Abstract
Objectives:
To evaluate impact of first therapy session, containing functional electrical stimulation (FES) and therapeutic exercises (TE) on erector spinae (ES) and rectus abdominis (RA) force generation in persons with spinal cord injury (SCI).
Methods:
Five men with SCI were divided in two groups - FES+TE received concurrent FES on ES and RA and TE, TE only TE. Participants performed exercises for improving sitting balance and posture. Muscles’ electrical activity was evaluated by electromyography; amplitude (AEMG) and median frequency (MF) were used for analysis.
Results:
AEMG of ES left (L) increased 292.9% (g=-0.92), right (R) 175% (g=-1.01), RA L 314.3% (g=-0,81, P<0.05), R 266.7% (g=-0.08) in FES+TE. AEMG of ES L increased 47.6% (g=-0.46), R 96.4% (g=-0.95); RA L 7.1% (g=-0.97), but R decreased 6.7% (g=0.12) in TE. MF of ES L increased 108.5% (g=-0.74), R 184% (g=-1.25); RA L 886.7% (g=3-05, P<0.05), R 817.6% (g=-2.55, P<0.05) in FES+TE. MF of ES L increased 95.2% (g=-1.02), R 161.4% (g=-1.64); RA L 3,2% (g=-0.06), R 30.8% (g=-0.46) in TE.
Conclusions:
In SCI persons, single session exercises and concurrent functional electrical stimulation may be more effective on muscles` force generation than only exercises. However, replication of the results is needed before clinical implementation.
Keywords: Force Generation, Functional Electrical Stimulation, Muscle Fatigue, Spinal Cord Injury, Therapeutic Exercises
Introduction
Traumatic spinal cord injury (SCI) is caused by different types of trauma and results in damage to sensory, motor and/or autonomic function. Injury may cause long-term disability, that can have an impact on persons’ physical, social and psychological well-being and therefore on quality of life[1,2]. The quality of life also depends on the ability to sit unsupported, which is very important in SCI persons’ everyday life, as sitting balance is needed to perform functional activities[3]. Persons with SCI often sit on the front edge of the wheelchair or on bed for different activities, therefore good postural stability is critical[4]. After SCI, compensatory strategies (e.g. posterior pelvic tilt) are used to maintain sitting balance[5,6]. In SCI persons with high thoracic injury, the latissimus dorsi, the ascending part of trapezius, the cranial section of the pars sternocostalis of the pectoralis major, the serratus anterior and the cranial parts of the thoracic erector spinae (ES) play an important role in unsupported sitting during activities of daily living (ADL)[5,7]. Voluntary activation of muscles that remain below the level of injury is impaired and therefore muscles’ force generation is reduced[8,9]. Rapid and significant loss of muscle mass occurs followed by a transformation of muscle fibers to fast fatigable type (Type II) and subsequently a change in muscle contractile properties[10]. Type II motor units (MU) have axons with relatively large diameter and muscle fibers with large cross-sectional area, representing fast and forceful contractions, but they fatigue quickly[11]. In paralyzed muscles proportionally more muscle fibers are of Type II than Type I, therefore muscles are more susceptible to fatigue and their voluntary force output decreases[12].
Strength training is commonly used to improve muscles’ force generation, but in case of SCI, functional electrical stimulation (FES) can also be applied to activate muscle recruitment[13] and thus increase muscles’ resistance to fatigue[14]. FES is used to elicit muscle contractions in upper motor neuron lesions[5,13] and it has been potentially useful also in rehabilitation of persons with SCI[9]. Thus, stimulation of paralyzed muscles produces functional contractions and therefore improving SCI persons’ functions[9,15] such as trunk stability[5]. Evidence shows conflicting results in combing FES and progressive resistance training because of methodological and treatment differences, which is confirmed by comparison of four randomized controlled trials, where only one study demonstrates promising results[16]. Unfortunately, there is lack of high-quality studies to state the useful therapeutic effects of electrical stimulation[16]. Several published studies show good results in combing lower limb FES with functional activities like cycling[17,18], but there is limited information about the effect of concurrent FES and therapeutic exercises (TE) on trunk muscles’ force generation and fatigue during the one therapy session.
