conclusions:
The consumption of green tea supplements 90 min before aerobic exercise may decrease inflammation and oxidative stress factors and improve VO2 Max in summer.
Keywords: Exercise, hot temperature, inflammation, oxidative stress, tea
Introduction
Exercise-induced oxidative stress is potentially influenced by exercise intensity and duration and the subject's physical capacity and environmental conditions.[1] Workers, athletes, soldiers, firefighters, and law enforcement officials are routinely required to exercise or work in hot, humid environments.[2] Exposure to environmental heat stress is dangerous because of the onset of heat-related illness. Complications from heat-related illness result in loss of practice and work time,[2] which is particularly detrimental to athletes in the field of sports science.[3]
Several studies have determined that exercise toleration in a hot environment is lower than the cold environment. It has also been observed in animal studies that exposure to hot environments may result in increased oxidative stress.[4] Moreover, human surveys show that high-temperature environments can increase exercise-induced oxidative stress. One study shows that exercising in a hot environment can increase inflammatory factors. This increase has been observed in both acute and chronic conditions.[5]
During exhaustive exercise, increases reactive oxygen species (ROS) leads to altered redox state in the muscle during exhaustive exercise.[6,7,8] Oxidative stress affects the proteins, lipids, and DNA, possibly, leading to contractile muscle dysfunction, accelerated muscle fatigue, and reduced exercise performance,[9,10] as well as damaging the cells and inhibiting muscle growth.[11]
Implementation of long-term and high-intensity exercises are associated with safety changes, including the release of inflammatory mediators, activation of the subunits of the white blood cells (WBCs), acute phase proteins, and proinflammatory and anti-inflammatory cytokines.[12] Increased levels of inflammatory cytokines in the blood increase (augment) the risk of chronic diseases, such as cardiovascular disease (CVD), osteoporosis, diabetes, insulin resistance, and cognitive disorders.[13,14]
Today, researchers concentrate on finding more effective and safer antioxidants that are derived from natural sources. Catechin compounds in green tea by harvesting free radicals lead to this plant which was introduced as a natural antioxidant.[15,16] Besides, green tea can increase the anti-inflammatory[15] effects on slight systemic inflammation, which leads to the decreased level of C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6).[17]
Studies have shown that green tea increases superoxidase in serum and catalase expression in the aorta, which protects the cells against ROS.[18,19] In addition, it has been observed that green tea reduced the malondialdehyde (MDA), which is a sign of oxidative stress.[15,20] In another study, Jówko et al. (2015) showed that daily consumption of 980 mg green tea catechins (GTCs) for 4 weeks increases the total antioxidant activity, MDA, and superoxide dismutase in runners after performing ergometer training.[21]
While exercising in a hot environment, a large increase in skin blood flow is required. It affects cardiovascular function by decreasing the central blood volume and impairing muscle blood flow. So, the core-to-skin temperature gradient could be an important factor that may influence the maximum oxygen uptake (VO2 Max).[22] Malaguti et al. (2013) reported that GTCs could increase endurance in performance and VO2 Max, especially in untrained individuals.[23] Novozhilov et al. (2015) showed that the intake of 6 mg of purified green tea extract per kilogram of body weight in rats (for 7 days) increased their performance (swimming).[24]
Bogdanski et al. (2012) investigated the daily intake of 379 mg of green tea for 3 months on 56 obese subjects and observed that CRP and TNF-α levels in green tea group (GT) are lower than the placebo group. In this study, there was no exercise intervention.[25] Namita et al., (2012) reported that daily consumption of soft drinks with green tea extract for 8 weeks does not affect the CRP, IL-6, and IL 1 beta (IL-1β).[26] Although studies about the impact of green tea on summer performance are scarce, physical activity in summer is inevitable, which can be accompanied by many health problems.
Methods
Subjects
The present study was a double-blind randomized controlled trial (RCT)â€"pretest-posttest design with a control group. Fifteen untrained male participated in this study. The untrained participants were defined as those who had neverbeen involved in any type of regular exercise training. The mean age of the participants was 25.06 ± 2.1. They were recruited from the university campus. The participants were fully informed of any risks and discomforts associated with the experiments before they provided informed written consent to participate and then divided into green tea (n = 8) and placebo (n = 7) groups.
