Abstract
Decades of population-based health outcomes data highlight the importance of understanding how environmental exposures in pregnancy affect maternal and neonatal outcomes. Animal model research and epidemiological studies have revealed that such exposures are able to alter fetal programming through stable changes in the epigenome, including altered DNA methylation patterns and histone modifications in the developing fetus and infant. It is similarly known that while microbes can biotransform environmental chemicals via conjugation and de-conjugation, specific exposures can also alter the community profile and function of the human microbiome. In this review, we consider how alterations to the maternal and or fetal/infant microbiome through environmental exposures could directly and indirectly alter fetal programming. We highlight two specific environmental exposures, cadmium (Cd) and polycyclic aromatic hydrocarbons (PAHs), and outline their effects on the developing fetus and the perinatal (maternal and fetal/infant) microbiome. We further consider how chemical exposures in the setting of natural disasters may be of particular importance to environmental health.
Introduction
Human exposure to environmental chemicals has changed in significant ways in recent decades. These changes broadly include both quantitative disruptions (the number and concentration of environmental chemicals) as well as qualitative variations (the nature, timing and duration of the exposure, largely as a result of prolonged exposures across the lifespan). Epidemiologists, toxicologists, reproductive biologists and clinicians have long studied how environmental exposures influence maternal and fetal/neonatal outcomes, inclusive of both teratogenic and non-teratogenic sequelae. Some exposures, such as that of methylmercury, a known neurotoxin, have been extensively studied [1–4]. However, the effects on fetal outcomes and the potential for fetal programming later in life disease via common and generally non-teratogenic compounds remains largely unknown. Furthermore, determining precise levels and duration or temporality of either maternal or fetal exposures following several routes of exposure to environmental chemicals during pregnancy remains a challenge. Specifically, in the environment, we are exposed to mixtures of chemicals through the air we breathe (inhalation exposures), the food and water we consume (ingestion exposures), and the specific conditions of the environment where we live and work (largely absorption exposures through our skin). In the case of pregnancy, these exposures may be secondarily vertically transmitted to the developing fetus, both as a product of transplacental exposure as well placental deposition. Qualitative and quantitative estimates of these exposures is both understudied and continuously challenging.
In this review we will first provide a very brief overview of environmental chemical biotransformations, which are known to occur both in humans and other vertebrate animals as well as in bacteria. We will then provide a brief overview of key molecular mechanisms which are thought to mediate the adverse effects of environmental exposures in pregnancy. We will consider two specific environmental exposures of interest, cadmium (Cd) and polycyclic aromatic hydrocarbons (PAHs), and consider two possibilities. First, how their disruption of the maternal and perinatal microbiome may subsequently modify risk of later in life disease. Second, we will consider how a disrupted microbiome may limit the biotransformation of these environmental chemicals to similarly modify occurrence of perinatal disease. Finally, we will consider the occurrence of particularly adverse environmental exposures which may accompany natural disasters.
Biotransformation of environmental chemicals occurs in vertebrates and bacteria
Human metabolism and biotransformation of environmental chemicals.
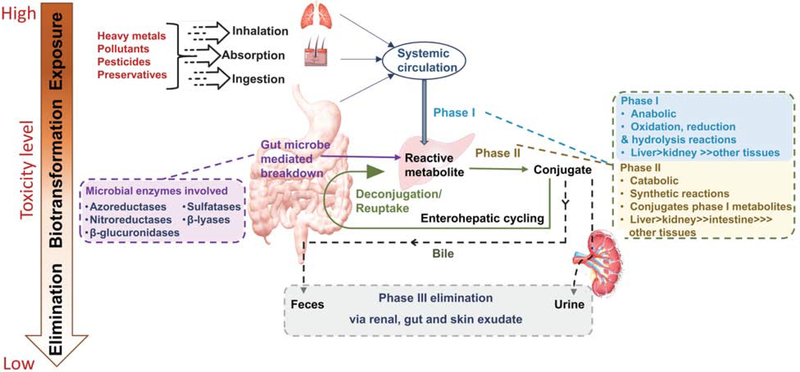
In vitro and in vivo studies have highlighted the role of the molecular machinery of the human liver, gut and kidney in the metabolism and elimination of environmental chemicals (Figure 1). It typically involves biotransformation of these chemicals (via Phase I, II and/or III pathways) to form a more hydrophilic compound which can be readily excreted out of the body [5]. We and others have previously published examples of genomic and epigenomic variants which can affect the ability or activity of these biotransformation reactions in pregnancy [6, 7]. However, although genomic structural variations and single nucleotide polymorphisms are known to occasionally alter an individual’s susceptibility to disease occurrence resulting from exposures to environmental chemicals, the overwhelming majority of biotransformation reactions are shared among the population at-large. Phase I enzymes belong to the cytochrome P450 (CYP) superfamily and are usually expressed in hepatic cells and some enterocytes. Metabolism through the Phase II pathway mainly involves formation of water soluble products by conjugation of functional groups to the products formed in Phase I pathway. Liver, gut and kidney are the site of the majority of Phase II reactions. Phase III pathways primarily employ cellular and efflux transporters which enable the metabolic products of Phase I and II to be eliminated from the body.
