Abstract
Metabolic alterations serve a significant role in the pathogenesis of kidney disease. Long non-coding RNA (lncRNA) taurine upregulated gene 1 (TUG1) is a known regulator of podocyte health and mitochondrial biogenesis. Although TUG1 protects against podocyte loss in models of diabetic nephropathy, it is unknown if urinary TUG1 expression is associated with clinical and histopathological findings in non-diabetic patients diagnosed with glomerulonephritides. In the present study, the expression of TUG1, podocyte-specific markers (nephrin and podocin) and mitochondrial biogenesis-associated mRNAs (transcription factor A mitochondrial, cytochrome C oxidase subunit 5A and peroxisome proliferator-activated receptor γ coactivator 1α) were examined in urinary sediment of non-diabetic patients with biopsy-confirmed glomerulonephritides and healthy controls. Urinary expression of TUG1 was significantly lower in patients with glomerulonephritides, particularly those diagnosed with Focal Segmental Glomerulosclerosis (FSGS). Furthermore, TUG1 levels were associated with urinary expression of podocyte-specific markers and mRNAs associated with mitochondrial biogenesis. Loss of TUG1 expression in urinary sediment was strongly associated with FSGS, highlighting the potential of this lncRNA and its mitochondrial biogenesis-associated targets as non-invasive biomarkers of assessing podocytopathy.
Keywords: TUG1, lncRNA, glomerulonephritides, FSGS, urinary sediment
Introduction
Glomerulonephritides are a group of rare diseases that often affects younger individuals and can lead to end-stage renal disease (ESRD) (1). Although the clinical presentation of glomerulonephritides and their outcomes are variable, well-established factors such as persistent proteinuria, hypertension, diabetes and cardiovascular disease increase the risk of ESRD progression (2). Podocyte injury is a hallmark of renal diseases, presenting clinically with proteinuria, glomerulosclerosis and kidney failure (3). Terminally differentiated podocytes have a limited capacity to self-replicate; hence, direct cellular damage contributes to the onset and progression of glomerular diseases such as Focal Segmental Glomerulosclerosis (FSGS), Minimal Change Disease and Diabetic Nephropathy (DN) (4). Additionally, immune-mediated damage is responsible for the establishment and progression of secondary glomerulonephritides, including lupus nephritis (LN) and ANCA-associated vasculitis (AAV) (5).
The gold standard for confirming a diagnosis of glomerulonephritides diagnosis is renal biopsy (6). However, biopsies are invasive, may have complications and are hard to interpret due to the presence of highly complex pathogenic mechanisms that translate into limited histological responses of kidney injury (7). Thus, identifying non-invasive biomarkers of glomerular disease may result in improved diagnosis and guide therapeutic choices in the field of nephrology (8).
Long non-coding RNAs (lncRNAs) are RNA molecules comprised of >200 nucleotides with no protein-coding capacity. They are a class of RNAs comprised of heterogeneous intergenic transcripts, sense or antisense transcripts that overlap with other genes, or enhancer RNAs with a range of functions that includes the regulation of gene expression, chromatin remodelling, microRNA (miRNA/miR)-sponging and protein scaffolding (9,10). LncRNAs modulate several biological processes, including homeostasis, cellular metabolism, proliferation, apoptosis and differentiation (10,11). Dysregulated expression of lncRNAs such as metastasis-associated lung adenocarcinoma transcript 1, LOC105374325, LOC105375913, X-inactive specific transcript and RP11-2B6.2 contribute to the pathogenesis of various kidney diseases (12-17). Moreover, circulating lncRNAs are stable and easily detectable in plasma, serum and urine, characteristics that make them convenient for use diagnostically (18,19).
The lncRNA taurine upregulated gene 1 (TUG1), located on chromosome 22q12, regulates podocyte health and glomerulosclerosis by altering the expression of peroxisome proliferator-activated receptor γ Coactivator 1α (PGC1A) (20-23). PGC1A is a transcriptional coactivator that controls mitochondrial biogenesis and is encoded by the PPARGC1A gene in humans (24,25). A decrease in PGC1A expression contributes to the onset of metabolic diseases such as DN, and transgenic expression of PPARGC1A in tubular cells protects mice from developing acute and chronic forms of kidney disease (26,27). Long et al (28) found that podocyte-specific transgenic expression of lncRNA TUG1 protects against DN in a mouse model, and demonstrated that lower glomerular filtration rates (GFR) were correlated with a decrease in TUG1 intrarenal expression in human subjects. However, it is unknown if TUG1 can be detected in urine and if its expression levels are correlated with histopathological findings in kidney biopsies from patients diagnosed with glomerulonephritides other than DN. In the present study, the urinary expression of TUG1 in non-diabetic patients with glomerulonephritides was characterized and it was shown that a decrease in TUG1 expression was significantly associated with FSGS.
Materials and methods
Ethical considerations
The present study complied with the ethical principles for medical research specified in the Declaration of Helsinki (29) and was approved by the Local Ethics and Research Committee at the Hospital de Especialidades NUM. 1, Bajio, Leon, Guanajuato, Instituto Mexicano del Seguro Social (approval no. CLIEIS R-2018-1001-114). Each participant provided written informed consent prior to enrolment in the study.