The exclusion criteria were the inability to perform exercise training; previous gastrointestinal (GI) surgery; serious medical problems; cancer; abnormal laboratory tests; history of severe injury; use of any herbal plant; and abuse of drugs or any supplementation in prior 6 months. The study was approved by the Research Ethics Committee of Baqiyatallah University of Medical Sciences, Tehran, Iran (Code: IR.BMSU.REC.1395.181).
Subjects were consumed 640 mg green tea polyphenols[27] or maltodextrin as a placebo in a double-blind design. They consumed supplements 90 min before exercise. To have a placebo that was indistinguishable for participants, it was necessary to be similar in amount, color, and shape.[28] Green tea polyphenols were purified in the laboratory of medical sciences. Quantities are within healthy limits and considered nontoxic. In addition, the subjects were asked to avoid strenuous physical activity 48 h before the tests. The necessary notice was given to both the groups regarding no change in their diet programs. Before the first session, the participants recorded a food diary for 3 days to check the intake of polyphenols and were asked to follow the same diet before the second session. Measurement of the dependent variables were in the pretest and posttest in hot climate (38°C, 60% relative humidity). These conditions are similar to the environmental heat load of many regions in Iran. The flowchart of the study is shown in Figure 1.
Figure 1.
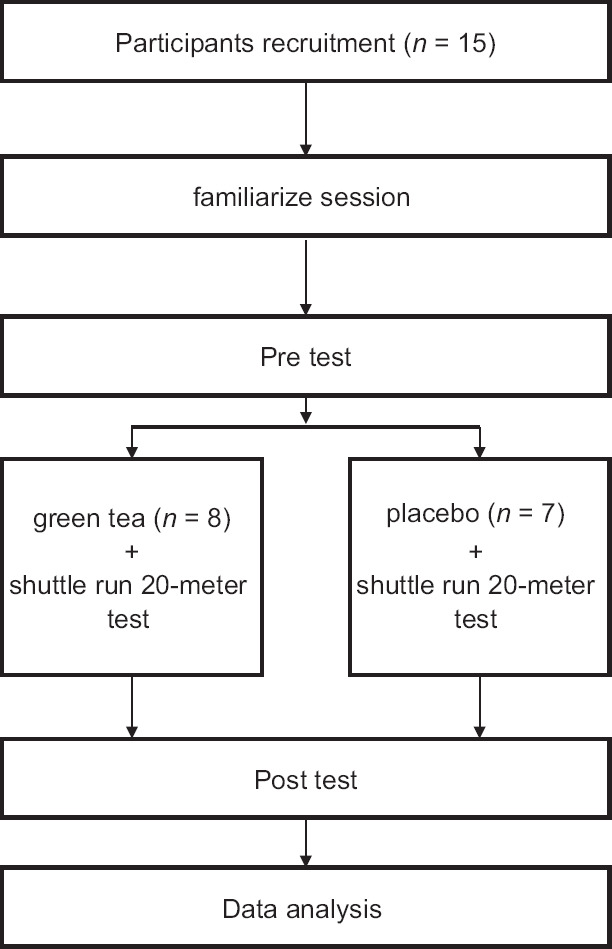
Flowchart of the study protocol
Exercise protocol
Twenty-meter shuttle runs were used as a maximal aerobic exercise. The test was made of 23 levels where each level lasted for approximately 1 min. Each level comprised of a series of 20 m shuttle runs [Table 1], where the starting speed was 8.5 km/h and increased by 0.5 km/h at each level. On the tape/CD, a single beep indicated the end of a shuttle and three beeps indicated the start of the next level. In addition, the maximum oxygen consumption per person was calculated by the below formula.[29]
Table 1.