Figure 1.
Diagrammatic representation of environmental chemical exposure routes, human and microbial biotransformation pathways, and elimination of metabolites and small molecular intermediates.
Maternal, placental and fetal metabolism—implications for understanding the potential for fetal harm.
As a result of metabolism and biotransformation reactions, the fetus will not necessarily experience maternal dose levels of environmental chemicals. Compounds which do not undergo first pass metabolism in the maternal liver and are renal excreted and transported across the placenta will hemoconcentrate in the fetus (due to its smaller circulating blood volume). One such example is the commonly used diabetes medication, metformin. Some compounds maybe metabolized by the placenta, either generating inert metabolites or metabolites which may cause placental damage. We have previously shown that this occurs with maternal tobacco use, with both Cd and PAHs being implicated as the harmful agents [6,7]. Some environmental chemicals have the potential to direct affect the fetus. In fact, we have previously shown that PAHs from maternal smoking are both associated with histologic evidence of oxidative damage in the placenta and can cause fetal growth restriction when the fetus is missing the Phase II activity of GSTT1 [8]. In this scenario, differential methylation and increased expression of CYP1A1 in the placenta occurs as a result of maternal smoking. This then generates more harmful DNA adducts and intermediates, driving the observed placental oxidative damage [6]. When the fetus lacks the GSTT1 enzyme activity, it cannot excrete these harmful intermediates. This renders relative weight differences in the fetus only when maternal smoking occurs [7]. In sum, fetal harm can occur through multiple mechanisms including via placental damage and/or direct fetal exposure.
Microbial biotransformation reactions: help from our smallest friends.
Classical environmental chemical biotransformation studies on human health largely considered the role of human cells and their molecular machinery on metabolism, catabolism, and elimination of environmental chemicals. However, we have long understood the importance of bacteria in biotransformation reactions and several studies have recently highlighted the importance of both bacteria in the environment and our gut microbes on the biotransformation of environmental chemicals of importance to human health (Figure 1).
Five enzymatic families of gut microbial origin (azoreductases, nitroreductases, β-glucuronidases, sulfatases and β-lyases) have been identified as key players in metabolizing many environmental chemicals (Figure 1) [8, 9]. Although most of our current and working knowledge pertaining to environmental chemicals and actions of microbial enzymes have resulted from studies of drugs at high therapeutic concentrations, several examples of gut-microbiome involvement in metabolism of mutagenic and carcinogenic chemicals has been demonstrated both in vitro and in vivo [8, 10]. Furthermore, in vitro studies of human and rodent gut bacteria show that gut microbiomes can modify bioavailability and toxicity of metals like arsenic, mercury and cadmium, generally by complex and multi-step enzymatic reactions [10]. More than 800 microbial genera have been identified as playing active roles in such biotransformation reactions, which collectively occur via either direct or indirect mechanisms and can modulate the toxicity, absorption and bioavailability of environmental chemicals [9]. Akin to first pass metabolism in the human liver, many chemicals undergo direct binding and/or degradation and deactivation by the gut microbiota. These small molecule intermediates may constitute a more or (ideally) a less toxic compound which can be further transformed in the human liver via Phase II reactions, or form conjugated or deconjugated products with a varied or altered potential for endocrine or metabolic disruption [11–13]. Indirect mechanisms include up- or down-regulation of human metabolism and biotransformation via phase I and II/III reactions and excretions [8]. This is schematically depicted in Figure 1. While the precise nature of these are outside the scope of the current review, it is important to advance the concept that there are both direct and indirect means by which the presence or absence of a certain strain or species of bacteria in the human gut can alter the molecular capacity of human cells and their molecules to biotransform environmental chemicals (Figure 1).
General importance of biotransformation reactions and their intermediates on pregnancy and perinatal outcomes.