Patient enrolment
A total of 11 patients with biopsy-confirmed glomerulonephritides (7 females and 4 males; median age, 31 years; range 19-59 years), and 10 healthy controls (6 females and 4 males; median age 34 years; range 22-54) were enrolled in the present study at UMAE-Hospital de Especialidades, CMNO in Guadalajara, Mexico. Inclusion criteria were non-diabetic patients aged ≥18 years. Exclusion criteria were intake of non-steroid anti-inflammatory drugs or platelet anti-aggregation medication a week before sampling, administration of anticoagulant medication 96 h before the biopsy, blood pressure >160/90 mmHg, active urinary infection or anaemia. Urine sample collection was taken shortly before biopsy collection. Only 1 patient enrolled in the study was unable to provide a urine sample and, therefore, was excluded from further analysis.
Data collection
Biochemical parameters corresponding to each glomerulonephritides patient at the time of biopsy were assayed by routine laboratory techniques at UMAE-Hospital de Especialidades and retrieved from patients' electronic medical records.
Reverse transcription-quantitative (RT-q)PCR
Urine specimens from patients and healthy controls were collected and centrifuged at 4,500 x g for 30 min at 4˚C. The urinary sediments were recovered by discarding the supernatant, and total RNA was extracted using RiboZol reagent (Amresco LLC) according to the manufacturer's protocol. cDNA was synthesized by reverse transcription using 1 µg total RNA and a QuantiTect Reverse Transcription kit according to the manufacturer's protocol (Qiagen, Inc.). The cDNA was used to perform quantitative PCR using EvaGreen 5x qPCR mix (qARTA Bio Inc.) and 10 pmol/µl each of the reverse and forward primers in a Lightcycler 96 (Roche Diagnostics). The sequences of the primers used to amplify human lncRNA TUG1(30), nephrin (NSPH1), podocin (NSPH2), transcription factor A, mitochondrial (TFAM), cytochrome C oxidase subunit 5A (COX5A), PPARGC1A and GAPDH are shown in Table I. The amplification conditions consisted of 1 cycle of initial denaturation at 95˚C for 15 min; followed by 40 cycles of denaturation at 95˚C for 15 sec, annealing at 56˚C for 20 sec and extension at 72˚C for 20 sec. GAPDH was used as the internal control to normalize gene expression data. The relative mRNA expression was calculated using the 2-∆∆Cq method (31).
Table I.
Sequences of the primers.
| Gene | Sequence, 5'-3' | Size, bp |
|---|---|---|
| TUG1 | 150 | |
| Forward | TAGCAGTTCCCCAATCCTTG | |
| Reverse | CACAAATTCCCATCATTCCC | |
| NPHS1 | 124 | |
| Forward | GTGCACTATGCTCCCACCAT | |
| Reverse | TCTCCCAGTTGAACATGCCC | |
| NPHS2 | 160 | |
| Forward | GAGGAAGGTACCAAATCCTCCG | |
| Reverse | GCAGATGTCCCAGTCGGAATA | |
| TFAM | 105 | |
| Forward | ATGGAGGCAGGAGTTTCGTT | |
| Reverse | CCTAGATGAGTTCTGCCTGCT | |
| COX5A | 160 | |
| Forward | AGATGCCTGGGAATTGCGTA | |
| Reverse | AGGTCCTGCTTTGTCCTTAACA | |
| PPARGC1A | 158 | |
| Forward | TGTGGAGTCCCTGGAATGGA | |
| Reverse | AAGATCGTGTTGGGCGAGAG | |
| GAPDH | 153 | |
| Forward | CCCACTCCTCCACCTTTGAC | |
| Reverse | TGGTCCAGGGGTCTTACTCC |
In silico identification of TUG1/miR-204-5p axis mRNA targets
The VarElect phenotype program from GeneAnalytics (geneanalytics.genecards.org), a pathway enrichment analysis tool, was used to identify predicted mRNA targets of the TUG1/miR-204-5p axis involved in mitochondrial biogenesis based on the genomic information stored in GeneCards (32). In total, 453 predicted mRNA targets of hsa-miR-204-5p downloaded from TargetScan version 7.2 (targetscan.org) (33) were profiled with GeneAnalytics. The entries were scored and ranked using an algorithm that enables matching of genomic expression, sequencing and microarray datasets to tissues and cells.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Non-parametric variables were compared using a Mann-Whitney U-test or a Kruskal-Wallis test with a Dunn's post-hoc test, as appropriate. A Spearman's rank correlation analysis was used to analyse correlations. Statistical analysis was performed in GraphPad Prism Version 6.01 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical and biochemical characteristics of glomerulonephritides patients and healthy controls
The present study included 10 patients with biopsy-confirmed diagnosis, 4 males and 6 females, and 10 sex-matched healthy controls. The mean age at renal biopsy was 34.4±11.1 years old, the mean body mass index was 30.1±5.1, and the diagnoses were as follows: 5 cases of FSGS, 3 cases of LN, 1 case of AAV and 1 case of advanced glomerular sclerosis (AGS). Table II shows the biochemical parameters of the glomerulonephritides patients. A total of 80% of the patients had haemoglobin values within the normal range. All glomerulonephritides patients had proteinuria, with the highest levels present in the patient diagnosed with AGS. However, CKD staging, classified according to the level of GFR (34) varied greatly. Only 2 patients had a normal GFR of >90 ml/min/1.73 m2; 3 FSGS patients were Stage 2; 1 AAV patient was Stage 3B; 3 patients were Stage 4 and only 1 LN patient was Stage 5 with a GFR <15 ml/min/1.73m2. The presence of glomerular damage in the absence of renal failure highlights the importance of identifying novel circulating markers able to detect glomerulonephritides during the early stages of CKD.
Table II.