Total number of shuttles in each level
| Level | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shuttles | 8 | 16 | 24 | 33 | 42 | 52 | 62 | 73 | 84 | 95 | 107 |
| Level | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| Shuttles | 119 | 132 | 145 | 158 | 172 | 186 | 201 | 216 | 232 | 248 | 264 |
VO2 Max = 18.043461 + (0.3689295 × TS) + (â'0.000349 × TS × TS)
Where TS was the total number of shuttles completed.
Biochemical analysis
Before and after each test, blood samples (5 ccs) were collected from the brachial vein by a doctor and then evaluated in the biochemistry laboratory. For evaluating, the TNF-α and IL_1β factors were used from Cohesion Biosciences Kit (CEK1347 and CEK1200), and for analysis, the total antioxidant capacity (TAC) used was ZellBio GmbH kit (Lot No: ZB-A116110).
Statistical analysis
In this study, Kolmogorov–Smirnov tests were used for the normality of the data and the natural assumption parametric tests were used for data. The differences between groups were assessed by using a one-way analysis of variance (ANOVA) in the Statistical Package for the Social Sciences (SPSS) software, version 18.0. In addition, the demographic characteristics and pretest differences were compared by independent t-test. The minimal level of significance adopted was P â 0.05 and data was expressed as mean ± standard deviation (SD).
Results
Descriptive characteristics of the subjects are presented in [Table 2]. There was no observed significant difference between the demographic characteristics of the subjects. As pretests were not significantly different from each other, only posttests were compared. According to the test results, including the amounts presented in Table 3 and Figure 2, it was concluded that VO2 Max of GT was significantly increased compared with the placebo (44.43 ± 3.06 ml/kg/min and 34.88 ± 1.30 ml/kg/min) after maximal aerobic activity (P = 0.001). The TNF-α in green tea supplementation (Pretest: 13.28 ± 1.94 pg/ml, Posttest: 15.03 ± 4.31 pg/ml) significantly increased less compared with placebo conditions (Pretest: 15.72 ± 5.18 pg/ml, Posttest: 31.38 ± 7.18 pg/ml) (P = 0.001). No significant difference was seen in the plasma levels of IL-1β in the green tea supplementation (Pretest: 15.40 ± 3.12 pg/ml, Posttest: 26.86 ± 5.05 pg/ml) and placebo group (Pretest: 18.02 ± 1.96 pg/ml, Posttest: 23.47 ± 3.16 pg/ml) after aerobic activity maximum (P = 0.251) [Figure 3]. The plasma TAC levels in the green tea supplementation (Pretest: 0.85 ± 0.04 mm, Posttest: 1.04 ± 0.06 mm) was significantly higher than placebo group (Pretest: 0.75 ± 0.15 mm, Posttest: 0.72 ± 0.04 mm) (P = 0.001) [Figure 4]. All data were represented as mean ± SD and P ≥ 0.05.
Table 2.
Primary characteristics of the subjects (Mean±SD)
| Groups | Green Tea | Placebo |
|---|---|---|
| n | 8 | 7 |
| Age (years) | 26.12±1.88 | 24.00±2.1 |
| Height (cm) | 175.12±4.61 | 176.14±3.84 |
| Weight (kg) | 73.00±2.07 | 76.42±4.82 |
| BMI (kg/m2) | 23.85±1.51 | 24.62±1.06 |
SD=Standard deviation, BMI=Body mass index
Table 3.
Results of maximum oxygen consumption, TNF-α, IL-1β, and TAC in green tea and placebo groups after exercise (mean±SD)
| Variable | Placebo group (mean±SD) | GT (mean±SD) | F | P |
|---|---|---|---|---|
| VO2 Max (ml/kg/min) | 34.88±1.30 | 44.43±3.06 | 26.76 | 0.001+ |
| TNF-α (pg/ml) | 31.38±7.18 | 15.03±4.31 | 31.209 | 0.001+ |
| IL-1β (pg/ml) | 23.47±3.16 | 26.86±5.05 | 3.67 | 0.251 |
| TAC (mm) | 0.72±0.04 | 1.04±0.06 | 11.18 | 0.001+ |
+Significant differences between placebo and green tea at P≤0/05, One way analysis of variance test, SD=Standard deviation, GT=Green tea group, VO2 Max=Maximum oxygen uptake, TNF-α = Tumor necrosis factor alpha, IL-1β = Interleukin 1 beta, TCA=Total antioxidan capacity
Figure 2.