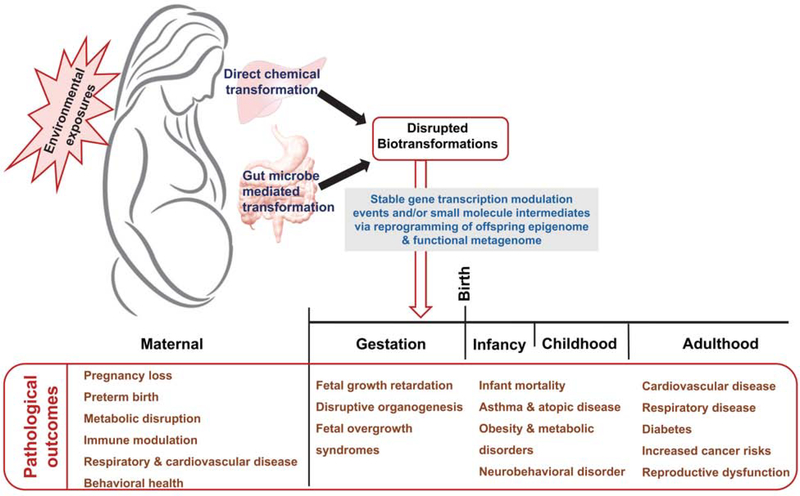
Some of the small molecule intermediates and their related metabolites and compounds formed as a result of microbial biotransformation will ultimately manifest as endocrine disrupting agents [11–13]. As such, these will in turn increase the risk of certain disorders in women, such as miscarriage, pregnancy loss and preterm birth among gravidae, as well as reproductive tract cancers and dysfunction and metabolic, cardiovascular and respiratory disorders (Figure 2) [11]. These exposure risks extend to the placenta and fetus, since certain environmental chemicals or their metabolic byproducts have been found to be transplacentally transported and may enter the fetal circulation [14, 15]. This in turn imparts many similar and often anticipated disorders among the offspring, which can extend throughout the lifespan (Figure 2).
Figure 2.
Diagrammatic representation of the outcomes of environmental exposures across the life stage.
Detection of environmental chemicals in the cells of the placenta [6,16], as well as from maternal and umbilical cord blood in association with preterm birth and developmental disruptions [7, 17, 18] reveal the importance of focused investigations into the level or dose of an exposure, as well as the importance of timing relative to embryogenesis and throughout the developing offspring’s lifespan, including critical windows for programming cardiovascular and metabolic health. Rapid growth of organ and physiologic system developments in fetuses and infants make them particularly vulnerable to the effects of organic pollutants, as we and others have previously demonstrated [6, 7, 9–12, 19–35]. The chemical burden circulating through the mother’s blood is shared with her fetus or neonate, and the child may, in some cases, be exposed to heavy doses relative to the body weight, which can lead to developmental defects and life-long functional deficits [19].
Environmental exposures in pregnancy: Cd and PAH as two relevant examples
While summarizing decades of research on the effects of environmental exposures in pregnancy is outside the scope of this review, we direct the reader to several excellent recent reviews on this subject [20–23]. Suffice it to say in brief, we and others have demonstrated that epigenomic modifications to critical gene regulatory regions are one key set of molecular mechanism by which environmental exposures parlay an enduring impact on the developing fetus [11, 24–26]. Maternal diet, lifestyle and environmental exposures have all been shown to persistently modulate gene transcription in the offspring via stable epigenomic modifications, including DNA methylation and histone modifications leading to altered occupancy of critical promoter regions. These stable modifications in the epigenome and/or its molecular machinery render risk of later in life metabolic, respiratory, neurodevelopmental and immunological disorders (Figure 2) [15, 27–29]. However, considerably less attention has generally been given to the role of perinatal microbiome in modulating and mitigating environmental exposures. Nonetheless, there are two good examples (Cd and PAH exposure) where there is emerging evidence to suspect the microbiome may play a role in their transformation and metabolism.
Cadmium (Cd).
Several routes of Cd exposure occurs including ambient inhalation (cigarette smoking, fossil fuel combustion and mine tailings) and ingestion (bioaccumulation in plant foods like cereals, leafy and root vegetables, and animal products) [30]. In non-pregnant individuals, Cd is toxic to the brain and kidney [31] and has a half-life of 5–40 years [32]. In pregnant women, Cd is known to accumulate in the placenta, and is associated with both necrosis and diminished placental function, presumptively via a reduction in trophoblast cell proliferation with a concomitant permissive apoptosis [33, 34]. Furthermore, Cd exposure is associated with reduced fetal birth weight and a smaller head circumference in newborns [35] and in animal models has been shown to alter fetal central nervous system, liver and kidney end-organ development [36].
Polycyclic aromatic hydrocarbons (PAHs):
Similar to Cd, PAHs are toxic organic chemicals that are found in carbon combustion byproducts including vehicle emissions, ambient air, and cigarette smoke, as well as in plant and animal based foods, soil, and water. They arise from incomplete combustion of fossil fuels, and can contaminate soil and aquatic environments. PAH exposure in pregnancy is associated with preterm delivery, reduced birthweight, small for gestational age infants, an increased risk of neonatal bronchopulmonary dysplasia, higher risks of childhood asthma and lower cognitive test scores [37–43]. PAHs are also able to cross the placenta and are found in measurable quantities above ambient levels in cord blood [17, 18, 44]. Due to their Phase I metabolism, PAHs are converted to electrophilic species which covalently attach to nucleophilic sites on the DNA backbone forming bulky adducts on DNA [45, 46]. These bulky DNA adducts therefore can be mutagenic due to faulty base excision repair of the double helix [45]. It is unsurprising that PAH exposures in utero have also been associated with persistent fetal reprogramming to render risk of later in life disease (Figure 2).