Biochemical parameters of glomerulonephritides patients.
| Patient no. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Diagnosis | FSGS | FSGS | AAV | LN | AGS | FSGS | LN | LN | FSGS | FSGS |
| Hemoglobin, g/dl | 14.7 | 13.2 | 12.8 | 11.6 | 8.7 | 10.5 | 15.6 | 13.8 | 16.7 | 16.3 |
| sCr, mg/dl | 0.84 | 0.57 | 1.5 | 3.7 | 3.9 | 2.1 | 0.86 | 8.4 | 1.4 | 1.5 |
| GFR, ml/min/1.73m2 | 90 | 126 | 38 | 17 | 15 | 28 | 107 | 8 | 66 | 63 |
| Proteinuria, g/24 h | 1.28 | 2.68 | 1.9 | 2.24 | 5.8 | 3.68 | 1.59 | 2.9 | 2.72 | 1.79 |
| Albumin, g/dl | 3.7 | 2.1 | 4.1 | 3.6 | 2.5 | 2.3 | 2.9 | 3 | 3.5 | 4.3 |
| pANCA | + | - | + | +++ | - | + | +++ | +++ | - | - |
| cANCA | - | - | - | - | - | - | - | - | - | - |
| Antinuclear antibodies | - | 0.09722 | 0.26389 | 0.48611 | 0.09722 | 0.09722 | 0.15278 | 0.15278 | 0.15278 | - |
| Anti-DNA antibodies | - | - | - | +++ | - | - | ++ | ++ | - | - |
| C3 fraction, mg/dl | 124 | 112 | 93.9 | 55 | 104 | 127 | 59.7 | 92.2 | 143 | 125 |
| C4 fraction, mg/dl | 28.6 | 38.4 | 24.4 | 7 | 31.8 | 28.9 | 9.29 | 19.4 | 33.5 | 33.5 |
| CRP, mg/l | 3.75 | <3.23 | 5.53 | <3.23 | 15.8 | <3.23 | 16 | 11.1 | <3.23 | <3.23 |
| ESR, mm/h | 37 | 42 | 16 | 12 | 18 | - | 28 | 26 | - | 10 |
FSGS, focal segmental glomerulosclerosis; LN, lupus nephritis; AVV, ANCA-associated vasculitis; AGS, advanced global sclerosis; sCr, serum creatinine; pANCA, perinuclear/nuclear antineutrophil cytoplasmic antibody; cANCA, cytoplasmic antineutrophil cytoplasmic antibody; C3, complement component 3; C4, complement component 4; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; -, negative; +, low; ++, moderate; +++, high.
Glomerulonephritides patients have low levels of urinary TUG1 expression
Cell-free lncRNAs are stable in urine and may potentially be used as non-invasive biomarkers to identify diseases, such as lupus nephritis and membranous nephropathy (18,19). To assess alterations in TUG1 expression in the urinary sediment of patients with glomerulonephritides, RT-qPCR was used. Urinary expression of TUG1 was reduced in glomerulonephritides patients compared with the healthy controls (Fig. 1A). Furthermore, significantly lower TUG1 expression levels in urinary sediment was observed in patients with FSGS when compared with all other diagnoses (Fig. 1B). These results suggest that the urinary expression of TUG1 decreases with podocytopathy.
Figure 1.
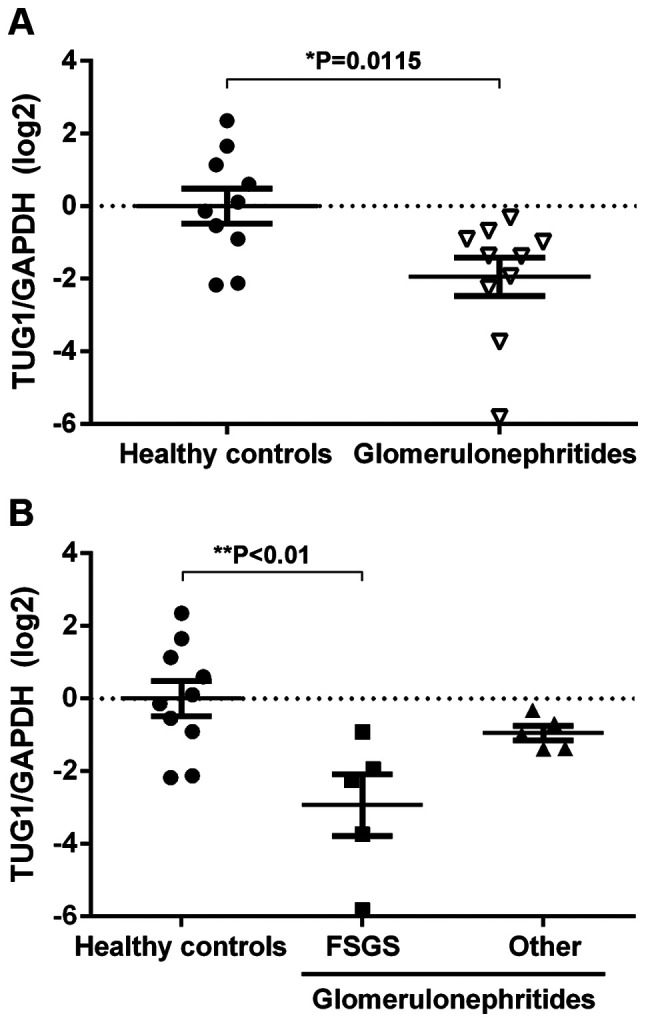
Urinary expression of TUG1 is decreased in FSGS patients. (A) RT-qPCR results showing the log2-fold change in expression of TUG1/GAPDH in the urinary sediment of healthy controls (n=10) and glomerulonephritides patients (n=10). (B) Reverse transcription-quantitative PCR results showing the log2-fold change in expression of TUG1/GAPDH in the urinary sediment of healthy controls (n=10), FSGS patients (n=5) and other diagnoses (lupus nephritis, n=3; ANCA-associated vasculitis, n=1; and advanced glomerular sclerosis, n=1). Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01. TUG1, taurine upregulated gene 1; FSGS, focal segmental glomerulosclerosis.