Due to long pre-study inactivity of SCI patients, related with local rehabilitation system, was the aim of the current study to evaluate the immediate impact of the first therapy session on paralyzed muscles activation. Throughout the study project consisted of twelve therapy sessions, but current paper compares the muscles force generation and fatigue characteristics before and after single physiotherapy session.
The aims of the study were to evaluate the force generation and fatigue of ES and rectus abdominis (RA) during one therapy session containing FES+TE and TE, and to compare the efficacy of both treatments based on the selected characteristics in persons with incomplete cervical SCI.
Methods
Study design and participants
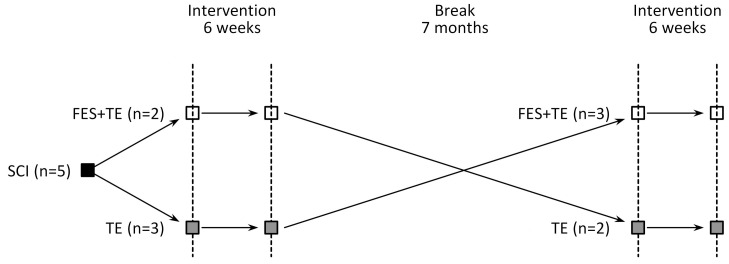
Current feasibility study was a preliminary research to estimate the selected assessment and treatment methods for full-scale study. Crossover study design was used for the research. The representation of the study design is presented in Figure 1.
Figure 1.
Schematic representation of crossover study design.
Eighteen SCI patient’s data were obtained from University hospital. The suitability for the study was initially specified with a telephone-interview, followed by the consultation of the neurosurgeon who confirmed that the participant can be included in the study. The inclusion and exclusion criteria of the study are presented in Table 1. Thirteen participants were excluded from the study due to inadequacy of inclusion criteria. A total of five participants completed the study.
Table 1.
Inclusion and exclusion criteria of the study.
| Criteria | Justification of the criteria |
|---|---|
| Inclusion criteria | |
| Age 18-55 | Age 18 defines the grown-up; therefore, participants are responsible for themselves. For participants over 55 years, age-related sarcopenia could have effect on results. |
| Chronic SCI (≥12 months’ post injury)18 | During acute or sub-acute phases spontaneous recovery could happen which is minimized in chronic stage. |
| Traumatic origin of the injury, located in the cervical region resulting in tetraplegia | Traumatic spinal cord injuries are more frequent and larger and more homogenous sample size could be achieved. |
| Scoring by American Spinal Injury Association Impairment Scale (AIS) at B or C | AIS B indicates that sensory but not motor functions are preserved below the neurological level, including the sacral segments S4-S5. Score C indicates that motor functions are preserved below the neurological level but more than half of key muscles below the neurological level have a muscle grades 0-2. Both of the Scores are classified as incomplete lesions. |
| Able to sit unsupported on a custom-made chair | Measurements are done unsupported. Multidirectional limits of stability are measured and also muscle activity in various directions. |
| Exclusion criteria | |
| Complete spinal cord injury (AIS A) | AIS A indicates that no motor or sensory function is preserved in the sacral segments S4-S5 and it is classified as complete lesion, therefore no voluntary muscle contraction is possible. |
| Cardiopulmonary insufficiency | Contraindication for electrical stimulation |
| Cardiac pacemaker | Contraindication for electrical stimulation |
| Oncological disease | Contraindication for electrical stimulation |
| Epilepsy | Contraindication for electrical stimulation |
| Open wounds or metal implants in the area, where FES was planned to be performed | Contraindication for electrical stimulation |
Participants were alternately assigned to two groups - group one performed simultaneous FES and TE (FES+TE) of ES and RA, group two performed only TE (TE). First participant enrolled FES+TE group, subsequent participant to TE group etc. Crossover study design was used - participants who were initially assigned to FES+TE group were seven months later assigned to TE group and vice versa.
Despite that one SCI person with AIS C was enrolled to study, the respective data was not compared with AIS B persons` data, whole group mean was used in data analysis. Considering the close area of denervation and the innervation of multiple muscles by one nerve root, the authors of current study assume that AIS C person`s data have minimal effect on results. Despite that whole therapy program lasted for 6 weeks consisted from 12 therapy session, in current research paper, data from only the first therapy session was used for analysis.