Cd and PAH mediated epigenetic modifications
In the non-pregnant individual, Cd bioaccumulates in bones [24, 47–50]. During pregnancy, this Cd is released from bone, increasing the risk of Cd exposure to the developing fetus [51]. Cd is thought to alter DNA methyl transferase activity, which could in turn alter epigenetic marks during development which are crucial for regulation of gene transcription events [52]. While the precise molecular mechanisms underlying Cd-driven associations in adverse pregnancy outcomes is not completely understood, in utero Cd exposures are associated with hypo-methylation of repetitive regions of the genome, specifically those of long interspersed nuclear elements (LINE-1) with increased Cd levels [53]. Given that maintenance of methylation levels of repetitive regions is important for genome stability, this may contribute to a number of pleiotropic measures. Interestingly, one study suggested sex-specificity with sexual dimorphism of altered DNA methylation patterns in the cord blood with in utero Cd exposures [54]. Specifically, DNA methylation patterns in male offspring were positively correlated with Cd exposure, while those of female offspring were negatively correlated.
Given that the reactive oxygen species generated from PAH metabolism are able to form bulky adducts to DNA, DNA methylation patterns can be altered during repair of the affected areas of the genome. Studies of epigenetic alterations of PAH exposure in pregnancy have therefore focused specifically on changes in DNA methylation patterns in the exposed offspring. Genomic hyper-methylation in cord blood was found in umbilical cord white blood cells of newborns exposed to PAHs in utero [55]. In these same subjects, PAH exposure, DNA methylation levels and neurodevelopmental delays were correlated at age 3 [56]. In a murine model of prenatal PAH exposures offspring revealed behavioral changes not observed in exposed animals and these behavioral changes were accompanied by alterations to DNA methylation patterns in the cortex [57].
Cd and PAH mediated microbiome alterations
Several studies to date have suggested that the microbiome interacts with and alters the end point measures of environmental toxicants like heavy metals (including Cd) and organic pollutants (including PAHs). Perhaps unsurprising given their crucial role in the biotransformation and turnover of small molecule intermediates and metabolites, microbes are key players in xenobiotic and environmental chemical metabolism [13]. Generally commensal microbiota can metabolize such compounds both directly following ingestion or inhalation, or secondarily following their initial conjugation in the human liver [13]. Similarly, ingested xenobiotics are able to interfere with microbial enzymatic activity of the gut, and have been shown to directly induce dysbiosis via alterations in the community profile of the gut niche and its microniches [13]. Thus, environmental chemicals can disrupt the human gut ecology, both at the levels of altering human microbial composition and its encoded metagenomic functions. The net effect of these compositional and functional changes to the gut microbiome is that it’s capacity to metabolize or biotransform environmental chemicals and their derivatives either towards human benefit or with risk of human harm occurs. Consequences of these interactions are only beginning to be appreciated and remain largely understudied and poorly characterized.
In developmental animal models, Cd administered through drinking water induced gut dysbiosis of both communities and their functions in the small intestine and distal colon [58]. However and interestingly, having an established gut microbiota (e.g. adult-like) was protective against heavy metal induced dysbiosis, including that of Cd [59]. Specifically, mice lacking a mature or diverse intestinal microbiota are more susceptible to accumulation (5–10 times) of Cd or lead (Pb) in their target organs including blood, liver, kidney and spleen compared to symbiotic control groups.
As one such example, BaP is an interesting organic pollutant with regards to its interaction with microbiome. It has been found that human gut microbiota metabolize BaP creating an estrogenic compound through hydroxylation to 7-hydroxybenzo[a]pyrene (7-OH-BaP), is able to activate human estrogen receptor while its parent compound cannot [60]. Therefore, the toxicity of ingested, inhaled, or absorbed PAHs may be enhanced through interactions with the human microbiome. In other words, members of the gut microbiome which would retain the capacity to hydroxylate BAP to 7-OH-BaP would be considered “dysbiotic” in this context. Conversely, absence of these microbes would confer potential symbiotic benefit to the human host. Thus, knowing the presence or absence of given microbes capable of not only beneficial but potentially detrimental biotransformation reactions would be crucial to understanding environmental health risks and potential mitigation benefits. At present, how this transformation into estrogenic compounds may affect pregnancy loss, preterm birth, and reproductive health are unknown at present. Clearly an understanding of this association and alterations in pregnancy outcomes and fetal programming remain an unexplored but potentially high impact area of study.