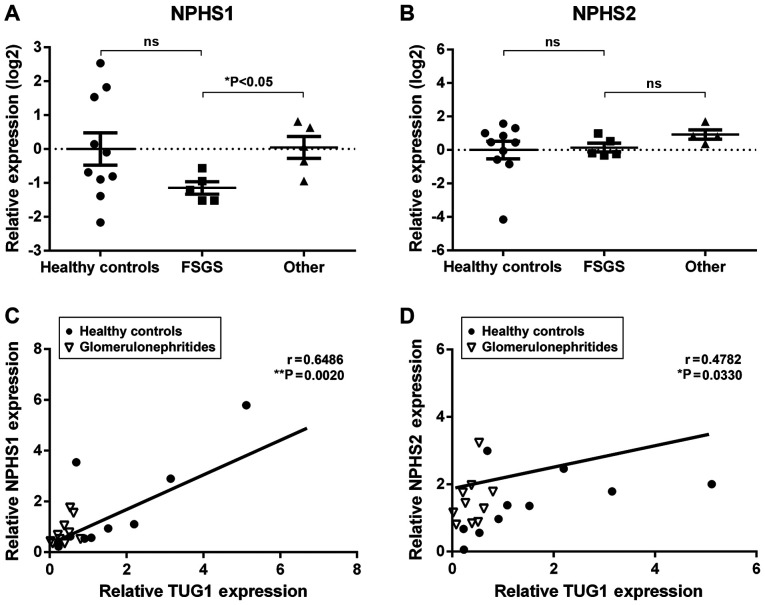
Urinary expression of TUG1 correlates with podocyte marker expression
Glomerular injury is associated with podocyte loss, a phenomenon that can be monitored by detecting podocyte-specific mRNAs in the urine (35). In the present study, two podocyte-specific mRNAs, NPHS1 and NPHS2, were detected using RT-qPCR. Although the expression levels of urinary NPHS1 was not significantly different between glomerulonephritides patients and healthy controls, NPHS1 urinary expression was significantly lower in the FSGS patients when compared with other diagnoses (Fig. 2A). In contrast, NPHS2 mRNA did not differ significantly amongst the groups (Fig. 2B). Interestingly, a positive correlation was observed between TUG1 urinary expression and both podocyte-specific mRNAs (NPHS1, r=0.6486, **P<0.01; NPHS2, r=0.4782, *P<0.05; Fig. 2C and D). These results further suggest an association between TUG1 urinary expression and podocyte damage.
Figure 2.
TUG1 urinary expression is correlated with podocyte marker mRNA expression in glomerulonephritides patients and healthy controls. Reverse transcription-quantitative PCR results showing the log2-fold change in expression of (A) NPHS1 and (B) NPHS2 in the urinary sediment of healthy controls (n=10), FSGS patients (n=5) and other diagnoses (lupus nephritis, n=3; ANCA-associated vasculitis, n=1; and advanced glomerular sclerosis, n=1). Data are presented as the mean ± standard error of the mean. *P<0.05. Correlation between lncRNA TUG1 transcript levels and (C) NPHS1 or (D) NPHS2 in the urinary sediment of healthy controls (n=10) and glomerulonephritides patients (n=10). *P<0.05, **P<0.01. TUG1, taurine upregulated gene 1; NSPH1, nephrin; NSPH2, podocin; ns, not significant.
Urinary expression of mitochondrial biogenesis markers is correlated with TUG1 expression levels
Several studies have validated the functional role of TUG1 as a lncRNA in directly binding to specific miRNAs to regulate post-transcriptional processing via a competing endogenous RNA mechanism (21,23,28,30). One such target is miR-204-5p (30). Thus, the VarElect phenotype program from Gene Analytics was used to predict mRNA targets of the TUG1/miR-204-5p axis involved in mitochondrial biogenesis. Through gene data profiling, a total of 27 genes significantly associated with mitochondrial biogenesis were identified (Table III).
Table III.
Predicted targets of TUG1/miR-204-5p axis involved in mitochondrial biogenesis.