Participants’ demographic data, duration and classification of SCI are presented in Table 2.
Table 2.
Participants’ anthropometric data, duration and classification of spinal cord injury.
| Subject | Age (years) | Weight (kg) | Height (cm) | BMI (kg·m-2) | Time post injury (years) | AIS score | Level of the injury |
|---|---|---|---|---|---|---|---|
| 1 | 44 | 119.3 | 181 | 36.42 | 12 | B | C6 |
| 2 | 44 | 77.2 | 188,5 | 21.61 | 6 | B | C6 |
| 3 | 41 | 64.3 | 177,5 | 20.29 | 19 | B | C5 |
| 4 | 27 | 62 | 195 | 16.31 | 4 | B | C6 |
| 5 | 39 | 61.5 | 173 | 20.55 | 13 | C | C5 |
| mean±SD | 39.0±7.0 | 76.9±24.6 | 183.0±8.8 | 23.0±7.7 | 10.8±6.0 | N/A | N/A |
| 95% CI | 30.3, 47.7 | 46.4, 107.4 | 172.1, 193.9 | 13.4, 32.7 | 3.4, 18.2 |
Abbreviations: SCI - spinal cord injury, BMI - body mass index, AIS - American Spinal Injury Association Impairment Scale, SD - standard deviation, N/A - not applicable.
Statement of ethics
We confirm that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The current study was approved by Research Ethics Committee of the University of Tartu (permit number: 263/T-4, 17.10.2016), all patients signed a written informed consent for participation in the study. The study protocol is registered in the United States National Library of Medicine, (ClinicalTrials.gov) ID NCT03517787, protocol ID Spinaal.
Intervention
Therapy consisted of eight different exercises for improving sitting balance and upper body posture (Supplementary data). Each exercise had 12 repetitions and included three sets. Participants’ training was conducted in their home, in their own wheelchair with the guidance of physical therapist.
Pre-set program from four-channel FES device (I-Tech Medical Division, Martellago, Italy) was selected to deliver transcutaneous electrical stimulation using self-adhesive gel electrodes (5x5 cm) (I-Tech Medical Division, Martellago, Italy). Electrodes were placed on thoraco-lumbar area of the ES and RA muscles bilaterally. Proximal electrodes on ES were placed at the T8 level and distal electrodes at the L4 level. Proximal electrodes on RA were placed at the level of 10th costa and distal electrode at the level crista iliaca. ES and RA were chosen for stimulation as these muscles have been shown to contribute significantly to trunk stability[20]. The purpose of selecting pre-set FES program was to allow the participants to continue training with the same parameters. Program was selected mainly according to the frequency parameters. Lower frequency parameters postpone fatigue and discomfort[21] and they are also in compliance with previous studies[22]. FES program consisted of three phases: warm-up, work and recovery. Characteristics of the program were obtained from users’ manual[23]. Frequency was set to 3 Hz for warm-up and recovery phases. During work phase, frequency alternated according to the following scheme: 8 Hz for 18 sec, 2 Hz for 2 sec and 18 Hz for 10 sec, etc. Alternation of the frequency repeated continuously without a pause. Work phase lasted for 30 minutes and both warm-up and recovery lasted for 5 minutes. Pulse duration of the program was 275 μs. Intensity of current was individually increased and was set as high as strong visible evoked muscle contraction was obtained, but no unpleasant sensation was reported by the participants if some sensory function had remained. Muscles were activated simultaneously to generate co-activation and therefore to stiffen the trunk. To maintain correct alignment of the upper body during stimulation, the intensity of the stimulation on one muscle was bilaterally equal.