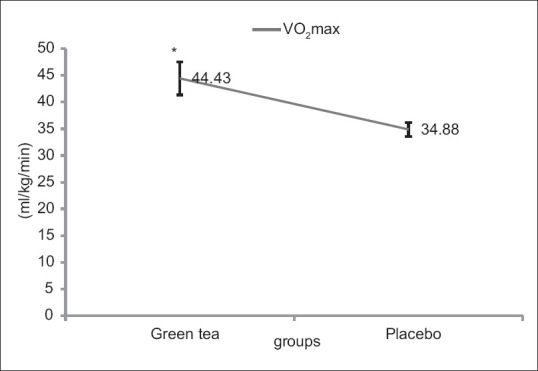
Maximal oxygen consumption (ml/kg/min) in green tea supplementation and placebo groups. *Significant differences between placebo and supplement group at P ≤ 0/05
Figure 3.
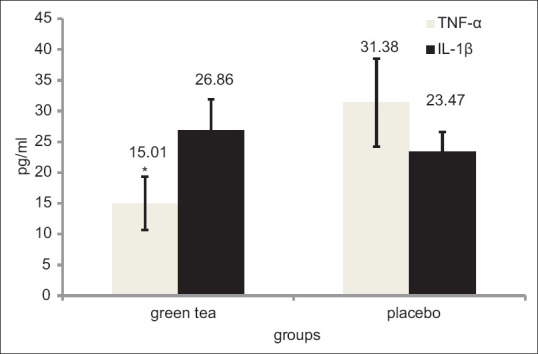
The concentrations of TNF-α and IL-1β. *Significant differences between placebo and supplement group at P ≤ 0/05
Figure 4.
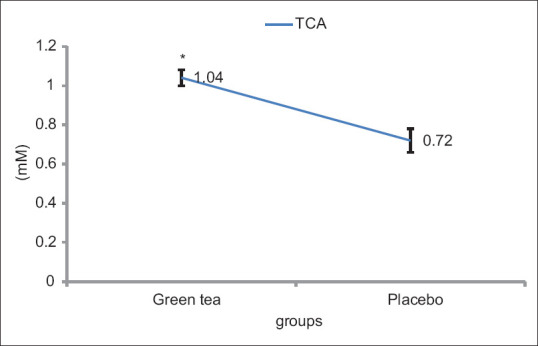
The concentrations of TAC. *Significant differences between the placebo and supplement group at P ≤ 0/05
Discussion
The results of this study showed that green tea consumption leads to a slight increase in the TNF-α level in the subjects after maximum aerobic activity at warm temperatures. After muscle damage (stressful situations, such as heat, damage, and extreme sports), inflammations occur, the resident tissue macrophages are activated, TNF-α and IL-1 are secreted locally, and release of IL steady-state conditions cause inflammation. It has been reported that exercising in hot environments and elevated circulating stress hormones and catecholamines would cause greater immune disturbance compared with exercise at normal temperatures.[30] Sureda et al. (2015) evaluated the anti-inflammatory responses in healthy male athletes after physical activity in hot weather. In this study, nine athletes perform 45 min running at 75 to 80% VO2 Max in both natural temperature of 10° to 12° and warm temperatures of 30° to 32°. Blood samples immediately before and 2 h after the exercise were collected. The results showed that inflammatory damage increased after exercising and anti-inflammatory responses were increased in recovery time.[31] On the other hand, Sureda et al. (2015) and Cosio et al. (2011), showed that exercise in hot temperatures can cause the secretion of inflammatory factors and the occurrence of inflammation.[31,32] Cao et al. (2007) examined the effect of green tea on the expression level of oxidative stress and inflammatory factors. They reported that 1 gm of purified green tea per kilogram of body weight could reduce the levels of TNF in the liver and skeletal muscles of mice. They suggested that green tea reduced inflammation by down-regulating the expression of TNF-α and reduced the oxidative stress by reducing the expression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).[33] Bogdanski et al. (2012) studied the effects of 379 mg daily dose of pure green tea for at least 3 months on 56 obese subjects. In this study, exercise intervention was not used and researchers finally stated that daily consumption of 379 mg of green tea could reduce levels of TNF-α, CRP, and oxidative stress, as well as reduce the markers of inflammation and oxidative stress.[25] Unfortunately, the ambient temperature has not been reported in the study, but according to the results of green tea in sedentary subjects, observed that these supplements can prevent the increasing levels of TNF-α in stressful conditions, such as heat and physical activity.