| Symbol | Description | Global Ranka | Scorea,b |
|---|---|---|---|
| PPARGC1A | PPARG Coactivator 1α | 1 | 18.76 |
| TFAM | Transcription Factor A, Mitochondrial | 4 | 11.67 |
| CREB1 | CAMP Responsive Element Binding Protein 1 | 25 | 4.98 |
| SIRT1 | Sirtuin 1 | 26 | 4.80 |
| COX5A | Cytochrome C Oxidase Subunit 5A | 39 | 3.13 |
| ATF2 | Activating Transcription Factor 2 | 52 | 2.42 |
| MAPK1 | Mitogen-Activated Protein Kinase 1 | 56 | 2.32 |
| ESR1 | Estrogen Receptor 1 | 61 | 1.96 |
| CAMK2D | Calcium/Calmodulin Dependent Protein Kinase II δ | 68 | 1.68 |
| NFATC3 | Nuclear Factor of Activated T Cells 3 | 68 | 1.68 |
| OGT | O-Linked N-Acetylglucosamine (GlcNAc) Transferase | 69 | 1.59 |
| MXI1 | MAX Interactor 1, Dimerization Protein | 69 | 1.59 |
| ESRRG | Estrogen Related Receptor γ | 75 | 0.68 |
| CPOX | Coproporphyrinogen Oxidase | 75 | 0.68 |
| ESR2 | Estrogen Receptor 2 | 80 | 0.36 |
| TFAP2A | Transcription Factor AP-2α | 80 | 0.36 |
| ZBTB20 | Zinc Finger and BTB Domain Containing 20 | 81 | 0.26 |
| NPAS3 | Neuronal PAS Domain Protein 3 | 81 | 0.26 |
| NR4A2 | Nuclear Receptor Subfamily 4 Group A Member 2 | 81 | 0.26 |
| RICTOR | RPTOR Independent Companion of mTOR Complex 2 | 81 | 0.26 |
| CAMK1 | Calcium/Calmodulin Dependent Protein Kinase I | 81 | 0.26 |
| TET2 | Tet Methylcytosine Dioxygenase 2 | 81 | 0.26 |
| ATXN1 | Ataxin 1 | 81 | 0.26 |
| VHL | Von Hippel-Lindau Tumor Suppressor | 81 | 0.26 |
| HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase | 81 | 0.26 |
| ZNF521 | Zinc Finger Protein 521 | 81 | 0.26 |
FSGS, focal segmental glomerulosclerosis; LN, lupus nephritis; AVV, ANCA-associated vasculitis; AGS, advanced global sclerosis; sCr, serum creatinine; CRP, C reactive protein; ESR, erythrocyte sedimentation rate.
aObtained from a total of 453 genes.
bBased on analysis in VarElect of miR-204-5p predicted targets from TargetScan.
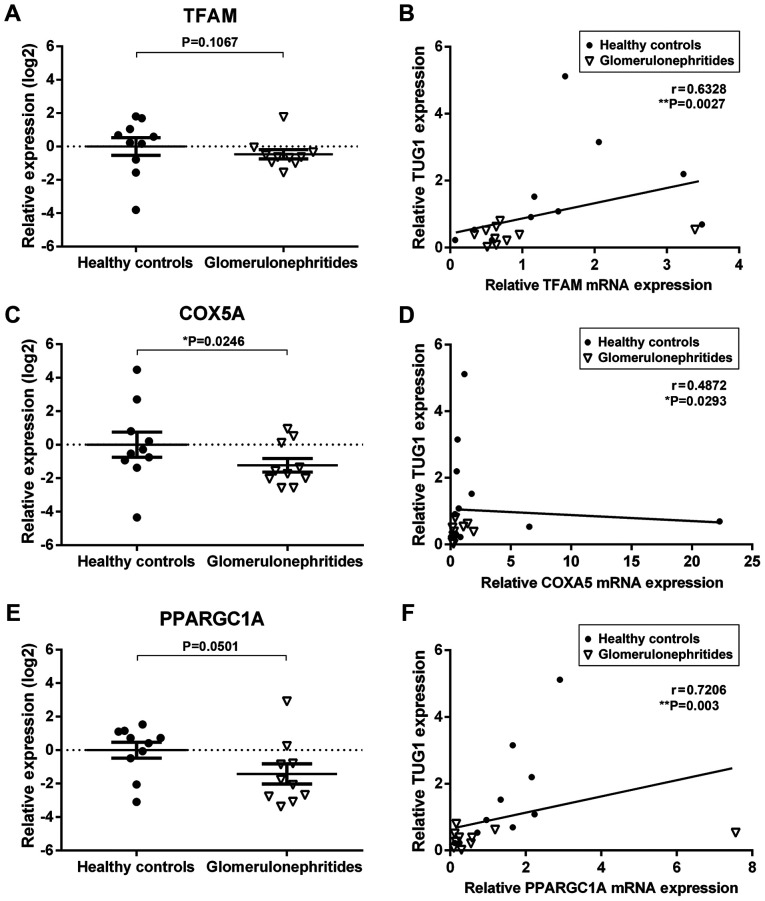
Next, the urinary expression of two predicted TUG1/miR-204 mRNA targets significantly associated with mitochondrial biogenesis, TFAM and COX5A, as well as one validated target, PPARGC1A (36), were quantified (Fig. 3). The relative expression levels of COXA5 and PPARGC1A were lower in the urinary sediment of glomerulonephritides patients when compared with healthy controls (Fig. 3C and E). Additionally, there was a significant correlation between TUG1 urinary expression and all three mitochondrial biogenesis mRNAs quantified in the present study (TFAM, r=0.6328, **P<0.01; COXA5, r=0.4872, *P<0.05; PPARGC1A, r=0.7206, **P<0.01; Fig. 3B-F). These results suggest that urinary RNAs may reflect molecular processes involved in the pathophysiology of renal dysfunction.
Figure 3.
Levels of urinary lncRNA TUG1 correlate with mitochondrial biogenesis mRNAs predicted to be modulated by the TUG1/miR-2014-5p axis. Reverse transcription-quantitative PCR results showing the log2-fold change in expression of (A) TFAM, (C) COX5A and (E) PPARGC1A in the urinary sediment of healthy controls (n=10) and glomerulonehritides patients (n=10). Data are presented as the mean ± standard error of the mean. Correlation between lncRNA TUG1 transcript levels and (B) TFAM, (D) COXA5 and (F) PARGC1A in the urinary sediment of healthy controls (n=10) and glomerulonephritides patients (n=10). *P<0.05, **P<0.01. TUG1, taurine upregulated gene 1; miR, microRNA; TFAM, transcription factor A mitochondrial, COX5A, cytochrome C oxidase subunit 5A; and PPARGC1A, peroxisome proliferator-activated receptor γ coactivator 1α.
