Abstract
Objective:
The lymphatic system plays an important role in joint diseases. This study aimed to evaluate the effects of ginsenoside Rg1 on lymphatic drainage and accumulation of inflammatory products in the joints.
Methods:
Two-month-old transgenic mice that overexpress tumor necrosis factor alpha (TNF-α; TNF-Tg) were used as the animal models. Ginsenoside Rg1 was administered for 12 weeks and the lymphatic drainage in the mice was evaluated using near infrared-indocyanine green (NIR-ICG) lymphatic imaging system. The clinical symptoms of arthritis were evaluated weekly. The ankle and knee joints were harvested for hematoxylin-eosin (HE), alcian blue/orange G (ABOG), and tartrate-resistant acid phosphatase (TRAP) staining, and the foot dorsal skin was used for whole-mount immuno-staining. Simultaneously, the serum levels of IL-6 and TNF-α were detected using enzyme-linked immunosorbent assay (ELISA).
Results:
Ginsenoside Rg1 significantly improved the lymphatic drainage function, reduced synovial inflammation and bone erosion, decreased serum IL-6 and TNF-α concentration, and increased smooth muscle coverage on the collecting lymphatic vessels in the foot skin of the TNF-Tg mice. Furthermore, ginsenoside Rg1 treatment for 12 weeks did not cause any damage to the liver and kidney tissues.
Conclusion:
Ginsenoside Rg1 improves lymphatic drainage and joint inflammation in TNF-Tg mice. Therefore, ginsenoside Rg1 has the potential to be a candidate drug for the treatment of inflammatory arthritis.
Keywords: Inflammatory Arthritis, Ginsenoside Rg1, Lymphatic Drainage, TNF-α
Introduction
Inflammatory arthritis (IA) refers to arthritis caused by inflammatory factors other than bone degeneration, and includes rheumatoid arthritis (RA), psoriatic arthritis, and ankylosing spondylitis[1]. RA is the most common[2] form of IA, with a prevalence rate of approximately 3% in adult[3]. The early stages of this disease are often characterized by synovial inflammation and secondary joint damage, and if not treated properly, can result in irreversible joint deformities that seriously affect a patient’s work ability and quality of life[4]. At present, the clinical treatment of IA mainly aims to prevent the development of joint damage and long-term disability by delaying disease development and reducing rheumatism[5,6]. However, most of the drug have serious side effects and do not meet the current clinical needs[7]. Therefore, identification and development of novel anti-inflammatory arthritis drugs has become an area of intensive research and has important clinical significance.
Recently, increasing number of studies have confirmed that lymphatic drainage plays an important role in the occurrence and development of IA[8]. A large number of cytokines and chemical factors can be found in the lymph of the inflammatory joint drainage[9]. The number of lymphatic vessels in the synovial tissue of the joint is also increased in patients with joint inflammation and edema[10]. Lymphatic contraction and chronic inflammatory-erosive joints are enhanced because of anti-TNF drugs during the treatment of arthritis[11]. The tumor necrosis factor-transgenic (TNF-Tg) inflammatory mouse models show a decrease in lymphatic drainage function[12]. In summary, the above studies demonstrate that the progression of arthritis is closely associated with lymphatic drainage[13]. Currently, very few drugs target the lymphatic system for the treatment of IA.
Tumor necrosis factor alpha (TNF-α) plays an important role in the pathogenesis of joint inflammation[14]. A variety of transgenic mice overexpressing TNF-α (TNF-Tg) present with chronic progressive arthritis of the joints and are therefore ideal animal models for studying IA[15].
Previous studies have confirmed a high correlation between lymphatic drainage function and IA[16]. Total saponins from Panax notoginseng were found to promote lymphatic drainage by promoting lymphangiogenesis and could treat IA in TNF-Tg mice by improving the lymphatic drainage[17]. Analysis of the active ingredients in the total saponins from P. notoginseng revealed ginsenoside Rg1 to be a major component, which has been shown to have anti-inflammatory effects[18,19]. In addition, previous studies have reported that ginsenoside Rg1 reduces collagen-induced arthritis (CIA) in mice. However, in the CIA model, the CIA tends to heal itself, and thus the therapeutic effect of ginsenoside Rg1 remains unclear. Therefore, we used the TNF-Tg mouse model to study the effect of ginsenoside Rg1 on lymphatic drainage and to evaluate its potential mechanism in chronic progressive IA.