Outcome measures
Telemetric 16-channels EMG device (Mega Electronics, Ltd, Kuopio, Finland) was used to measure electrical activity of ES and RA. Mega Electronics software was also used for analysis of data (Mega Electronics, Ltd, Kuopio, Finland). Skin was cleaned with alcohol and shaved if needed. After skin preparation, bipolar 8-shaped electrodes (Noraxon, Scottsdale, Arizona, USA), spaced 2 cm apart and oriented parallel to the long axis of the muscles, were placed above muscles bilaterally. Electrode for ES was placed at the L2 level and for RA two cm below the umbilicus bilaterally. Measurements were conducted prior to the therapy session and immediately after to obtain acute changes of the measured characteristics. Measurements were done on a custom-made chair, which enabled participants to sit with 90ο angle in their hip and knee joints. Participants were asked to keep hands on their thighs. EMG data were initially registered in the resting state, followed by a maximum voluntary isometric contraction (MVC) of ES and RA muscles for three seconds. Nicholas MMT hand-held dynamometer model 01160 (Lafayette Instrument, US) was used to register the values of MVC characteristics. For the measurement of MVC of ES, dynamometer was placed between participants’ scapulae and participant was instructed to push backwards against the dynamometer. For the measurement of MVC of RA, dynamometer was placed on the upper part of sternum and participant was asked to flex the trunk and tighten abdominal muscles. Three repetitions of MVC were performed, with one-minute resting time between the attempts. The data with the highest MVC value and therefore also with the greatest muscle activity were used for analysis. EMG sampling frequency was set to 1000 Hz and Raw Free measuring component was used for analysis. Measuring range was -3000 to 3000 μV. The duration of data used for analysis was two seconds. Average Spectrum algorithm was used for the analysis. EMG amplitude (AEMG, μV) was collected to measure electric potential differences within each muscle as a result of motor unit action potentials produced by the central nervous system and to imply muscles’ force generation. EMG median frequency (MF, Hz) was collected to characterize muscle fatigue. Higher post-intervention amplitude parameter reflects greater muscles’ force generation and lower MF shows greater fatigue level[24]. All testing procedures were conducted in participants’ homes.
Statistical analysis
Data analysis was performed using SPSS software (version 20, IBM Corporation, New York, NY, USA). Descriptive statistics were used to explain the demographics and SCI characteristics. Shapiro-Wilk test was used to analyze normality of data distribution. As the sample size was very small and data were not normally distributed, Kruskal-Wallis H was used to compare data between groups. Dunn’s post-hoc tests with Bonferroni correction were carried out for pairwise correction after a significant Kruskal-Wallis H test. Wilcoxon signed-rank test was used for within-group comparison. Probability value (p-value) less than 0.05 was statistically significant. Hegdes’ g was calculated to describe the effect size. A value of 0.2 of g was considered small, 0.5 medium and over 0.8 as a large and substantial difference[25].
Results
Pre- and post-intervention data for AEMG and MF for both study groups are presented in Table 3.
Table 3.
Mean (standard deviation) pre- and post-intervention results for amplitude and median frequency and effect size of the intervention.
| FES+TE (n=5) | TE (n=5) | |||||
|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | Effect size | Pre-intervention | Post-intervention | Effect Size | |
| Amplitude (µV) | ||||||
| ES L | 2.80 (2.49) | 11.0 (11.11) | -0.92 | 8.20 (8.64) | 12.20 (6.83) | -0.46 |
| ES R | 3.20 (3.19) | 8.80 (6.30) | -1.01 | 5.60 (4.45) | 11.00 (5.70) | -0.95 |
| RA L | 1.40 (0.89) | 5.80 (6.87) | -0.81* | 5.60 (3.65) | 6.00 (5.10) | -0.97 |
| RA R | 1.80 (1.79) | 6.60 (6.03) | -0.08 | 6.00 (4.30) | 5.40 (4.98) | 0.12 |
| Median Frequency (Hz) | ||||||
| ES L | 16.40 (21.16) | 34.20 (22.13) | -0.74 | 21.00 (19.61) | 41.00 (15.56) | -1.02 |
| ES R | 16.20 (21.24) | 46.00 (21.85) | -1.25 | 17.60 (17.60) | 46.00 (13.34) | -1.64 |
| RA L | 3.00 (4.47) | 29.60 (10.21) | -3.05* | 30.80 (19.15) | 31.80 (11.84) | -0.06 |
| RA R | 3.40 (5.37) | 31.20 (12.85) | -2.55* | 24.00 (16.46) | 31.40 (12.18) | -0.46 |
Abbreviations: SCI - spinal cord injury. FES - functional electrical stimulation, TE - therapeutic exercise, ES - m erector spinae, RA - m rectus abdominis, L - left, R - right,
- P<0.05.