Environmental exposures and fetal programming
The concept of fetal programming proposes that preconception and/or prenatal and postnatal nutrient and environmental chemical exposures (collectively referred to as the perinatal period) have persistent multigenerational and potentially transgenerational adverse effects on the offspring. Over the course of pregnancy, fetal nutrient requirements changes with time, leading to dynamic metabolic adaptations in pregnant women [61]. However, the gut microbiota can modulate the metabolic and physiology of utilization of both macro and micronutrient, parlaying implications on fetal programming across the lifespan [48, 62, 63]. The strategic location of the microbiome at almost all interfaces with external environment (e.g., the lung and nasopharyngeal microbiomes with respect to inhalation, the skin with respect to absorption, and the oral and gut with respect to ingestion). The net effect is that by measuring environmental chemicals and their intermediates alongside functional alterations in the microbiome of multiple body sites in parallel with measures of altered molecular, cellular, organ, and whole systems physiology across the lifespan, reasonable suppositions regarding the capacity to link risk from exposure to the functions of the microbiome can be made [64]. To this end, it is worthwhile to note that the majority of epigenetic modifications which result in detectable gene regulatory events, including alterations in site-specific DNA methylation and histone demarcations, are now correlated with examples regarding predominance or absence of certain microbial taxa and their resultant metagenomic functions [64, 65]. Whether this will hold true across the spectrum of environmental chemicals which have been linked to fetal reprogramming events remains to be seen.
Beyond just direct environmental chemical exposures, it is worthwhile to consider a “two hit” model of modulation in biotransformation of potential toxicants. For example, we and others have shown that the maternal diet has a profound and lasting influence on early developmental processes, including metabolic, behavioral, immune modulatory, and cardiovascular. Similarly, multiple recent publications from our lab in both humans and non-human primates demonstrates the concomitant lasting “footprint” of a high fat maternal diet on both the offspring gut microbiome and biologically relevant histone modifications, which parlay as risk of later in life disease [24, 47–50, 66, 67]. We and others have also correlated increased risks of spontaneous preterm birth with prenatal exposure to environmental PAHs [50, 68, 69]. Although several studies have shown that environmental chemicals can induce microbiome changes [58, 59, 65, 70] and that altered microbial communities contribute to changes in host phenotypes [48], it is equally possible that common exposures such as a maternal high fat diet can render alterations in the maternal and offspring microbiome which render later susceptibility to environmental chemicals [recently summarized in. Therefore embracing microbes in exposure science would play a key role in illuminating the relationships among secondary perinatal factors (such as the maternal diet), concomitant or latter environmental chemical exposure, functional alterations in the human microbiome, and fetal programming rendering risk of later in life disease.
Special Considerations: Natural Disasters
As previously noted, where we live and work can significantly determine the burden of environmental exposures we encounter. However, something that can drastically change this burden is the occurrence of a natural disaster which further disrupts environmental chemicals deposition in the environment and renders increased human exposure susceptibility. From disasters such as flooding in urban populations (i.e. from Hurricanes Katrina and Harvey) we know that the local microbial ecosystem is drastically modified [71, 72]. Flooded homes become vulnerable to molds and myotoxins [73, 74], which further risk disruption of the microbial community. In parallel with these potential microbial disruptions is the risk of increasing the load and intensity of environmental chemical exposures either as a result of leaching of ground deposits, compromise of mitigation efforts, or increased inhalation, ingestion and/or absorption. Moreover, both immediate and lasting clean-up and remediation efforts may negatively affect air quality increasing the risk of adverse environmental exposure burden via inhalation [75]. The end sequelae of these events have been partially measured in limited studies. For example, otherwise healthy individuals exposed to flood waters are at significant risk for skin infections [76], including those with pathogenic multi-drug resistant bacteria [77]. Exposed children are at an increased risk for upper and lower respiratory infections and reactive airway disease [78]. Whether this is a direct result of increased environmental irritants, the result of disrupted microbiome ecology, or a combination of the two remains to be determined.