According to the results, it was observed that green tea supplementation compared with the placebo has no significant effect on the IL-1β secretion in subjects after maximum aerobic exercise in a hot climate. Wheeler et al. found that epigallocatechin gallate (EGCG) inhibited NF-κB activation in human lung epithelial cells treated with IL-1β (IL-1β is a powerful activator of NF-κB).[34] However, some studies did not report temperature and other studies did not use exercising in their research. Therefore, the effects of green tea after exercising on inflammation factors in a hot environment are not clear and need more study. We can conclude that TNF-α decreased and no different in IL-1β observed, so it may lead to the declined of inflammation.[35]
Accordingly, the results of this study showed that supplementation of green tea before maximum aerobic activity could increase the body's antioxidant defense against oxidative stress in hot temperatures as compared with the placebo group. The antioxidant intake protecting the body against oxidative stress also hypothesized that exercise-induced oxidative stress upregulation of antioxidant defense and the adaptive responses to training.[36] Although in some studies, the consumption of GTC does not affect the superoxide dismutase (SOD) activity, however, preventing the activity of NF-κB by high levels of GTC can prevent rising of the oxidative enzyme.[37]
Also, the antioxidant activity of GTC lead to inhibited oxidation of NF-κB and activation of peroxisome is the most important factor in fat metabolism. The recent study examined the effects of green tea epigallocatechin (GTE) on intense oxidation of blood in trained athletes sprinting during the preparatory period and suggested that GTE supplementation increased the antioxidant potential of blood and prevented the increased oxidant in the sedentary state.[38,39]
In a stdy by Panza et al. (2008), healthy men involved in two intense training resistance: once after 7 days of consuming water and the other after 7 days consumption of green tea (decoction 600 ml, concentrations of phenol 771 mg/ml). After exercise, training xanthine oxidase activity increased (the main source of free radicals). However, as a result of consuming green tea, the increase of blood antioxidant activity in both sedentary and after exercising can be observed.[38] Jowko et al. (2012) investigated the consumption of GTE (640 mg in a day for 4 weeks) on trained people. They showed that the amount of blood polyphenol and blood antioxidant potential increased after the ingestion of GTE.[27]
In another study, Jowko et al. (2015) showed that daily consumption of 980 mg GTCs for 4 weeks after a bicycle ergometer, increases the antioxidant activity, MDA, and SOD.[21] NF-κB oxidation is activated by exercising, which releases the antioxidant enzyme gene. In addition, this adjustment is eliminated when the production of ROS is prevented, and it is known as the inhibiting oxidation of xanthine.[40]
Although in some studies, the consumption of GTE does not affect the SOD activity, preventing the activity of NF-κB by high levels of GTC can prevent increasing activities of other antioxidant enzymes. Taking a low intake of antioxidants and antioxidant-rich foods is the best advice,[38] and it may be one of the most important issues for professional athletes in sports with weight control. Jowko et al. (2012) have shown that by the consumption of GTE, there was no change in antioxidant parameters. In this study, subjects completed a 1.5-h exercise after ingesting a single dose of GTE (640 mg). After the ingestion of GTE, polyphenols increased in the blood, but the overall potential of antioxidants in the blood was not changed. Therefore, it is likely that the consumption of GTE may be severe enough that antioxidants in the blood and potential long-term GTE supplementation caused reduced oxidation.[27]
The assumption is that catechin raises the total antioxidant capacity and protects the cell from damage by antioxidant properties and enzymes (SOD and catalase).[18,19] This direct action on nitric oxide concentration is accompanied by a reduction in oxygen species.[20] It is observed that MDA, which is a sign of oxidative stress, falls after consumption of green tea.[20] Also, only regular exercising undermines the oxidative balance through the generation of ROS and adjusts the antioxidant enzymes. The findings of this study have revealed that the high environmental temperature, such as the ambient temperature, is the reason for the lack of alignment, which can cause an increase in oxidative stress.