Material and Methods
Animals
The TNF-Tg mouse model was successfully generated by George Kollias Laboratories in 1991[20]. These mice express human TNF-α transgene, which causes progressive arthritis in the animals. Polyarthritis causes swelling and deformation of joints at 2–3 months of age[21]. The TNF-Tg mice used in this study were donated by Dr. G. Kollias (Institute of Immunology, Alexander Fleming Biomedical Sciences Research Center, Vari, Greece), and were commissioned by the Shanghai Southern Model Animal Company for mating and breeding (SPF grade). Mice were housed in a temperature (25°C) and humidity (45–55%)-controlled environment and provided ad libitum access to maintenance diet and water. All animal procedures were performed according to the Guiding Principles for the Care and Use of Laboratory Animals approved by the Animal Regulations of the National Science and Technology Committee of China under the approval of the Longhua Hospital Animal Ethics Committee (Shanghai, China).
Reagents and drugs
Main reagents: Ginsenoside Rg1 (Chengdu Pufei De Biotech Co. Ltd., 22427-39-0), Isoflurane (Reward, 217140901), indocyanine green (Akorn, 17478-701-02), 10% formalin (Guangzhou Vigers Biotechnology, 14071005), xylene (Sinopharm Chemical Reagent Co. Ltd., 20140506), hydrochloric acid (Sinopharm Chemical Reagent Co. Ltd., 20140228), ammonia water (Sinopharm Chemical Reagent Co. Ltd., 20140607), phosphate buffered saline (PBS) (Medicago, 09-2052-100), hematoxylin (Sigma, H-3136), eosin (Sigma, E4009-5G), Alcian Blue (Sigma, A5268), orange G (Sigma, 1936-15-8), AS-BI phosphate (Sigma, 1919-91-1), ammonium aluminum sulfate (Sigma, A-2140), sodium iodate (Sigma, S-4007), tartaric acid (Sigma, 87-69-4), Mouse IL-6 ELISA Kit (Abcam, ab100713), Mouse TNF-α ELISA Kit (Abcam, ab208348).
Main instruments: Spray anesthesia machine (Matrx, 07149), NIR imaging system (Olympus, MVX10), and Olympus fully automated tissue scanner (Olympus, VS120-SL).
Drug administration
Twelve TNF-Tg female mice were randomly divided into two groups: TNF-Tg and TNF-Tg + ginsenoside Rg1 group, consisting of six mice each. A batch of six littermates of wild type (WT) mice were used as the WT group. TNF-Tg + ginsenoside Rg1 group mice were treated with 20 mg/kg ginsenoside Rg1 by daily gavage at a fixed time and continuously for 12 weeks. The TNF-Tg and WT mice were treated with the same volume of saline. The dosage of the drug used in the drug group was calculated according to the equivalent dose conversion table for animals and humans.
Evaluation of arthritis
Body weights reflect the health status of the mice most directly. After the experiment was carried out, the body weight of the mice was measured weekly using an electronic scale, and the circumference of the ankle joint was measured using a Vernier caliper to assess the degree of joint deformity in the mice.
Near infrared-indocyanine green (NIR-ICG) lymphatic imaging
After anesthetizing the mice with isoflurane, the lower extremities were depilated and 10 μL of ICG (0.1 μg/mL, dissolved in distilled water and stored in the dark) was injected intradermally into the footpads. The fluorescence signal was then observed as the dye gradually entered the axillary lymph node from the plantar lymphatics. One hour after the injection, the signal intensity in the lymphatic vessels tended to stabilize. The fluorescence was observed continuously for 600 s and recorded, and the pulse number was obtained based on the number of fluctuations in the lymphatic signal intensity. Pulse is a signal change caused by the rhythmic contraction of the lymphatic smooth muscle, which reflects the drainage function of the lymphatic vessels.