Discussion
The diagnosis of glomerulonephritides in current clinical practice is primarily dependent on the histological analysis of renal biopsies (6). However, this traditional approach is being challenged by molecular techniques that go beyond description and examine disease mechanisms (37,38). Multiple studies have shown the association between dysregulated expression of certain lncRNAs and the development and progression of pathological disease states that affect the kidney (12-17). Urinary non-coding RNAs are a promising non-invasive tool able to reflect renal disease, aid in its appropriate diagnosis, and guide therapeutic choices (12,39,40). In the present study, it was shown that lncRNA TUG1 was present in the urinary sediment, and its expression was significantly reduced in patients with biopsy-confirmed glomerulonephritides, particularly those diagnosed with FSGS. Moreover, there was a positive correlation between urinary TUG1 expression and mRNAs known to be involved in pathogenic mechanisms associated with podocyte loss.
Mutations in NPHS1 or NPHS2 lead to proteinuria and rapid ESRD progression, highlighting the critical role of these podocyte-specific structural proteins in glomerular filtration barrier function (41,42). Although NPHS1 and NPHS2 are detectable in the urine of glomerulonephritides patients and have been described as clinically valuable biomarkers of podocyte injury, the results between studies are inconsistent and raise questions regarding the reasons behind the contrasting findings (43). In the present study, a decrease in urinary NPHS1 expression in FSGS was observed when compared to other diagnoses, but no differences in NPHS2 levels within the groups was found. These results partially agree with previous studies that showed urinary NPHS1 and NPHS2 mRNA levels were reduced in patients with MCN and FSGS, a feature that correlates with the degree of proteinuria (44,45). However, TUG1 expression levels showed no correlation with GFR, proteinuria or albuminuria in the present study (data not shown). Although it has been proposed that these podocyte-specific biomarkers can detect nephropathy before the development of albuminuria (46-48), a larger patient cohort is required to assess the relationship between TUG1 urinary expression and clinical markers of renal dysfunction traditionally used in the diagnosis of glomerulonephritides. In the present study, a positive correlation between NPHS1 and NPHS2 mRNA levels and TUG1 expression was observed, which suggests that the molecular mechanisms described in vitro and in animal models of DN regarding the relationship between TUG1 and podocyte loss are reflected in the urine of non-diabetic patients (28).
A decrease in PGC1A, a transcriptional coactivator that regulates energy homeostasis and mitochondrial biogenesis, has been implicated in the development of acute kidney injury, DN and renal fibrosis (49). The expression levels and activity of PGC1A depend on multiple transcriptional and post-transcriptional mechanisms, some mediated by non-coding RNAs (22,28). Interactions between TUG1 and the promoter of PGC1A enhances its transcription, and increases mitochondrial content and cellular ATP levels whilst reducing mitochondrial reactive oxygen species levels. In addition, TUG1 acts as a competitive endogenous RNA for miRNAs such as miR-145, miR-144 and miR-204-5p (30,50,51). miRNA miR-204-5p is involved in multiple cellular processes, including angiogenesis, vascular disease, metabolism and glucose homeostasis (30,52). Through in silico analysis, targets of the TUG1/miR-204-5p axis involved in mitochondrial biogenesis were predicted and it was subsequently shown that PGC1A, COX5A and TFAM were also detectable in the urine and were correlated with TUG1 expression. PGC1A is a known posttranscriptional target of miR-204-5p (53,54); however, COX5A and TFAM still require experimental validation. Nonetheless, the results suggest that the TUG1/miR-204 axis and their mitochondrial biogenesis targets are relevant in FSGS pathogenesis and may serve as potential biomarkers.
The present study has some limitations, including the small number of glomerulonephritides patients enrolled and the variability of diagnosis, which included primary and secondary glomerulonephritides. Nonetheless, our findings support a role for lncRNA TUG1 in the development of glomerulonephritides, and justify further exploration of urinary TUG1 as a potential biomarker of FSGS. More extensive studies, with carefully selected subjects are required to confirm the relationship between urinary TUG1 expression, clinical markers of renal dysfunction and glomerular damage observed in kidney biopsies; and to validate lncRNA TUG1 as a biomarker of FSGS. Additionally, more in vitro and animal experiments focused on the TUG1/miR-204-5p axis should be performed to increase our understanding of these non-coding RNAs in mitochondrial bioenergetics and podocyte loss.
In conclusion, the results of the present study highlight the potential of urinary non-coding RNAs, such as TUG1, in the diagnosis of renal disease, and suggests that this lncRNA and its mitochondrial-associated pathways are relevant in glomerulonephritides other than DN. Further studies are required to evaluate urinary TUG1 as a potential biomarker of podocytopathy, and to determine its association with kidney dysfunction and patient prognosis.
Acknowledgements
We would like to thank Claudia Susana Meza Calvillo for her technical assistance as part of the Scientific Research Summer Program of the Mexican Academy of Science 2019.
Funding
This study was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico (grant no. SALUD-2018-02-B-S-42687).
