Abstract
Wilson’s disease (WD) is an autosomal recessive genetic disease linked to ATP7B, which is located on the chromosome 13q14.3. We presently report a hepatolenticular degeneration carrier whose clinical phenotype mainly included limb weakness and tremor with a novel WD mutation. The mutation in Exon 10 of ATP7B Gene [c.2480G>A p. (Arg827Gln)] was identified after gene sequencing. We have provided diagnostic analyses, such as muscle biopsy and electrophysiology, which would be helpful to deepen the understanding of the pathogenesis underneath nerve damage in WD heterozygote carriers(Hzc).
Keywords: ATP7B, Gene Mutation, Limb Weakness, Tremor, Wilson Disease
Introduction
Wilson’s disease (WD) is an autosomal recessive genetic disease linked to ATP7B[1], which is located on the chromosome 13q14.3. The genetic prevalence of WD is reported as 13.9 per 100,000 cases (95% CI: 12.9-14.9), or 1 per 7194 cases[1]. The most common age is 5-35 years old[2]. The ATP7B gene whose dysfunction may lead to many forms of liver conditions[2], corneal Kayser–Fleischer ring (K-F ring), progressive extra-pyramidal symptoms and/or psychiatric symptoms. Most patients with WD have complex heterozygotes mutation[3]. WD gene mutation hotspots vary in different regions. The p.His1069Gln is found mostly in European countries[4] and the United States[5]. The p.Arg778Leu mutation is the most common in China[6], Japan[7] and Korean[8]. In China, 67% of WD gene mutations are located in exons 8,12, and 13[9], of which p.Arg778Leu is also common. Its clinical symptoms are mostly related with the liver, such as elevated liver enzymes, liver cirrhosis, and hepatosplenomegaly. The next most common mutations are p.Pro992Leu and p.Thr935Met[6]. The p.Arg827Gln found in this article is a new mutation in China and has not been reported in China. At present, there is no large-scale study on the gene distribution of WD Hzc. In China, a study on the sequencing of ATP7B gene in 40 WD Hzc showed that there were 2975C>T (6 cases), 2333G>T (5 cases), 3443T>C (5 cases), 3426G>C (4 cases), etc. Out of these, 6 patients had clinical symptoms[10].
The heterozygotes mutation carriers often showed no liver symptoms or neurological dysfunctions[11].A small number of the heterozygotes mutation carriers often show limb tremors or liver symptoms[10]. Peripheral neuromuscular damage is rare in Chinese WD patients[12]. We describe a clinical case of young novel heterozygous carrier (Hzc) of ATP7B mutation, it’s main symptoms include double upper limb weakness and tremor. We also provide the patient’s muscle pathological tissue biopsy and electromyography, as we report this case as follows.
Case presentation
A 30-year-old male was admitted to the hospital due to paroxysmal bilateral upper limb weakness for six years and tremor of both hands for two months. Muscle strength and tension as well as tendon reflex were normal at the time patient was admitted by the hospital (Figure 1). Liver function was evaluated after admission. In this context, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were normal. Ceruloplasmin [16.10 mg/dL (normal range: 22.0-58.0)]. MRI results of the brain were normal. Brain MRS showed that creatine levels of the right lenticular nucleus slightly decreased.
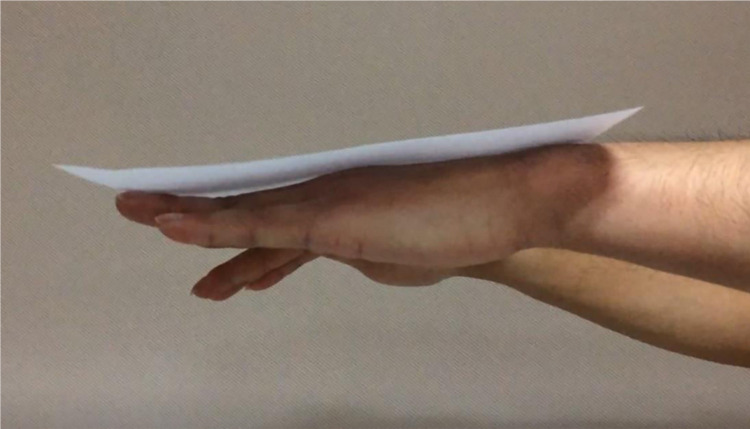
Figure 1.