Whole-mount staining of the back of the foot
The distribution of the lymphatic vessels in the skin of the back of the foot of the mice was analyzed using podoplanin/ α-smooth muscle actin (α-SMA) double fluorescent staining. The fur was removed with a hair removal lotion and the dorsal skin of the foot was fixed in 10% formalin, and then blocked with 3% BSA. Tissues were incubated with 1:1000 dilution of podoplanin (PDPN) antibody and 1:400 dilution of fluorescein isothiocyanate labeled anti-SMA antibody at 4°C overnight. Tissues were imaged using a fluorescence microscope. The α-SMA stained the blood vessels with green fluorescence, while the flat foot protein, PDPN, stained the capillary lymphatic vessels with red fluorescence. The double fluorescence label was visible as yellow fluorescence, reflecting a mature lymphatic vessel. The percentage of vessels covered by α-SMA+ SMCs was used to evaluate the changes in the lymphatic vessel. The percentage of SMC coverage was calculated as follows: (SMC coverage area/whole lymphatic vessel area) × 100%.
Histological examination
Following 12 weeks of treatment with ginsenoside Rg1, the knee and ankle joints of the mice were harvested, and the specimens were fixed in 10% formalin for 24 h. Then the specimens were continuously washed in water for 2 h and transferred to 10% ethylene diamine tetraacetic acid (EDTA) decalcification solution for 20 days. The decalcification solution was replaced once every 48 h. After decalcification, the specimens were washed in water continuously for 2 h, dehydrated with a series of ethanol, and embedded in paraffin. After paraffin embedding, serial sagittal sections of 4 μm were obtained and stained with HE, alcian blue/orange G (ABOG), and tartrate-resistant acid phosphatase (TRAP). After a full-length scan using an Olympus VS120-SL, the target area of the ankle talus and knee joints were analyzed with Olympus VS120 image analysis software. The cartilage (blue zone) and synovial inflammation areas were measured in the ABOG-stained sections, and TRAP-positive areas were evaluated in the TRAP-stained sections.
Hepatic and nephrotic HE staining
The liver and kidneys of the mice were fixed in 10% formalin for 24 h and washed with water continuously for 2 h. The soft tissue was then dehydrated. After paraffin embedding, 3 μm cross sections were prepared. The sections were subjected to HE staining to detect the tissue structure of the liver and the kidney.
ELISA
All the mice were sacrificed under anesthesia. A total of 0.5 ml of blood was collected from each mouse and centrifuged. Serum was collected for the determination of TNF-α and IL-6 levels using the corresponding ELISA kits according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8.0 statistical software. All data are the result of three independent experiments and expressed as mean ± sstandard deviation (±SD). One-way ANOVA followed by Dunnett’s t-test was used to determine the significance of the differences between groups. A value of P<0.05 was considered statistically significant.
Results
Effect of ginsenoside Rg1 on body weight and ankle joint swelling in mice
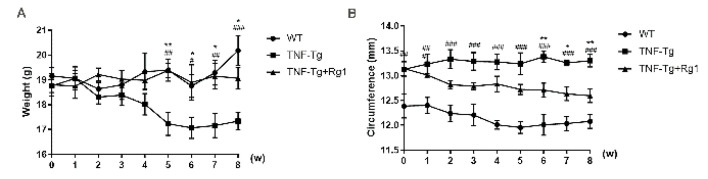
The initial body weight and ankle joint circumference of each experimental group was recorded at the start of the experiment (0 weeks), and then recorded once a week thereafter. The results showed a significant decrease in the body weights of the mice in the TNF-Tg group at the beginning of the fifth week (P<0.05), consistent with the findings of a previous study. At the same time, the body weight of the mice increased after five weeks of intervention with ginsenoside Rg1 (P<0.05; Figure 1A). Compared to the WT group, the TNF-Tg group showed a significant increase in the degree of ankle joint swelling, with ginsenoside Rg1 showing protective effect on inflammation and swelling of the joints to a certain extent. After six weeks of treatment, the degree of swelling was significantly reduced (P<0.05; Figure 1B).
Figure 1.
Effect of ginsenoside Rg1 on body weight and ankle joint swelling in mice. Body weight and ankle circumference were assessed weekly after Rg1 treatment. (A) Body weight results showed a significant increase in the Rg1 treated group starting at the fifth week (P<0.05). (B) From the sixth week, the degree of swelling of the ankle joint in the Rg1 treatment group was significantly reduced (P<0.05). (*P<0.05 vs. Rg1-treated samples. #P<0.05 vs. WT samples.)