Amplitude
Effect size values for AEMG change of left and right ES demonstrated large difference from baseline measures for FES+TE and small to large difference for TE. Comparison between FES+TE and TE indicated that AEMG of both left and right ES did not differ significantly pre- (P>0.05) and post-intervention (P>0.05).
Effect size values for AEMG change of left and right RA demonstrated that the therapy had substantial effect on FES+TE characteristics. Therapy had almost no effect on TE. Comparison between groups revealed that pre-intervention AEMG of left RA was significantly lower for FES+TE (P<0.05, g=-1.43, 95% CI -2.82, -0.04). Post-intervention AEMG characteristics did not differ significantly between groups.
Median frequency
In current study, median frequency characteristics increased as a result of single session therapy, indicating that muscle activation increased, and activated muscles’ fatigue did not occur.
Effect size values for MF of left and right ES demonstrated that therapy had medium to considerable effect on both groups. Comparison between groups did not show any statistically significant differences for pre- and post-intervention.
Effect size values for median frequency of left and right RA demonstrate that therapy had considerable effect on FES+TE and almost no effect on TE. Comparison between groups indicated that pre-intervention MF was significantly lower for FES+TE as compared to TE for both left (P<0.05, g= -1.80, 95% CI -3.28, -0.33) and right RA (P<0.05, g=-1.52, 95% CI -2.93, -0.11). Post-intervention characteristics did not differ between groups (P>0.05).
Discussion
The most important finding of the present feasibility study was that combined functional electrical stimulation and therapeutic exercises improved trunk muscles’ force generation and postponed their fatigue more than therapeutic exercises alone in incomplete spinal cord injured persons.
Due to limited published information about paralyzed muscles’ force generation and fatigue during concurrent FES and TE for trunk muscles, the aim of the study was to assess the electrical activity of ES and RA during one therapy session in cervical SCI persons.
It is known that FES is a method applied in physiotherapy to induce contraction of paralyzed muscles[13], but at the same time the main FES training limitation is the inability to maintain sufficient contraction force over the training period, whereby electrical stimulation with multiple repetitions will result in significant force decline[26]. The authors of current study combined FES+TE to assess whether two methods conducted simultaneously have better outcome than TE alone on improving trunk muscles’ force generation and fatigue.
In both SCI groups, pre-intervention AEMG characteristics of ES and RA were minimal, but they were slightly higher for TE group. Post-intervention characteristics increased more noticeably in FES+TE group. The increase of post-intervention AEMG characteristics is explained by the knowledge that very few MUs need to be recruited for EMG to register any activity[27]. Before the therapy, some of the SCI persons were not able to recruit ES and RA, but after FES and TE, activation in muscle recruitment was identified. Changes were more evident in the FES+TE group and more for the RA, as named muscle is innervated by lower thoracic spinal nerves. ES is innervated by dorsal rami of lumbar spinal nerves, which originates from the lower part of spinal cord[28]; therefore, innervation of ES could be more impaired. Current study revealed that FES+TE group activated their RA muscle significantly more after single session therapy compared with pre-intervention characteristics, which is explained by combined muscle activation therapies and partial innervation of the muscle. According to the study results confirming, that TE group post-intervention RA muscles’ force generation was not as extensive as in FES+TE group, then it can be assumed that FES generates much powerful muscle contractions and recruit more fibers in paralyzed muscle than voluntary contraction during therapeutic exercise.
AEMG can provide an indirect measure of the magnitude of muscle force[29] and therefore it is an appropriate to describe recovery of voluntary contraction and muscle activation[30]. Comparison of the effect size results confirms that combined FES+TE has more influence on muscles’ force generation than TE only. AEMG of ES and RA was higher in FES+TE as compared to TE. Although the post-intervention results of AEMG were significantly higher for only RA muscle, the effect size characteristics showed a substantial effect of FES+TE therapy, which confirms that FES has more influence on force generation than only TE in SCI persons.