Tremor could be seen in this WD patient when his hands were raised horizontally.
Electromyogram of the patient showed that most of the nerve conductive velocity (NCV), related to the limb nerves, presented a decreased speed. Accordingly, the F-wave incubation period was prolonged, and this observation was obvious for both upper limbs. Acupuncture electromyogram (EMG) showed that most of the muscles were electromyographically damaged and, as a result, myogenic damage was diagnostically considered.
Biopsies of the deltoid muscle on the right upper limb indicated (i) mild myogenic changes (mitochondrial disease could not be excluded), (ii) unbalanced proportion of muscle fibers, and mild neurogenic pathological changes (Figure 2). Ophthalmic examination suggested negative K-F ring. The penicillamine challenge test showed that, after administration penicillamine (500 mg po q12h), 24-hour urine copper was 1405.6 ug /24h. No obvious abnormalities were detected in regard to folic acid and vitamin B12 levels, Human Immunodeficiency Virus (HIV) plus rapid plasma reagin (RPR) tests, serum tumor markers, and ultrasound examination of the liver, gallbladder and heart. Targeted DNA sequencing revealed a mutation in the ATP7B gene [c.2480G> A p. (Arg827Gln)] (Figure 3). Upon penicillamine treatment, bilateral weakness of the upper limbs and tremor were relieved. At 1-year follow-up, the patient presented no obvious clinical symptoms.
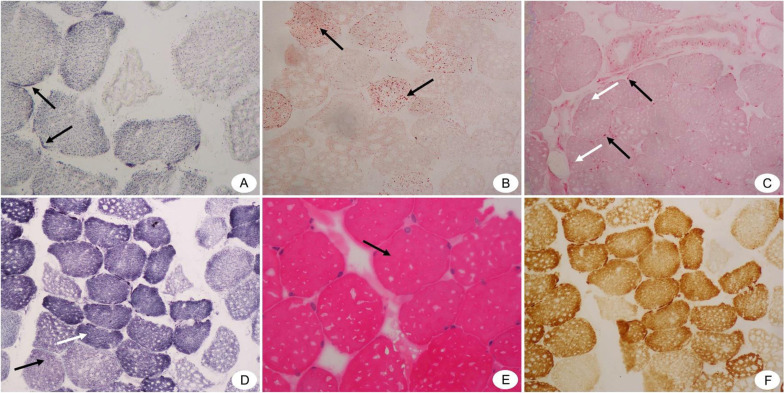
Figure 2.
Pathological results of biopsies from right deltoid muscle of the WD patient (Under light microscope). (A) SDH staining (×400) shows hyperchromatism around type I muscle fibers (shown by black arrows). (B) ORO staining(×200) shows that Lipid droplets in some type I muscle fibers are slightly finer (shown by black arrows). (C) ACP staining(×200) shows that A small number of positive particles are seen in the perimysium (shown by black arrows) and endomysium (shown by white arrows). (D) NADH staining(×200) shows that the muscle fiber structure is a little disordered. Type I muscle fibers are dominant, and there is a small group of group distribution phenomenon (shown by white arrow). The black arrow indicates the type 2 muscle fibers. (E) HE staining(×400)shows that the muscle fibers are blunt round (shown by black arrow). (F) There is no obvious abnormality in COX staining(×200).