Effect of ginsenoside Rg1 on lymphatic drainage in TNF-Tg mice
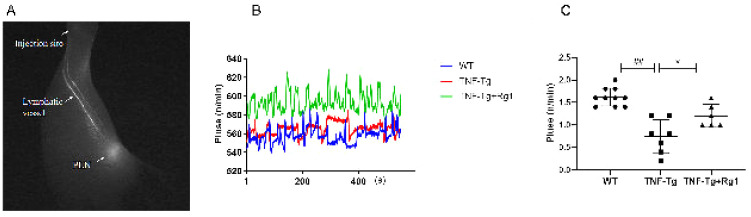
The number of beats in the lymphatic vessels of the lower limbs from the plantar to the axillary lymph nodes constitutes a pulse. The lymphatic signal changes in the lower limbs of mice were measured for 600 s and reflect the regular contraction and relaxation of the lymphatic vessels (Figure 2A). The results showed that compared to the WT group (Figure 2B), the number of lymphatic vessels in the TNF-Tg mice decreased significantly (P<0.05), and the number of beats after ginsenoside Rg1 treatment increased significantly (P<0.05).
Figure 2.
Effect of ginsenoside Rg1 on lymphatic drainage in TNF-Tg mice. (A, B) Lymphatic pulses were measured at the region of interest. Changes in the lymphatic signal of the lower limbs of mice were measured within 600 s, reflecting the process of regular contraction and relaxation of the lymphatic vessels (pulse). (C) Quantitation of lymphatic pulses/minute. Compared with WT mice, the lymphatic unit time pulsation in the TNF-Tg mice reduced (P<0.05), and Rg1 treatment promoted an increase in the lymphatic unit time beats (P<0.05). (*P<0.05 vs. Rg1-treated samples. #P<0.05 vs. WT samples.)
Effect of ginsenoside Rg1 on lymphatic function based on observation of the lymphatic vessels in the skin of the back of the foot
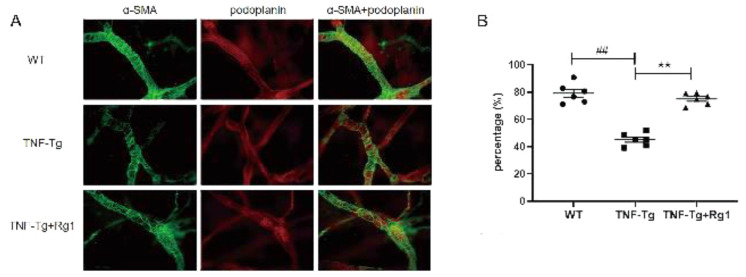
The distribution of lymphatic vessels in the skin of the back of the mice was analyzed using podoplanin/α-SMA double fluorescent staining. α-SMA was labeled with a green fluorescence marker, and flat foot protein (PDPN) with red. Double fluorescence labeling is visible in yellow indicating the mature lymphatic vessels (Figure 3A). Compared to the WT mice, the TNF-Tg mice had fewer lymph nodes and more damaged lymphatic vessels in the back of the foot, and the area covered by lymphatic smooth muscle was reduced. The area covered by lymphatic smooth muscle was significantly increased after ginsenoside Rg1 treatment (P<0.05; Figure 3B).
Figure 3.
Effect of ginsenoside Rg1 on lymphatic function based on observation of the lymphatic vessels in the skin of the back of the foot. The distribution of the lymphatic vessels in the skin of the back of the mice’s foot was detected by Podoplanin/α-smooth muscle actin (α-SMA) double fluorescent staining. (A) Representative images showing Podoplanin+/ α -smooth muscle actin (α-SMA)+ mature lymphatic vessels. (B) SMC coverage (%). The area covered by lymphatic smooth muscle was reduced in the TNF-Tg mice. After Rg1 treatment, and the area covered by lymphatic smooth muscle was significantly increased (P<0.05). (*P<0.05 vs. Rg1-treated samples. #P<0.05 vs. WT samples.)