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
FJS-T, MM-P and RE conceived the study. FJS-T and MM-P collected and analysed the data. ZM performed the experiments. CM-C performed the histopathological analysis of renal biopsies. RE drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study complied with the ethical principles for medical research specified in the Declaration of Helsinki and was approved by the Local Ethics and Research Committee at the Hospital de Especialidades NUM. 1, Bajio, Leon, Guanajuato, Instituto Mexicano del Seguro Social (approval no. CLIEIS R-2018-1001-114). Each participant provided written informed consent prior to enrolment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Floege J, Amann K. Primary glomerulonephritides. Lancet. 2016;387:2036–2048. doi: 10.1016/S0140-6736(16)00272-5. [DOI] [PubMed] [Google Scholar]
- 2.Caliskan Y, Kiryluk K. Novel biomarkers in glomerular disease. Adv Chronic Kidney Dis. 2014;21:205–216. doi: 10.1053/j.ackd.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matovinović MS. Podocyte injury in glomerular diseases. EJIFCC. 2009;20:21–27. [PMC free article] [PubMed] [Google Scholar]
- 4.Mallipattu SK, He JC. The podocyte as a direct target for treatment of glomerular disease? Am J Physiol Renal Physiol. 2016;311:F46–F51. doi: 10.1152/ajprenal.00184.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2009;21:489–494. doi: 10.1097/BOR.0b013e32832efff1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan J, Mohan P, Appel GB. Diagnostic tests and treatment options in glomerular disease: 2014 update. Am J Kidney Dis. 2014;63:656–666. doi: 10.1053/j.ajkd.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Parikh SV, Ayoub I, Rovin BH. The kidney biopsy in lupus nephritis: Time to move beyond histology. Nephrol Dial Transplant. 2015;30:3–6. doi: 10.1093/ndt/gfu348. [DOI] [PubMed] [Google Scholar]
- 8.Rovin BH, Almaani S, Malvar A. Reimagining the kidney biopsy in the era of diagnostic biomarkers of glomerular disease. Kidney Int. 2019;95:265–267. doi: 10.1016/j.kint.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongelli A, Martelli F, Farsetti A, Gaetano C. The dark that matters: Long Non-coding RNAs as master regulators of cellular metabolism in Non-communicable diseases. Front Physiol. 2019;10(369) doi: 10.3389/fphys.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzen JM, Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12:360–373. doi: 10.1038/nrneph.2016.51. [DOI] [PubMed] [Google Scholar]
- 12.Ignarski M, Islam R, Müller RU. Long Non-coding RNAs in kidney disease. Int J Mol Sci. 2019;20(3276) doi: 10.3390/ijms20133276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian H, Wu M, Zhou P, Huang C, Ye C, Wang L. The long non-coding RNA MALAT1 is increased in renal ischemia-reperfusion injury and inhibits hypoxia-induced inflammation. Ren Fail. 2018;40:527–533. doi: 10.1080/0886022X.2018.1487863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu S, Han R, Shi J, Zhu X, Qin W, Zeng C, Bao H, Liu Z. The long noncoding RNA LOC105374325 causes podocyte injury in individuals with focal segmental glomerulosclerosis. J Biol Chem. 2018;293:20227–20239. doi: 10.1074/jbc.RA118.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han R, Hu S, Qin W, Shi J, Zeng C, Bao H, Liu Z. Upregulated long noncoding RNA LOC105375913 induces tubulointerstitial fibrosis in focal segmental glomerulosclerosis. Sci Rep. 2019;9(716) doi: 10.1038/s41598-018-36902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin LW, Pan M, Ye HY, Zheng Y, Chen Y, Huang WW, Xu XY, Zheng SB. Down-regulation of the long non-coding RNA XIST ameliorates podocyte apoptosis in membranous nephropathy via the miR-217-TLR4 pathway. Exp Physiol. 2019;104:220–230. doi: 10.1113/EP087190. [DOI] [PubMed] [Google Scholar]
- 17.Liao Z, Ye Z, Xue Z, Wu L, Ouyang Y, Yao C, Cui C, Xu N, Ma J, Hou G, et al. Identification of renal long Non-coding RNA RP11-2B6.2 as a positive regulator of type I interferon signaling pathway in lupus nephritis. Front Immunol. 2019;10(975) doi: 10.3389/fimmu.2019.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santer L, López B, Ravassa S, Baer C, Riedel I, Chatterjee S, Moreno MU, González A, Querejeta R, Pinet F, et al. Circulating long noncoding RNA LIPCAR predicts heart failure outcomes in patients without chronic kidney disease. Hypertension. 2019;73:820–828. doi: 10.1161/HYPERTENSIONAHA.118.12261. [DOI] [PubMed] [Google Scholar]
- 19.Huang YS, Hsieh HY, Shih HM, Sytwu HK, Wu CC. Urinary Xist is a potential biomarker for membranous nephropathy. Biochem Biophys Res Commun. 2014;452:415–421. doi: 10.1016/j.bbrc.2014.08.077. [DOI] [PubMed] [Google Scholar]
- 20.Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14:291–312. doi: 10.1038/nrneph.2018.9. [DOI] [PubMed] [Google Scholar]