It is commonly accepted that greater fatigue as a result of electrical stimulation is explained by the Hennemann size principle, where MU recruitment is reverse of the physiological recruitment order[26] and constant muscle fibers recruitment order[31]. In contrast, according to Gregory & Bickel[32] the muscle recruitment pattern during electrical stimulation could be random, causing more fatigue than during voluntary exercise. The authors stated that the greater the force decline with electrical stimulation, the greater is the fatigue. In the current study, FES was used in combination with TE and therefore greater muscle fatigue was expected. Similarly, to the AEMG, MF was also minimal during the initial assessment, indicating that almost no electrical activity in ES and RA was noticed in both SCI groups and therefore, any decline in MF characteristics was impossible to register. The obtained results were opposite - MF increased in both SCI groups, showing that both FES and TE are effective in activating muscles. The results were also more significant for FES+TE and for RA indicating that FES+TE is more effective than only TE. Increase in MF also confirms that more MUs are activated and therefore voluntary muscle contraction improves[33,34]. As the voluntary muscle activation progresses, larger and faster MUs are gradually recruited, and the change can be seen as an increase in spectral parameters like MF[32]. The recruitment of new MUs results in greater force generation which is in accordance with the results of this study. The increase of MF was also more noticeable in FES+TE group, showing that FES is more effective in recruiting muscle fibers. This result is in accordance with previous published work stating that regular physical activity consisting of TE and preferably FES to paralyzed body parts should be conducted to maintain the current state and to promote functional plasticity[13]. Functional exercise has shown good results in improving SCI persons’ unsupported sitting[4] but adding FES to the therapy could bring even better results in long term as more efficient muscle activation occurs. Unfortunately, there is little evidence to support these findings. According to recent systematic review, there are very few high-level studies, confirming that physical therapy increases muscle strength in paralyzed muscles[35]. Another systematic review that compared different SCI persons physiotherapy methods, includes only one study that met their criteria[36]. It has been found that resistance training versus no intervention, also resistance training combined with FES versus no intervention has statistically significant differences. Again, it must be noted that there is limited or absent information about FES and strength training, generating trunk muscles force in SCI persons.
The effect of single session FES is relevant for the therapist to analyze the course of therapy and to understand whether the selected training characteristics are appropriate or not. Immediate effect of FES and TE gives knowledge whether the previously inactive muscles could be activated with one therapy session or repetitive therapy is needed for significant activation of muscles.
The clinical importance of the study is the knowledge that appropriately selected FES combined with TE could be an effective method to use in SCI persons’ rehabilitation to improve participants’ muscle recruitment. This feasibility study showed that selected research methodology was appropriate, but before clinical implementation, the study should be replicated with larger sample size.
Limitations of the study
Limitation of the study is relatively small sample size, which may not be representative of all SCI participants of this age. However, in accordance with this current study, there are number of studies conducted with similar sample sizes investigating persons with SCI and the application of FES[5,37,38]. Several therapies need to be conducted to conclude whether concurrent FES and TE has an advantage over TE for muscles’ force generation during longer period. Also, an absence of a non-training group is considered as a limitation of the study.
Conclusion
Based on the results of the current study, it can be concluded that single session functional electrical stimulation in combination with therapeutic exercises could be more effective in improving trunk muscles’ force generation and postponing their fatigue as compared with exercises therapy alone in chronic, incomplete spinal cord injured persons.