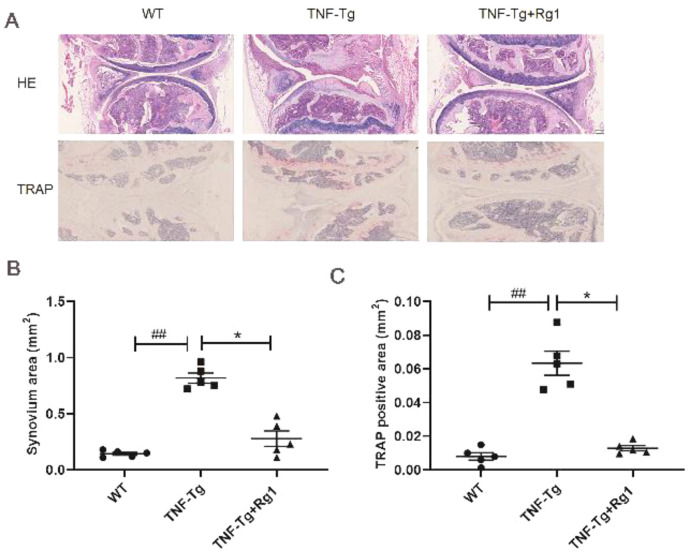
Effect of ginsenoside Rg1 on synovial volume and bone erosion in ankle joints of TNF-Tg mice
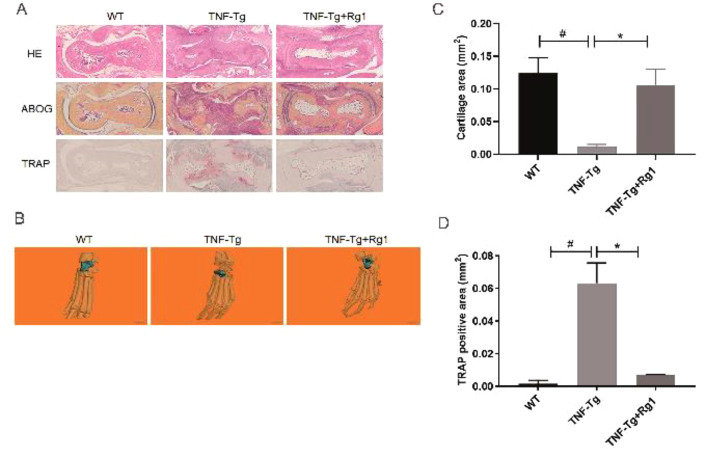
HE staining of the ankle joint showed that, compared to the WT group, the joint surface of the TNF-Tg group was severely damaged, and the bone erosion was severe. After Rg1 treatment, the area of inflammatory erosion was significantly reduced (Figure 4A). Compared to the TNF-Tg group, the bone erosion area at the ankle joints of mice was significantly reduced (Figure 4B). ABOG staining showed that the cartilage of the TNF-Tg group was severely reduced, and the cartilage structure was restored after ginsenoside Rg1 treatment (P<0.05; Figure 4C). TRAP staining showed that more red-stained osteoclasts were observed in the TNF-Tg group, and the osteoclast area was significantly reduced following ginsenoside Rg1 treatment (P<0.05; Figure 4D).
Figure 4.
Effect of ginsenoside Rg1 on synovial volume and bone erosion in ankle joints of TNF-Tg mice. (A) Representative HE staining, ABOG staining, and TRAP staining sections. (B) Micro-CT of ankle joints. (C) Quantitation of cartilage area (blue zone). ABOG staining showed that the cartilage of the TNF-Tg group was severely damaged but was obviously restored after Rg1 treatment (P<0.05). (D) Quantitation of TRAP+ osteoclast area. TRAP staining showed that more red-stained osteoclasts were observed in the TNF-Tg group and the osteoclast area was significantly reduced after Rg1 treatment (P<0.05). (*P<0.05 vs. Rg1-treated samples. #P<0.05 vs. WT samples.)
Effect of ginsenoside Rg1 on the pathological morphology of the knee joint in TNF-Tg mice
HE staining of the knee joints showed that the TNF-Tg group had larger inflammatory infiltration areas compared to the WT group (P<0.05; Figure 5A). Osteopenia, incomplete articular surface, and slippery membrane areas were also significantly increased (Figure 5A). The inflammatory infiltrate and synovial areas were significantly reduced after ginsenoside Rg1 treatment (P<0.05; Figure 5B). TRAP staining showed more osteoclasts in the TNF-Tg group, and the osteoclast area was significantly reduced following ginsenoside Rg1 treatment (P<0.05; Figure 5C).
Figure 5.
Effect of ginsenoside Rg1 on the pathological morphology of the knee joint in TNF-Tg mice. (A) Representative HE staining and TRAP staining sections. (B) Quantitation of synovial areas. The inflammatory infiltrating and synovial areas of the ginsenoside Rg1 group were significantly reduced (P<0.05). (C) Quantitation of TRAP+ osteoclast area. TRAP staining showed that more osteoclasts were seen in the TNF-Tg group and the osteoclast area was significantly reduced after Rg1 treatment (P<0.05). (*P<0.05 vs. Rg1-treated samples. #P<0.05 vs. WT samples.)