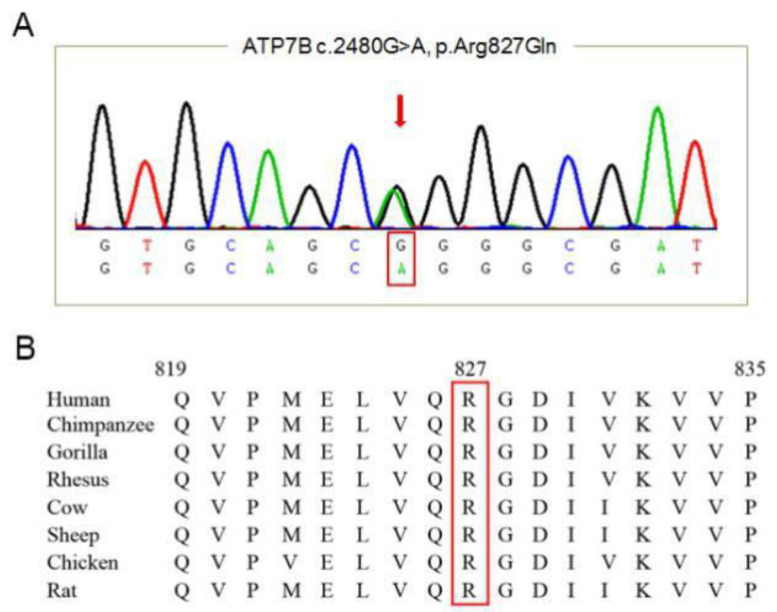
Figure 3.
Mutation analysis of WD patient genome. (A) control DNA sequence; (B) A missense mutation along the sequence of exon 10 of ATP7B was detected [c.2480G>A p. (Arg827Gln)].
Discussion
In our current study, the patient had muscle weakness and tremor symptoms, with abnormal results of serum ceruloplasmin and urinary copper, which complied with the WD diagnosis scoring system[13]. The K-F ring which is caused by deposition of excess copper in Descemet’s membrane of the cornea was negative in our case[14]. An examination by an experienced physician using a slit lamp is required to identify the K-F ring, which can be present in 95% of WD patients with neurologic symptom[15]. We have also detected a heterozygous mutation in this patient, which was specifically located in the exon 10 of the ATP7B gene [c.2480g>a p.(arg827GIn)]. The gene mutation was a missense mutation. In the structure of ATP7B, the CPC cation transmembrane transport region on the 6th transmembrane domain is related to exon 10 and is mainly responsible for the transport of copper ions. The mutation will cause ATP7B to stay in the perinuclear Golgi apparatus and endoplasmic reticulum. It is degraded by ubiquitin protease and cannot discharge copper ions to the cell membrane. The mutation of the 827th amino acid on ATP7B has been reported previously[16,17], but the amino acid after mutation is different from our patient. Due to the refusal of family members, it was not possible to further determine whether the current ATP7B mutation had an inheritance pattern. While this patient presented only one allele abnormality, about 90% of WD patients are known to have two or more pathogenic mutations along the genome6. So, it is feasible that this subject would be a WD Hzc with clinical manifestations rather than a WD patient per se.
Currently, there is no common understanding of WD Hzc’s clinical symptoms. Kumar S et al. believe that Hzc has only one recessive pathogenic gene, so the disease will not develop for life and no treatment is needed18. However, Tarnacka B and others believe that some WD Hzc may find a slight increase in transaminases, but abdominal ultrasound has not found abnormalities[11]. Simple heterozygous mutations cause half of ATP7B dysfunction, decreased ceruloplasmin levels and decreased copper transport capacity. Most WD Hzc have subclinical phenotypes without symptoms, but it is found that some patients will have mild neurological symptoms which are considered to be related to decreased ceruloplasmin levels. So WD Hzc may show mild clinical symptoms, which are easily misdiagnosed as neurological and/or liver diseases. Some WD Hzc may present tremors[11], but to a lesser extent. Another study has shown that WD Hzc can manifest phenotypic symptoms similar to Parkinson’s disease (PD)[19]. This data reiterates the importance for the proper clinical identification of WD.