Acknowledgement
Author contribution: Conceptualization, M.B.; D.V.; methodology, M.B.; D.V.; H.G.; J.E.; formal analysis, M.B.; J.E.; investigation, M.B.; A.Z.; T.A..; resources, D.V.; H.G.; J.E.; T.A.; data curation, M.B.; J.E.; writing - original draft preparation, M.B.; writing - review and editing, A.Z.; J.E.; H.G.; T.A.; D.V.; supervision, D.V.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Sabre L, Pedai G, Rekand T, Asser T, Linnamägi U, Kõrv J. High Incidence of traumatic spinal cord injury in Estonia. Spinal Cord. 2012;50:755–759. doi: 10.1038/sc.2012.54. [DOI] [PubMed] [Google Scholar]
- 2.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CL, Yeung KT, Bih LI, Wang CH, Chen MI, Chien JC. The relationship between sitting stability and functional performance in patients with paraplegia. Arch Phys Med Rehabil. 2003;84(9):1276–1281. doi: 10.1016/s0003-9993(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 4.Boswell-Ruys CL, Harvey LA, Barker JJ, Ben M, Middleton JW, Lord SR. Training unsupported sitting in people with chronic spinal cord injuries:a randomized controlled trial. Spinal Cord. 2010;48(2):138–43. doi: 10.1038/sc.2009.88. [DOI] [PubMed] [Google Scholar]
- 5.Kukke SN, Triolo RJ. The effects of trunk stimulation on bimanual seated workspace. IEEE Trans Neural Syst Rehabil Eng. 2004;12:177–185. doi: 10.1109/TNSRE.2004.827222. [DOI] [PubMed] [Google Scholar]
- 6.Milosevic M, Masani K, Kuipers MJ, Rahouni H, Verrier MC, McConville KM, et al. Trunk control impairment is responsible for postural instability during quiet sitting in individuals with cervical spinal cord injury. Clin Biomech (Bristol. Avon) 2015;30(5):507–512. doi: 10.1016/j.clinbiomech.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Seelen HAM, Potten YJM, Drukker J, Reulen JPH, Pons C. Development of new muscle synergies in postural control in spinal cord injured subjects. J Electromyogr Kines. 1998;8:23–34. doi: 10.1016/s1050-6411(97)00002-3. [DOI] [PubMed] [Google Scholar]
- 8.Bochkezanian V, Newton RU, Traiano GS, Vieira A, Pulverenti TS, Blazevich AJ. Effect of tendon vibration during wide-pulse neuromuscular electrical stimulation (NMES) on muscle force production in people with spinal cord injury (SCI) BMC Neurol. 2018;18:17. doi: 10.1186/s12883-018-1020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CW, Chen SC, Chen CH, Chen TW, Chen JJ, Lin CS, et al. Effects of functional electrical stimulation on peak torque and body composition in patients with incomplete spinal cord injury. Kaohsiung J Med Sci. 2007;23(5):232–40. doi: 10.1016/S1607-551X(09)70403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelletier CA, Hicks AL. Muscle characteristics and fatigue properties after spinal cord injury. Crit Rev Miomed Eng. 2009;37:139–164. doi: 10.1615/critrevbiomedeng.v37.i1-2.40. [DOI] [PubMed] [Google Scholar]
- 11.Grater Jr DR, Dolbow D, Tsui B, Gorgey AS. Functional electrical stimulation therapies after spinal cord injury. Neurorehabil. 2011;28:231–248. doi: 10.3233/NRE-2011-0652. [DOI] [PubMed] [Google Scholar]
- 12.Peckham PH, Mortimer JT, Marsolais EB. Alterations in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin Orthop. 1976;114:326–334. [PubMed] [Google Scholar]
- 13.Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, et al. Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:631–654. doi: 10.1016/j.pmr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragnarsson KT. Functional electrical stimulation after spinal cord injury:current use, therapeutic effects and future directions. Spinal Cord. 2008;46:255–274. doi: 10.1038/sj.sc.3102091. [DOI] [PubMed] [Google Scholar]
- 15.Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36(7):463–469. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- 16.Harvey LA. Physiotherapy rehabilitation for people with spinal cord injuries. J Physiother. 2016;62:4–11. doi: 10.1016/j.jphys.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Harvey LA, Fornusek C, Bowden JL, Pontifex N, Glinsky J, Middleton JW, et al. Electrical stimulation plus progressive resistance training for leg strength in spinal cord injury:A randomized controlled trial. Spinal Cord. 2010;48:570–575. doi: 10.1038/sc.2009.191. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoleni S, Stampacchia G, Gerini A, Tombini T, Carrozza MC. FES-cycling training in spinal cord injured patients. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5339–5341. doi: 10.1109/EMBC.2013.6610755. [DOI] [PubMed] [Google Scholar]