With regards to environmental exposures per se, flooding is able to redistribute soil and water contaminants such as PAHs and toxic metals. This was measured as seen in the aftermath of Hurricane Harvey, which flooded the greater Houston area in August of 2017 [79, 80]. During Harvey, 51 inches of rain fell over a span of two days and flash flooding drove over 100,000 evacuees through contaminated floodwaters following unprecedented residential home damage. How exposures to these floodwaters, and their associated contaminants have affected population health are still being studied and remain largely unexplored. However, a study from our lab constituting of over 40,000 deliveries occurring before, during and after Hurricane Harvey revealed that pregnant women and their infants had a higher likelihood of adverse outcomes if they delivered after landfall of the Hurricane [81]. We continue to study a longitudinal cohort to determine specific changes to the maternal and neonatal microbiomes from exposure to the floodwaters of Harvey, and how this is further associated with maternal and neonatal outcomes (ClinicalTrials.gov Identifier: NCT02392650). Suffice it to say, with the ongoing threat of climate change being realized as increased flooding in coastal communities, it is of imminent need to be understood what the risks are, how and why they are conferred on human health, and what potential mitigations may be of benefit.
Future Directions
We are in an exciting and impactful time in our understanding of the role human play on the environment, and how the environment in turn affects human health. As our working knowledge increases, we are beginning to understand the potential limitations to mitigation efforts. As is only too often the case with respect to these deepening understandings of the role human activity plays on negatively impacting our increasingly fragile environment, the time for focused efforts with a sense of crisis and urgency has arrived. Considering that environmental exposures interact with and modify the microbiome, and that the microbiome interacts and modifies environmental chemicals, we believe that there is no more important opportunity for practical and relevant translational research than perinatal environmental microbial health. We can imagine no more important activity than interrogations which are dedicated and committed to understanding if, how, and when there are opportunities for correcting the health of the current and coming generations in response to environmental exposures. Alternately, if these processes are largely irreversible, then we must ardently undertake large scale efforts to mitigate these exposures at a local, regional and global levels. The very health of not only our species but nearly every living and reproducing species depends on such efforts.
Acknowledgments
The authors acknowledge funding from the NIH to support this work (HD075858 to MAS and P42ES027725, P50MD015496, R01HD091731, R21 ES029462 & R01DK089201 to KMA)
Footnotes
The authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*special interest
**outstanding interest
- [1].Myers GJ, Davidson PW, Prenatal methylmercury exposure and children: Neurologic, developmental, and behavioral research, Environmental Health Perspectives 106 (1998) 841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sakamoto M, Kakita A, Wakabayashi K, Takahashi H, Nakano A, Akagi H, Evaluation of changes in methylmercury accumulation in the developing rat brain and its effects: A study with consecutive and moderate dose exposure throughout gestation and lactation periods, Brain Research 949(1–2) (2002) 51–59. [DOI] [PubMed] [Google Scholar]
- [3].Huang C-F, Liu S-H, Hsu C-J, Lin-Shiau S-Y, Neurotoxicological effects of low-dose methylmercury and mercuric chloride in developing offspring mice, Toxicology Letters 201(3) (2011) 196–204. [DOI] [PubMed] [Google Scholar]
- [4].Oliveira CS, Joshee L, Zalups RK, Pereira ME, Bridges CC, Disposition of inorganic mercury in pregnant rats and their offspring, Toxicology 335 (2015) 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Almazroo OA, Miah MK, Venkataramanan R, Drug metabolism in the liver, Clinics in Liver Disease 21(1) (2017) 1–20.* An in-depth review of drug metabolism in liver explaining the Phase I, II & III and the disease states and altered physiologic conditions can affect the efficiency of the drug metabolic or transport processes
- [6].Sbrana E, Suter MA, Abramovici AR, Hawkins HK, Moss JE, Patterson L, Shope C, Aagaard-Tillery K. Maternal tobacco use is associated with increased markers of oxidative stress in the placenta. Am J Obstet Gynecol. 2011. September;205(3):246.e1–7. doi: 10.1016/j.ajog.2011.06.023.*
- [7].Aagaard-Tillery K, Spong CY, Thom E, Sibai B, Wendel G Jr, Wenstrom K, Samuels P, Simhan H, Sorokin Y, Miodovnik M, Pharmacogenomics of maternal tobacco use: Metabolic gene polymorphisms and risk of adverse pregnancy outcomes, Obstetrics and Gynecology 115(3) (2010) 568.* References 6 and 7 demonstrate that oxidative stress in the placenta occurs with maternal smoking, and fetal growth restriction as a result of maternal smoking occurs with the fetus has a deletion polymorphysm in GSTT1. This suggests that both placental damage and direct fetal harm occur as a result of maternal inhalation of several potent environmental chemicals (such as PAHs) via smoking.
- [8].Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP, Gut Reactions: Breaking Down Xenobiotic–Microbiome Interactions, Pharmacological Reviews 71(2) (2019) 198–224. [DOI] [PubMed] [Google Scholar]
- [9].Das A, Srinivasan M, Ghosh TS, Mande SS, Xenobiotic metabolism and gut microbiomes, PLoS One 11(10) (2016) e0163099.* This review provides a focused and detailed description of the contribution of the gut microbiome in health and disease to xenobiotic metabolism emphasising on therapeutic interventions, pharmacological drug action, and chemical biotransformations that will collectively implict the future practice of precision medicine.