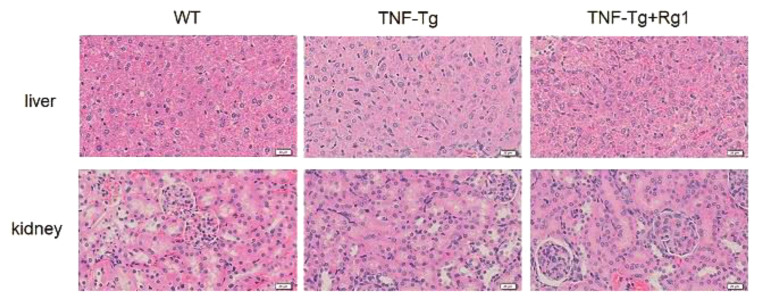
Effect of ginsenoside Rg1 on liver and kidney toxicity in TNF-Tg mice
HE staining results showed that the hepatocytes of the WT, TNF-Tg, and ginsenoside Rg1 groups maintained intact cell structures, uniform cell gaps, non-significant expansion of the renal tubules, intact glomerular structures, and no inflammatory edema in the renal interstitium. No obvious liver or kidney toxicity was observed (Figure 6).
Figure 6.
The effect of Rg1 on liver and kidney toxicity in mice. HE staining results showed that the hepatocytes of the WT, TNF-Tg, and ginsenoside Rg1 groups maintained intact cell structures, uniform cell gaps, non-significant expansion of the renal tubules, intact glomerular structures, and no inflammatory edema in the renal interstitial. No obvious liver and kidney toxicity were observed.
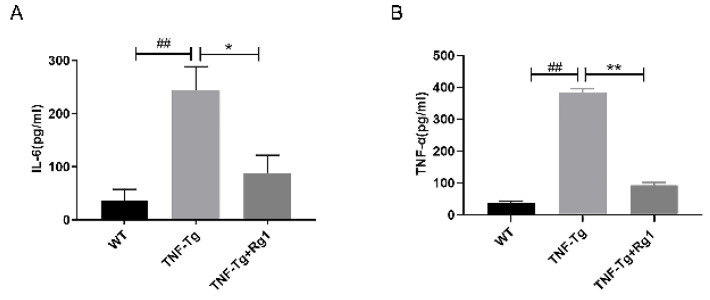
Effect of ginsenoside Rg1 on serum levels of TNF-α and IL-6
Compared to the WT group, the concentration of IL-6 and TNF-α was significantly increased in the TNF-Tg group (P<0.05; Figure 7). However, ginsenoside Rg1 significantly reduced IL-6 and TNF-α serum concentration in the TNF-Tg mice (P<0.05; Figure 7).
Figure 7.
Effect of ginsenoside Rg1 on serum levels of TNF-α and IL-6. (A, B) Compared with the WT group, the concentration of IL-6 and TNF-α in the TNF-Tg group was significantly increased (P<0.05). Rg1 significantly reduced IL-6 and TNF-α expression (P<0.05). (*P<0.05 vs. Rg1-treated samples. #P<0.05 vs. WT samples.)
Discussion
This is the first study to use the TNF-Tg mice to evaluate the effect of ginsenoside Rg1 on arthritis and lymphatic function in chronic IA. A previous study indicated that ginsenoside Rg1 reduces joint synovial hyperplasia, inflammatory infiltration, and destruction of articular cartilage in a CIA mice model[22]. In this study, we showed that ginsenoside Rg1 significantly improved the lymphatic drainage function, ankle and knee joint inflammation, and tissue damage in TNF-Tg mice without liver and nephrotoxicity. This and other studies suggest that ginsenoside Rg1 attenuates joint inflammation not only in a collagen-induced arthritis model, but also in chronic progressive IA caused by TNF-α overexpression. Furthermore, our study shows that ginsenoside Rg1 also promotes lymphatic drainage function and mature structure of TNF-Tg mice.