Tremor is the most common neurological manifestations of WD[2]. Due to the difference of copper deposition and tolerance in affected organs and tissues, the symptoms and signs of the disease can largely diverge. Patients with WD Hzc can also develop brain damage. WD Hzc can manifest phenotypic symptoms similar to PD[20]. In China, Zhou et al. conducted a study on 40 WD Hzc and found that 6 WD Hzc had clinical symptoms. Among them, the most common neurological symptom was tremor (4 cases), but WD Hzc had mild neurological symptoms[10]. Our patient had tremor similar to Zhou’s results of the study, and ceruloplasmin was lower than normal. Meanwhile, MRI of the patient was normal in our study, while MRS showed that creatine level of the right lenticular nucleus was slightly decreased. As a result, neuronal metabolism could be slightly reduced, as previously reported[11]. Creatine plays a vital role in the storage and transport of cellular energy[20]. Creatine in the systemic circulation can be transported into the brain through the creatine transporter in capillary endothelial cells[21]. The reduction or absence of creatine in the brain will lead to the weakening of the buffering capacity of ATP peak demand and the transport capacity of energy-rich phosphate compounds, which will lead to mitochondrial dysfunction[22]. This is also consistent with our muscle biopsy results that shows mitochondrial injury. The decrease of creatine in the brain might be a result of the deposition of copper ions in the lenticular nucleus.
Mitochondrial damage caused by excessive copper is one of the main pathological mechanisms of WD-related cell injury[23]. Results of muscle biopsy from the WD Hzc in our case suggested mitochondrial damage, which could act as a major cause of muscle weakness. According to the nerve examination along the extremities, NCV was largely retarded, the latency of F-wave was prolonged, especially in the upper limbs. Our data was consistent with WD neuropathological examination, which indicated destruction of the myelin sheath[24]. It could be one of the reasons for muscle weakness.
Conclusion
Presently, we have found a novel WD mutation with muscle weakness accompanied with tremor. This WD Hzc presented a novel genetic mutation, which enriches the collection ATP7B gene mutations putatively linked to WD pathology. Moreover, we have provided diagnostic analyses, such as muscle biopsy and electrophysiology, which would be helpful to deepen the understanding of the pathogenesis underneath nerve damage in WD Hzc.
Ethics statement
This paper was approved by the Ethics Committee of the first affiliated hospital of Zhejiang Chinese Medical University, with the ethical approval number (2019-zx-001-01). We obtained written informed consent from the patient for the publication of this case report.
Author contributions
Zhengxiang Zhang participated in the topic selection and design of this article, and was responsible for collecting clinical data and samples as well as writing the manuscript; Jiayi Liu participated in the writing of the thesis; Wenjing Zheng participated in data analysis; Qun Hou participated in the design of the article and further revision. Liping Zhang was responsible for directing the whole article.
Funding
This study was founded by: National Natural Science Foundation (CN) (Grant number 81302938), Zhejiang provincial natural science foundation of China (Grant number LY18H270009).
Acknowledgments
Thanks to the patient and Hangzhou KingMed Diagnostics for their help and participation in this study. Thanks to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Gao J L, Brackley S, Mann J P. The global prevalence of Wilson disease from next-generation sequencing data. Genet Med. 2019;21(5):1155–1163. doi: 10.1038/s41436-018-0309-9. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines:Management of chronichepatitis B virus infection. J Hepatol. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Ferenci P. Wilson's Disease. Clin Gastroenterol Hepatol. 2005;3(8):726–733. doi: 10.1016/s1542-3565(05)00484-2. [DOI] [PubMed] [Google Scholar]
- 4.Ferenci P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease:impact on genetic testing. Hum Genet. 2006;120(2):151–159. doi: 10.1007/s00439-006-0202-5. [DOI] [PubMed] [Google Scholar]