The results of this study showed that the GT had better performance compared with the placebo and GT was higher in VO2max than the placebo group. The authors tried to explain the mechanisms for the increase of VO2 Max after ingestion of EGCG, and they believe that EGCG may increase arterial oxygen difference in the skeletal muscle but the cardiac output is not increased and did not aerobic energy production, which is done by EGCG. Richards (2010) did not find any changes in respiratory exchange ratio after the oral administration of EGCG.[39] Among the studies done, the following studies were consistent with our study.
Ichinose et al. studied 19 healthy adults after taking 945 mg of EGCGâ€"3 capsules per dayâ€"48 h before exercise. They showed that short-term use of EGCG enhances athletic performance (this increase was observed in 14 of 19 patients.).[41] Roberts et al. (2015) observed that the consumption of 571 mg catechins for 4 weeks improved the exercising performance (running with 50% VO2 Max).[13] Malaguti et al. (2013) suggested that green tea can increase the VO2 Max and particular endurance performance in the untrained subjects.[23] Novozhilov et al. (2015) showed that taking 6 mg of purified green tea extract per kilogram of body weight of the rats (for 7 days) increased the performance (swimming).[24] Roberts also found that taking 571 mg of green tea for 4 weeks can improve the exercising performance in trained men.[13] Unfortunately, in all the studies, the temperature has not been reported. Finally, we can say that reducing the production of oxidants by using too many antioxidants needed adapting the specific cell in the exercise (one of the most critical factors in the performance is VO2 Max). In addition, the results of this study can be used for health promotion whom (as, athlete, soldier, and worker) that begins the intense physical activity in hot environments as well as useful for other researchers to create more research and routes.
Conclusions
Concerning our results, green tea supplementation may lead to a decrease of TNF-α and have no impact on IL-1β after aerobic exercise in summer that may prevent inflammation. In addition, these supplements can probably increase recruiting free fatty acid β-oxidation and carbohydrate storage. Green tea has a lipid oxidative character that may improve aerobic performance. On the other hand, green tea increases antioxidant capacity that prevents the body from oxidative damage.
The results of this study showed that using green tea supplementations 90 min before exercising could slow down the increasing of TNF-α and increase the total antioxidant capacity in aerobic exercising in summer. In addition, these supplements enhanced the VO2 max in summer. It is observed that using antioxidant supplements can limit the oxidative responses in heavy training or competition of professional athletes. Focusing on the antioxidant defense system by antioxidant supplements leads to reducing muscle damage and improves athletic performance.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ahn N, Kim K. The influence of obesity and ambient temperature on physiological and oxidative responses to submaximal exercise. Biol Sport. 2014;31:139–44. doi: 10.5604/20831862.1097482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohe LS. Exertional heat illness in a Marine training on the endurance course. JAAPA. 2012;25:34–6. doi: 10.1097/01720610-201206000-00007. [DOI] [PubMed] [Google Scholar]
- 3.McFarlin BK, Venable AS, Williams RR, Jackson AW. Comparison of techniques for the measurement of skin temperature during exercise in a hot, humid environment. Biol Sport. 2015;32:11–4. doi: 10.5604/20831862.1124569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Tan GY, Fu YQ, Feng JH, Zhang MH. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:204–8. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Quindry JC, Miller L, McGinnis G, Kliszczewiscz B, Slivka DR, Dumke C, et al. Environmental temperature and exercise-induced blood oxidative stress. Int J Sport Nutr Exerc Metab. 2013;23:128–36. doi: 10.1123/ijsnem.23.2.128. [DOI] [PubMed] [Google Scholar]