- [10].E. National Academies of Sciences, Medicine, Environmental chemicals, the human microbiome, and health risk: A research strategy, The National Academies Press, Washington, DC, 2018.* * This Consensus Study Report of The National Academy of Sciences, highlights key aspects of the human microbiome and its relation to health, describes potential interactions between environmental chemicals and the human microbiome, reviews the risk-assessment framework and reasons for incorporating chemical–microbiome interactions, and outlines its research strategy. It focuses on addressing questions about the interactions of environmental chemicals with the human microbiome and the implications for human health risk.
- [11].Onuzulu CD, Rotimi OA, Rotimi SO, Epigenetic modifications associated with in utero exposure to endocrine disrupting chemicals BPA, DDT and Pb, Reviews on Environmental Health 34(4) (2019) 309–325.* This review provides a brief overview of epigenetics and describe the various epigenetic mechanisms: DNA methylation, histone modifications and non-coding RNAs, and how each of them affects gene expression
- [12].Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ, The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism, Nature Reviews Microbiology 14(5) (2016) 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Claus SP, Guillou H, Ellero-Simatos S, The gut microbiota: A major player in the toxicity of environmental pollutants?, Npj Biofilms and Microbiomes 2 (2016) 16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tohyama C, Maternal exposure to environmental chemicals and health outcomes later in life, Pre-emptive Medicine: Public Health Aspects of Developmental Origins of Health and Disease, Springer; 2019, pp. 3–19. [Google Scholar]
- [15].Schug TT, Erlebacher A, Leibowitz S, Ma L, Muglia LJ, Rando OJ, Rogers JM, Romero R, vom Saal FS, Wise DL, Fetal programming and environmental exposures: Implications for prenatal care and preterm birth, Ann N Y Acad Sci 1276 (2012) 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Drwal E, Rak A, Gregoraszczuk EL, Review: Polycyclic aromatic hydrocarbons (PAHs)—Action on placental function and health risks in future life of newborns, Toxicology 411 (2019) 133–142. [DOI] [PubMed] [Google Scholar]
- [17].Ni W, Yang W, Jin L, Liu J, Li Z, Wang B, Wang L, Ren A, Levels of polycyclic aromatic hydrocarbons in umbilical cord and risk of orofacial clefts, Science of the Total Environment 678 (2019) 123–132. [DOI] [PubMed] [Google Scholar]
- [18].Sexton K, Salinas JJ, McDonald TJ, Gowen RM, Miller RP, McCormick JB, Fisher-Hoch SP, Polycyclic aromatic hydrocarbons in maternal and umbilical cord blood from pregnant Hispanic women living in Brownsville, Texas, International Journal of Environmental Research and Public Health 8(8) (2011) 3365–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grandjean P, Bellinger D, Bergman Å, Cordier S, Davey-Smith G, Eskenazi B, Gee D, Gray K, Hanson M, Van Den Hazel P, The faroes statement: Human health effects of developmental exposure to chemicals in our environment, Basic & Clinical Pharmacology & Toxicology 102(2) (2008) 73–75. [DOI] [PubMed] [Google Scholar]
- [20].Guo L-Q, Chen Y, Mi B-B, Dang S-N, Zhao D-D, Liu R, Wang H-L, Yan H, Ambient air pollution and adverse birth outcomes: a systematic review and meta-analysis, Journal of Zhejiang University-SCIENCE B 20(3) (2019) 238–252. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].Melody SM, Ford J, Wills K, Venn A, Johnston FH, Maternal exposure to short-to medium-term outdoor air pollution and obstetric and neonatal outcomes: A systematic review, Environmental Pollution 244 (2019) 915–925. [DOI] [PubMed] [Google Scholar]
- [22].Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A, Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges, Environmental Research 167 (2018) 144–159. [DOI] [PubMed] [Google Scholar]
- [23].Wang A, Padula A, Sirota M, Woodruff TJ, Environmental influences on reproductive health: The importance of chemical exposures, Fertility and Sterility 106(4) (2016) 905–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, Grove K, Lane R, Aagaard-Tillery K, Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates, The FASEB Journal 25(2) (2011) 714–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Singh S, Li SS-L, Epigenetic effects of environmental chemicals bisphenol a and phthalates, International Journal of Molecular Sciences 13(8) (2012) 10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Suter MA, Abramovici AR, Griffin E, Branch DW, Lane RH, Mastrobattista J, Rehan VK, Aagaard K, In utero nicotine exposure epigenetically alters fetal chromatin structure and differentially regulates transcription of the glucocorticoid receptor in a rat model, Birth Defects Research Part A: Clinical and Molecular Teratology 103(7) (2015) 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Faa G, Manchia M, Pintus R, Gerosa C, Marcialis MA, Fanos V, Fetal programming of neuropsychiatric disorders, Birth Defects Research Part C: Embryo Today: Reviews 108(3) (2016) 207–223. [DOI] [PubMed] [Google Scholar]