- 5.Kuppala D, Deng J, Brewer GJ, et al. Wilson disease mutations in the American population:identification of five novel mutations in ATP7B. Open Hepatol J. 2009;1(1):1–4. [Google Scholar]
- 6.Dong Y, Ni W, Chen WJ, et al. Spectrum and Classification of ATP7B Variants in a Large Cohort of Chinese Patients with Wilson's Disease Guides Genetic Diagnosis. Theranostics. 2016;6(5):638–649. doi: 10.7150/thno.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatsumi Y, Hattori A, Hayashi H, et al. Current state of Wilson disease patients in central Japan. Intern Med. 2010;49(9):809–815. doi: 10.2169/internalmedicine.49.2931. [DOI] [PubMed] [Google Scholar]
- 8.Bae S H, Kim J W, Seo JK. Haplotype analysis and possible founder effect at the R778L mutation of the ATP7B gene in Korean patients with Wilson's disease. Korean J HEPATOL. 2009;15(3):309–319. doi: 10.3350/kjhep.2009.15.3.309. [DOI] [PubMed] [Google Scholar]
- 9.Li XH, Lu Y, Ling Y, et al. Clinical and molecular characterization of Wilson's disease in China:identification of 14 novel mutations. BMC Med Genet. 2011;12:6. doi: 10.1186/1471-2350-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou XX, He RX, Pu XY, et al. Clinical characteristics of the Wilson disease carrier. Natl Med J China. 2019;99(11):806–811. doi: 10.3760/cma.j.issn.0376-2491.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Tarnacka B, Szeszkowski W, Buettner J, et al. Heterozygous carriers for Wilson's disease-magnetic spectroscopy changes in the brain. Metab Brain Dis. 2009;24(3):463–468. doi: 10.1007/s11011-009-9145-6. [DOI] [PubMed] [Google Scholar]
- 12.Xie JJ, Wu ZY. Wilson's Disease in China. Neurosci Bull. 2017;33(3):323–330. doi: 10.1007/s12264-017-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poujois A, Woimant F. Wilson's disease:A 2017 update. Clin Res Hepatol Gastroenterol. 2018;42(6):512–520. doi: 10.1016/j.clinre.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL Clinical Practice Guidelines:Wilson's disease. J Hepatol. 2012;56(3):671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Steindl P, Ferenci P, Dienes HP, et al. Wilson's disease in patients presenting with liver disease:a diagnostic challenge. Gastroenterology. 1997;113(1):212–218. doi: 10.1016/s0016-5085(97)70097-0. [DOI] [PubMed] [Google Scholar]
- 16.Ferenci P. Phenotype–genotype correlations in patients with Wilson's disease. Ann N Y Acad Sci. 2014;1315:1–5. doi: 10.1111/nyas.12340. [DOI] [PubMed] [Google Scholar]
- 17.Lee BH, Kim JH, Lee SY, et al. Distinct clinical courses according to presenting phenotypes and their correlations toATP7B mutations in a large Wilson's disease cohort. Liver Int. 2011;31(6):831–9. doi: 10.1111/j.1478-3231.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Thapa B, Kaur G, et al. Analysis of most common mutations R778G, R778L, R778W, I1102T and H1069Q in Indian Wilson disease patients:correlation between genotype/ phenotype/ copper ATPase activity. Mol Cell Biochem. 2007;294(1-2):1–10. doi: 10.1007/s11010-005-9028-z. [DOI] [PubMed] [Google Scholar]
- 19.Ilyechova E, Sankova T, Milyukhina I, et al. Association of Parkinson's disease (PD) with heterozygous carriers of the Wilson disease (WD) gene. Parkinsonism Relat D. 2016;22:e175. [Google Scholar]
- 20.Snow RJ, Murphy RM. Creatine and the creatine transporter:a review. Mol Cell Biochem. 2001;224(1-2):169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- 21.Braissant O, Henry H, Loup M, et al. Endogenous synthesis and transport of creatine in the rat brain:an in situ hybridization study. Mol Brain Res. 2001;86(1-2):193–201. doi: 10.1016/s0169-328x(00)00269-2. [DOI] [PubMed] [Google Scholar]
- 22.Stagg CJ, Rothman DL. Magnetic Resonance Spectroscopy:Tools for Neuroscience Research and Emerging Clinical Applications. San Diego: Academic Press; 2014. [Google Scholar]
- 23.Dusek P, Litwin T, Czlonkowska A. Wilson Disease and Other Neurodegenerations with Metal Accumulations. Neurol Clin. 2015;33(1):175–204. doi: 10.1016/j.ncl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Jung KH, Ahn TB, Jeon BS. Wilson disease with an initial manifestation of polyneuropathy. Arch Neurol. 2005;62(10):1628–1631. doi: 10.1001/archneur.62.10.1628. [DOI] [PubMed] [Google Scholar]