- 6.Bentley DJ, Dank S, Coupland R, Midgley A, Spence I. Acute antioxidant supplementation improves endurance performance in trained athletes. Res Sports Med. 2012;20:1–12. doi: 10.1080/15438627.2011.608050. [DOI] [PubMed] [Google Scholar]
- 7.Sachdev S, Davies KJ. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215–23. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Zakizadeh E, Faghihimani E, Saneei P, Esmaillzadeh A. The effect of purslane seeds on biomarkers of oxidative stress in diabetic patients: A randomized controlled cross-over clinical trial. Int J Prev Med. 2015;6:95. doi: 10.4103/2008-7802.166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decroix L, Tonoli C, Soares DD, Descat A, Drittij-Reijnders MJ, Weseler AR, et al. Acute cocoa Flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J Int Soc Sports Nutr. 2017;14:28. doi: 10.1186/s12970-017-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabidi RV, Hosseinzadeh S, Mahjoub S, Hosseinzadeh M, Myers J. Endurance exercise training and diferuloyl methane supplement: Changes in neurotrophic factor and oxidative stress induced by lead in rat brain. Biol Sport. 2013;30:41–6. doi: 10.5604/20831862.1029820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Withee ED, Tippens KM, Dehen R, Tibbitts D, Hanes D, Zwickey H. Effects of Methylsulfonylmethane (MSM) on exercise-induced oxidative stress, muscle damage, and pain following a half-marathon: A double-blind, randomized, placebo-controlled trial. J Int Soc Sports Nutr. 2017;14:24. doi: 10.1186/s12970-017-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finaud J, Scislowski V, Lac G, Durand D, Vidalin H, Robert A, et al. Antioxidant status and oxidative stress in professional rugby players: Evolution throughout a season. Int J Sports Med. 2006;27:87–93. doi: 10.1055/s-2005-837489. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JD, Roberts MG, Tarpey MD, Weekes JC, Thomas CH. The effect of a decaffeinated green tea extract formula on fat oxidation, body composition and exercise performance. J Int Soc Sports Nutr. 2015;12:1. doi: 10.1186/s12970-014-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohseni M, Vafa M, Zarrati M, Shidfar F, Hajimiresmail SJ, Forushani AR. Beneficial effects of coenzyme Q10 supplementation on lipid profile and intereukin-6 and intercellular adhesion molecule-1 reduction, preliminary results of a double-blind trial in acute myocardial infarction. Int J Prev Med. 2015;6:73. doi: 10.4103/2008-7802.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozaffari-Khosravi H, Ahadi Z, Tafti MF. The effect of green tea versus sour tea on insulin resistance, lipids profiles and oxidative stress in patients with type 2 diabetes mellitus: A randomized clinical trial. Iran J Med Sci. 2014;39:424–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Pezeshki A, Safi S, Feizi A, Askari G, Karami F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int J Prev Med. 2016;7:28. doi: 10.4103/2008-7802.173051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera N, Ambigaipalan P, Shahidi F. Epigallocatechin gallate (EGCG) esters with different chain lengths fatty acids and their antioxidant activity in food and biological systems. J Food Bioact. 2018;1:124–33. [Google Scholar]
- 18.Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr. 2004;134:38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine. 2002;9:232–8. doi: 10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- 20.Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002;50:3549–52. doi: 10.1021/jf020029h. [DOI] [PubMed] [Google Scholar]
- 21.Jówko E, Długołęcka B, Makaruk B, Cieśliński I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur J Nutr. 2015;54:783–91. doi: 10.1007/s00394-014-0757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Lorenzo S, An N, Feng W, Lai L, Cui S. Effects of heat and different humidity levels on aerobic and anaerobic exercise performance in athletes. J Exerc Sci Fit. 2013;11:35–41. [Google Scholar]