- 21.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15:501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Li SY, Park J, Qiu C, Han SH, Palmer MB, Arany Z, Susztak K. Increasing the level of peroxisome proliferator-activated receptor γ coactivator-1α in podocytes results in collapsing glomerulopathy. JCI Insight. 2017;2(e92930) doi: 10.1172/jci.insight.92930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SY, Susztak K. The long noncoding RNA Tug1 connects metabolic changes with kidney disease in podocytes. J Clin Invest. 2016;126:4072–4075. doi: 10.1172/JCI90828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Finck BN, Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long J, Badal SS, Ye Z, Wang Y, Ayanga BA, Galvan DL, Green NH, Chang BH, Overbeek PA, Danesh FR. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126:4205–4218. doi: 10.1172/JCI87927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Association WM. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 30.Yu C, Li L, Xie F, Guo S, Liu F, Dong N, Wang Y. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc Res. 2018;114:168–179. doi: 10.1093/cvr/cvx180. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Ari Fuchs S, Lieder I, Stelzer G, Mazor Y, Buzhor E, Kaplan S, Bogoch Y, Plaschkes I, Shitrit A, Rappaport N, et al. GeneAnalytics: An integrative gene set analysis tool for next generation sequencing, RNAseq and microarray data. OMICS. 2016;20:139–151. doi: 10.1089/omi.2015.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4(e05005) doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC. Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant. 2012;27:4079–4087. doi: 10.1093/ndt/gfs313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Civelek M, Hagopian R, Pan C, Che N, Yang WP, Kayne PS, Saleem NK, Cederberg H, Kuusisto J, Gargalovic PS, et al. Genetic regulation of human adipose microRNA expression and its consequences for metabolic traits. Hum Mol Genet. 2013;22:3023–3037. doi: 10.1093/hmg/ddt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saez-Rodriguez J, Rinschen MM, Floege J, Kramann R. Big science and big data in nephrology. Kidney Int. 2019;95:1326–1337. doi: 10.1016/j.kint.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Kretzler M, Cohen CD, Doran P, Henger A, Madden S, Gröne EF, Nelson PJ, Schlöndorff D, Gröne HJ. Repuncturing the renal biopsy: Strategies for molecular diagnosis in nephrology. J Am Soc Nephrol. 2002;13:1961–1972. doi: 10.1097/01.asn.0000020390.29418.70. [DOI] [PubMed] [Google Scholar]
- 39.Sun IO, Lerman LO. Urinary microRNA in kidney disease: Utility and roles. Am J Physiol Renal Physiol. 2019;316:F785–F793. doi: 10.1152/ajprenal.00368.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandenburger T, Salgado Somoza A, Devaux Y, Lorenzen JM. Noncoding RNAs in acute kidney injury. Kidney Int. 2018;94:870–881. doi: 10.1016/j.kint.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 41.Patrakka J, Kestilä M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Männikkö M, Visapää I, Holmberg C, Rapola J, et al. Congenital nephrotic syndrome (NPHS1): Features resulting from different mutations in Finnish patients. Kidney Int. 2000;58:972–980. doi: 10.1046/j.1523-1755.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- 42.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 43.Sekulic M, Pichler Sekulic S. A compendium of urinary biomarkers indicative of glomerular podocytopathy. Patholog Res Int. 2013;2013(782395) doi: 10.1155/2013/782395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hara M, Yanagihara T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron. 2001;89:342–347. doi: 10.1159/000046097. [DOI] [PubMed] [Google Scholar]
- 45.Szeto CC, Wang G, Chow KM, Lai FM, Ma TK, Kwan BC, Luk CC, Li PK. Podocyte mRNA in the urinary sediment of minimal change nephropathy and focal segmental glomerulosclerosis. Clin Nephrol. 2015;84:198–205. doi: 10.5414/CN108607. [DOI] [PubMed] [Google Scholar]
- 46.Pätäri A, Forsblom C, Havana M, Taipale H, Groop PH, Holthöfer H. Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes. 2003;52:2969–2974. doi: 10.2337/diabetes.52.12.2969. [DOI] [PubMed] [Google Scholar]
- 47.Ng DP, Tai BC, Tan E, Leong H, Nurbaya S, Lim XL, Chia KS, Wong CS, Lim WY, Holthöfer H. Nephrinuria associates with multiple renal traits in type 2 diabetes. Nephrol Dial Transplant. 2011;26:2508–2514. doi: 10.1093/ndt/gfq738. [DOI] [PubMed] [Google Scholar]
- 48.Chang JH, Paik SY, Mao L, Eisner W, Flannery PJ, Wang L, Tang Y, Mattocks N, Hadjadj S, Goujon JM, et al. Diabetic kidney disease in FVB/NJ Akita mice: Temporal pattern of kidney injury and urinary nephrin excretion. PLoS One. 2012;7(e33942) doi: 10.1371/journal.pone.0033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch MR, Tran MT, Parikh SM. PGC1α in the kidney. Am J Physiol Renal Physiol. 2018;314:F1–F8. doi: 10.1152/ajprenal.00263.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan J, Qiu K, Li M, Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589:3175–3181. doi: 10.1016/j.febslet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Cai H, Xue Y, Wang P, Wang Z, Li Z, Hu Y, Li Z, Shang X, Liu Y. The long noncoding RNA TUG1 regulates blood-tumor barrier permeability by targeting miR-144. Oncotarget. 2015;6:19759–19779. doi: 10.18632/oncotarget.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores-Pérez A, Marchat LA, Rodríguez-Cuevas S, Bautista-Piña V, Hidalgo-Miranda A, Ocampo EA, Martínez M, Palma-Flores C, Fonseca-Sánchez MA, Astudillo-de la Vega H, et al. Dual targeting of ANGPT1 and TGFBR2 genes by miR-204 controls angiogenesis in breast cancer. Sci Rep. 2016;6(34504) doi: 10.1038/srep34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maniyadath B, Chattopadhyay T, Verma S, Kumari S, Kulkarni P, Banerjee K, Lazarus A, Kokane SS, Shetty T, Anamika K, Kolthur-Seetharam U. Loss of hepatic oscillatory fed microRNAs Abrogates Refed transition and causes liver dysfunctions. Cell Rep. 2019;26:2212–2226. doi: 10.1016/j.celrep.2019.01.087. [DOI] [PubMed] [Google Scholar]
- 54.Houzelle A, Dahlmans D, Nascimento EBM, Schaart G, Jörgensen JA, Moonen-Kornips E, Kersten S, Wang X, Hoeks J. MicroRNA-204-5p modulates mitochondrial biogenesis in C2C12 myotubes and associates with oxidative capacity in humans. J Cell Physiol. 2020;235:9851–9863. doi: 10.1002/jcp.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.