Lymphatic vessels are responsible for regulating tissue fluid balance, immune defense, metabolism, and cancer metastasis[23]. They are involved in pathological changes, such as inflammation, immunity, and tumor metastasis[24], and play an important role in joint inflammation[25]. Our previous studies showed that sufficient lymph drainage helps to improve joint inflammation, and drugs activating lymphatic function can attenuate synovial inflammation[26]. In the current study, ginsenoside Rg1 was shown to promote lymphatic drainage and reduce joint inflammation. Our previous study showed that in response to TNF, iNOS is produced by the lymphatic endothelial cells of lymphatic vessels efferent to inflamed joints of TNF-Tg mice, which inhibits lymphatic smooth muscle cell contraction and causes impaired lymphatic drainage[27]. It has been reported that ginsenoside Rg1 inhibits the production of iNOS in M1-polarized macrophages and microglia. It is possible that ginsenoside Rg1 promotes lymphatic drainage by inhibiting iNOS production in lymphatic endothelial cells. However, the mechanism needs further research. It is well known that lymphatic vessels drain the peripheral inflammatory factors to the lymph nodes and finally to the blood circulatory system. Another question that needs to be addressed is whether promoting lymphatic drainage increases serum inflammatory cytokine levels. We tested the serum concentration of two important inflammatory cytokines, IL-1β and TNF-α, and found that Rg1 significantly reduced the serum concentration of these inflammatory factors in the peripheral blood. Several other studies have also shown that ginsenoside Rg1 exhibits anti-inflammatory effects[28,29]. This and other studies indicate that ginsenoside Rg1 promotes lymphatic drainage without increasing the serum inflammatory cytokine concentration.
Several studies have shown that ginsenoside Rg1 has a protective effect on liver and kidney damage[30]. Our current study also did not find any adverse effect of ginsenoside Rg1 on liver and kidney tissues. Thus, our study (along with other previous studies) suggests that ginsenoside Rg1 may be a safe therapeutic drug for IA.
Conclusion
We demonstrate that ginsenoside Rg1 effectively promotes the recovery of lymphatic drainage function, and reduces joint swelling, deformity of synovial inflammation, and bone erosion in TNF-Tg mice without impairment of the liver and kidney. Ginsenoside Rg1 may thus be a potential drug candidate to promote lymph flow and improve IA.
Acknowledgements
This work was sponsored by research grants from the National Natural Science Foundation (81822050 and 81920108032 to LQQ, 81904227 to WXY), Leading Medical Talents in Shanghai (2019LJ02 to LQQ), Dawn Plan of Shanghai Municipal Education Commission (19SG39 to QL), Shanghai Famous Chinese Medicine Research Studio Project (SHGZS-2017006), Shanghai Traditional Chinese Medicine Specialized Medical Treatment Technology Improvement Project (Zyjx-2017059), Shanghai TCM Medical Center of Chronic Disease (2017ZZ01010 to WYJ), The program for innovative research team of ministry of science and technology of China (2015RA4002 to WYJ), “Innovation Team” development projects (IRT1270 to WYJ), Three Years Action to Accelerate the Development of Traditional Chinese Medicine Plan (ZY(2018-2020)-CCCX-3003 to WYJ).
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
Authors’ Contributions
TH, YL, and DJ conceived the study, TH ,YL and WX performed electronic literature searches, and HX and XW performed manual literature searches. TH, YL performed study selection and data extraction, QL and HX performed quality check. TH and YL undertook the analyses with YW, QS, and WL. TH, YL, and DJ wrote the initial draft. QL, WL, and TW critically revised the manuscript. All authors have read and approved the final manuscript.