- 23.Malaguti M, Angeloni C, Hrelia S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid Med Cell Longev. 2013;2013:825928. doi: 10.1155/2013/825928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novozhilov A, Tavrovskaya T, Voitenko N, Maslova M, Goncharov N, Morozov V. Efficacy of green tea extract in two exercise models. Bull Exp Biol Med. 2015;158:342–5. doi: 10.1007/s10517-015-2757-4. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–7. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Namita P, Mukesh R, Vijay KJ. Camellia Sinensis (green tea): A review. Global J Pharmacol. 2012;6:52–9. [Google Scholar]
- 27.Jówko E, Sacharuk J, Balasinska B, Wilczak J, Charmas M, Ostaszewski P, et al. Effect of a single dose of green tea polyphenols on the blood markers of exercise-induced oxidative stress in soccer players. Int J Sport Nutr Exerc Metab. 2012;22:486–96. doi: 10.1123/ijsnem.22.6.486. [DOI] [PubMed] [Google Scholar]
- 28.Martin BJ, MacInnis MJ, Gillen JB, Skelly LE, Gibala MJ. Short-term green tea extract supplementation attenuates the postprandial blood glucose and insulin response following exercise in overweight men. Appl Physiol Nutr Metab. 2016;41:1057–63. doi: 10.1139/apnm-2016-0169. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie B. Performance Evaluation Tests. London: Electric World plc; 2005. [Google Scholar]
- 30.Ibrahim NS, Chen CK, Ayub A, Muhamad AS. Effects of prolonged running in the heat and cool environments on selected physiological parameters and salivary lysozyme responses. J Exerc Sci Fit. 2017;15:63–9. doi: 10.1016/j.jesf.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sureda A, Mestre-Alfaro A, Banquells M, Riera J, Drobnic F, Camps J, et al. Exercise in a hot environment influences plasma anti-inflammatory and antioxidant status in well-trained athletes. J Therm Biol. 2015;47:91–8. doi: 10.1016/j.jtherbio.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Cosio-Lima LM, Desai BV, Schuler PB, Keck L, Scheeler L. A comparison of cytokine responses during prolonged cycling in normal and hot environmental conditions. Open Access J Sports Med. 2011;2:7–11. doi: 10.2147/OAJSM.S15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao H, Kelly MA, Kari F, Dawson HD, Urban JF, Coves S, et al. Green tea increases anti-inflammatory tristetraprolin and decreases pro-inflammatory tumor necrosis factor mRNA levels in rats. J Inflamm (Lond) 2007;4:1. doi: 10.1186/1476-9255-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea–derived polyphenol, inhibits IL-1β-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 2004;134:1039–44. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- 35.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, Ferrando B, Viña J. Redox modulation of mitochondriogenesis in exercise Does antioxidant supplementation blunt the benefits of exercise training? Free? Radic Biol Med. 2015;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Shixian Q, VanCrey B, Shi J, Kakuda Y, Jiang Y. Green tea extract thermogenesis-induced weight loss by epigallocatechin gallate inhibition of catechol-O-methyltransferase. J Med Food. 2006;9:451–8. doi: 10.1089/jmf.2006.9.451. [DOI] [PubMed] [Google Scholar]
- 38.Panza VS, Wazlawik E, Schütz GR, Comin L, Hecht KC, da Silva EL. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition. 2008;24:433–42. doi: 10.1016/j.nut.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Richards JC, Lonac MC, Johnson TK, Schweder MM, Bell C. Epigallocatechin-3-gallate increases maximal oxygen uptake in adult humans. Med Sci Sports Exerc. 2010;42:739–44. doi: 10.1249/MSS.0b013e3181bcab6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Cabrera MC, Borrás C, Pallardó FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–20. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichinose T, Nomura S, Someya Y, Akimoto S, Tachiyashiki K, Imaizumi K. Effect of endurance training supplemented with green tea extract on substrate metabolism during exercise in humans. Scand J Med Sci Sports. 2011;21:598–605. doi: 10.1111/j.1600-0838.2009.01077.x. [DOI] [PubMed] [Google Scholar]