- 19.Gao KL, Chan KM, Purves S, Tsang WWN. Reliability of dynamic sitting balance tests and their correlations with functional mobility for wheelchair users with chronic spinal cord injury. J Orthop Translat. 2014;3:44–49. doi: 10.1016/j.jot.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milosevic M, McConville V, Seidic E, Masani K, Kvan MJ, Popovic MR. Visualization of trunk muscle synergies during sitting perturbations using self-organizing maps (SOM) IEEE Trans Biomed Eng. 2012;59:2516–2523. doi: 10.1109/TBME.2012.2205577. [DOI] [PubMed] [Google Scholar]
- 21.Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. 2012;85:201–215. [PMC free article] [PubMed] [Google Scholar]
- 22.Ibitoye MO, Hamzaid NA, Hasnan N, Abdul Wahab AKA, Davis GM. Strategies for rapid muscle fatigue reduction during FES exercise in individuals with spinal cord injury:a systematic review. Plos One. 2016;11:e0149024. doi: 10.1371/journal.pone.0149024. doi:10.1371/journal.pone.0149024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.I-Tech Medical Division. Electrotherapy model T-One Rehab, MNPG236 Rev.0 of 21/06/16. 2016 USER MANUAL. [Google Scholar]
- 24.Boccia G, Dardanello D, Rosso V, Pizzigalli L, Rainoldi A. The application of sEMG in aging:a mini review. Gerontology. 2015;61:477–484. doi: 10.1159/000368655. [DOI] [PubMed] [Google Scholar]
- 25.McGough JJ, Faraone SV. Estimating the size of treatment effects:Moving beyond P values. Psychiatry (Edgmont) 2009;6:21–29. [PMC free article] [PubMed] [Google Scholar]
- 26.Dolbow DR, Holcomg WR, Gorgey AS. Improving the efficiency of electrical stimulation activities after spinal cord injury. Curr Phys Med Rehabil Rep. 2014;2:169–175. doi: 10.1007/s40141-014-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calancie B, Molano MR, Broton JG. EMG for assessing the recovery of voluntary movement after acute spinal cord injury in man. Clin Neurophysiol. 2004;115:1748–1759. doi: 10.1016/j.clinph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Hochschild J. Functional anatomy for physical therapists. Thieme. 2016 [Google Scholar]
- 29.Roberts TJ, Gabaldon AM. Interpreting muscle function from EMG:lessons learned from direct measurements of muscle force. Integ Comp Biol. 2008;48:312–320. doi: 10.1093/icb/icn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DGL, Harkema SJ. Neurophysiological characterization of motor recovery in acute spinal cord injury. Spinal Cord. 2011;49:421–429. doi: 10.1038/sc.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashibe M, Zhang Q, Guiraud D, Fattal C. Evoked EMG-based torque prediction under muscle fatigue in implanted neural stimulation. J Neural Eng. 2011;8:064001. doi: 10.1088/1741-2560/8/6/064001. doi:10.1088/1741-2560/8/6/064001. [DOI] [PubMed] [Google Scholar]
- 32.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–364. [PubMed] [Google Scholar]
- 33.Bernardi M, Felici F, Marchetti M, Montellanico F, Piacentini MF, Solomonow M. Force generation performance and motor unit recruitment strategy in muscles of contralateral limbs. J Electromyog Kines. 1999;9:121–130. doi: 10.1016/s1050-6411(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 34.Roman-Liu D. The influence of confounding factors on the relationship between muscle contraction level and MF and MPF values on EMG signal:a review. Int J Occup Saf Ergo. 2016;22:77–91. doi: 10.1080/10803548.2015.1116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bochkezanian V, Newton RU, Trajano GS, Blazevich AJ. Effects of Neuromuscular Electrical Stimulation in People with Spinal Cord Injury. Med Sci Sports Exerc. 2018;50:1733–1739. doi: 10.1249/MSS.0000000000001637. [DOI] [PubMed] [Google Scholar]
- 36.Harvey LA, Glinsky JV, Bowden JL. The effectiveness of 22 commonly administered physiotherapy interventions for people with spinal cord injury:a systematic review. Spinal Cord; 54:914–923. doi: 10.1038/sc.2016.95. [DOI] [PubMed] [Google Scholar]
- 37.Audu ML, Lombardo LM, Schnellenberger JR, Foglyano KM, Miller MR, Triolo RJ. A neuroprosthesis for control of seated balance after spinal cord injury. J NeuroEng Rehabil. 2015;12:8. doi: 10.1186/1743-0003-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krause P, Szecsi J, Straube A. Changes in spastic muscle tone increase in patients with spinal cord injury using functional electrical stimulation and passive leg movements. Clin Rehabil. 2008;22:627–634. doi: 10.1177/0269215507084648. [DOI] [PubMed] [Google Scholar]