References
- 1.Murphy CL, Awan S, Sullivan MO, et al. Major cost savings associated with biologic dose reduction in patients with inflammatory arthritis. Ir Med J. 2015;108:19. [PubMed] [Google Scholar]
- 2.Zangi HA, Ndosi M, Adams J, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis. 2015;74:954–962. doi: 10.1136/annrheumdis-2014-206807. [DOI] [PubMed] [Google Scholar]
- 3.Conigliaro P, Chimenti MS, Triggianese P, et al. Autoantibodies in inflammatory arthritis. Autoimmun Rev. 2016;15:S158156⅙. doi: 10.1016/j.autrev.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:D8951. doi: 10.1002/14651858.CD008951.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colebatch AN, Marks JL, Edwards CJ. Safety of non-steroidal anti-inflammatory drugs, including aspirin and paracetamol (acetaminophen) in people receiving methotrexate for inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis) Cochrane Database Syst Rev. 2011;1:D8872. doi: 10.1002/14651858.CD008872.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Leung E, Al Efraij K, FitzGerald JM. The safety of mepolizumab for the treatment of asthma. Expert Opin Drug Saf. 2017;16:397–404. doi: 10.1080/14740338.2017.1286327. [DOI] [PubMed] [Google Scholar]
- 7.Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis:current evidence and future directions. Ann Rheum Dis. 2016;75:1428. doi: 10.1136/annrheumdis-2016-209201. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Edwards J, Banerji S, et al. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis. 2004;62:1227–1229. doi: 10.1136/ard.2003.005876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olszewski WL, Pazdur J, Kubasiewicz E, et al. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Wauke K, Nagashima M, Ishiwata T, et al. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J Rheumatol. 2002;29:34–38. [PubMed] [Google Scholar]
- 11.Zhang Q, Lu Y, Proulx ST, et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9:R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebert JR, Joss B, Jardine B, et al. Randomized trial investigating the efficacy of manual lymphatic drainage to improve early outcome after total knee arthroplasty. Arch Phys Med Rehabil. 2013;94:2103–2111. doi: 10.1016/j.apmr.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Guo R, Wood R, et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum. 2011;63:2318–2328. doi: 10.1002/art.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol. 2017;39:365–383. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 15.Liao X, Cui H, Wang F. Establishment of a transgenic mouse model of corneal dystrophy overexpressing human BIGH3. Int J Mol Med. 2013;32:1110–1114. doi: 10.3892/ijmm.2013.1480. [DOI] [PubMed] [Google Scholar]
- 16.Rahimi H, Bell R, Bouta EM, et al. Lymphatic imaging to assess rheumatoid flare:mechanistic insights and biomarker potential. Arthritis Res Ther. 2016;18:194. doi: 10.1186/s13075-016-1092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Chen Y, Zhang L, et al. Total saponins of panaxnotoginseng promotes lymphangiogenesis by activation VEGF-C expression of lymphatic endothelial cells. J Ethnopharmacol. 2016;193:293–302. doi: 10.1016/j.jep.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radad K, Gille G, Moldzio R, et al. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Cheng B, Zhu X, et al. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol. 2011;187:942–950. doi: 10.4049/jimmunol.1002579. [DOI] [PubMed] [Google Scholar]
- 20.Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor:a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Schwarz EM. The TNF-alpha transgenic mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25:19. doi: 10.1007/s00281-003-0125-3. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Fan W, Yin G. The study of mechanisms of protective effect of Rg1 against arthritis by inhibiting osteoclast differentiation and maturation in CIA mice. Mediators Inflamm. 2014;2014:305071. doi: 10.1155/2014/305071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo KP, Angeli V. Bidirectional Crosstalk between Lymphatic Endothelial Cell and T Cell and Its Implications in Tumor Immunity. Front Immunol. 2017;8:83. doi: 10.3389/fimmu.2017.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesler CT, Liao S, Munn LL, et al. Lymphatic vessels in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2013;5:111–124. doi: 10.1002/wsbm.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouta EM, Li J, Ju Y, et al. The role of the lymphatic system in inflammatory-erosive arthritis. Semin Cell Dev Biol. 2015;38:90–97. doi: 10.1016/j.semcdb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo R, Zhou Q, Proulx ST, et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2010;60:2666–2676. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Q, Ju Y, Chen Y, et al. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis Res Ther. 2016;18:62. doi: 10.1186/s13075-016-0963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu XD, Guo T, Liu L, et al. MiR-23a targets RUNX2 and suppresses ginsenoside Rg1-induced angiogenesis in endothelial cells. Oncotarget. 2017;8(35):58072–58085. doi: 10.18632/oncotarget.19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao S, Zou Y, Wang B, et al. Ginsenoside Rg1 improves lipopolysaccharide-induced acute lung injury by inhibiting inflammatory responses and modulating infiltration of M2 macrophages. Int Immunopharmacol. 2015;28(1):429–34. doi: 10.1016/j.intimp.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Q, Yang M, Deng Y, et al. The Safety Evaluation of Salvianolic Acid B and Ginsenoside Rg1 Combination on Mice. Int J Mol Sci. 2015;16(12):29345–56. doi: 10.3390/ijms161226176. [DOI] [PMC free article] [PubMed] [Google Scholar]