- [28].Heindel JJ, Skalla LA, Joubert BR, Dilworth CH, Gray KA, Review of developmental origins of health and disease publications in environmental epidemiology, Reproductive Toxicology 68 (2017) 34–48. [DOI] [PubMed] [Google Scholar]
- [29].Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B, Fetal programming of the metabolic syndrome, Taiwanese Journal of Obstetrics and Gynecology 56(2) (2017) 133–138. [DOI] [PubMed] [Google Scholar]
- [30].Kim K, Melough MM, Vance TM, Noh H, Koo SI, Chun OK, Dietary Cadmium intake and sources in the US, Nutrients 11(1) (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rinaldi M, Micali A, Marini H, Adamo EB, Puzzolo D, Pisani A, Trichilo V, Altavilla D, Squadrito F, Minutoli L, Cadmium, organ toxicity and therapeutic approaches: A review on brain, kidney and testis damage, Current Medicinal Chemistry 24(35) (2017) 3879–3893. [DOI] [PubMed] [Google Scholar]
- [32].Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, Rudisill C, Toxicological profile for cadmium, (2012). [PubMed]
- [33].Wier PJ, Miller RK, Maulik D, di Sant’Agnese PA, Toxicity of cadmium in the perfused human placenta, Toxicology and Applied Pharmacology 105(1) (1990) 156–171. [DOI] [PubMed] [Google Scholar]
- [34].Geng H-X, Wang L, Cadmium: Toxic effects on placental and embryonic development, Environmental Toxicology and Pharmacology (2019).* This review explicitly explains how the changes in the epigenetic modification patterns induced by cadmium exposure contributes to placental and fetal development.
- [35].Khoshhali M, Rafiei N, Farajzadegan Z, Shoshtari-Yeganeh B, Kelishadi R, Maternal exposure to Cadmium and fetal growth: A systematic review and meta-analysis, Biological Trace Element Research (2019) 1–11. [DOI] [PubMed] [Google Scholar]
- [36].Jacobo-Estrada T, Santoyo-Sánchez M, Thévenod F, Barbier O, Cadmium handling, toxicity and molecular targets involved during pregnancy: lessons from experimental models, International Journal of Molecular Sciences 18(7) (2017) 1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Duarte-Salles T, Mendez MA, Meltzer HM, Alexander J, Haugen M, Dietary benzo(a)pyrene intake during pregnancy and birth weight: Associations modified by vitamin C intakes in the Norwegian Mother and Child Cohort Study (MoBa), Environment International 60 (2013) 217–23. [DOI] [PubMed] [Google Scholar]
- [38].Langlois PH, Hoyt AT, Desrosiers TA, Lupo PJ, Lawson CC, Waters MA, Rocheleau CM, Shaw GM, Romitti PA, Gilboa SM, Malik S, Maternal occupational exposure to polycyclic aromatic hydrocarbons and small for gestational age offspring, Occup Environ Med 71(8) (2014) 529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Polanska K, Dettbarn G, Jurewicz J, Sobala W, Magnus P, Seidel A, Hanke W, Effect of prenatal polycyclic aromatic hydrocarbons exposure on birth outcomes: the Polish mother and child cohort study, Biomed Res Int 2014 (2014) 408939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Thakur VS, Liang YW, Lingappan K, Jiang W, Wang L, Barrios R, Zhou G, Guntupalli B, Shivanna B, Maturu P, Welty SE, Moorthy B, Couroucli XI, Increased susceptibility to hyperoxic lung injury and alveolar simplification in newborn rats by prenatal administration of benzo[a]pyrene, Toxicology Letters 230(2) (2014) 322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, Sowa A, Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children, Environ Sci Pollut Res Int (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lovasi GS, Eldred-Skemp N, Quinn JW, Chang HW, Rauh VA, Rundle A, Orjuela MA, Perera FP, Neighborhood social context and Iindividual polycyclic aromatic hydrocarbon exposures associated with child cognitive test scores, J Child Fam Stud 23(5) (2014) 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jedrychowski WA, Perera FP, Majewska R, Camman D, Spengler JD, Mroz E, Stigter L, Flak E, Jacek R, Separate and joint effects of tranplacental and postnatal inhalatory exposure to polycyclic aromatic hydrocarbons: Prospective birth cohort study on wheezing events, Pediatr Pulmonol 49(2) (2014) 162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang X, Li X, Jing Y, Fang X, Zhang X, Lei B, Yu Y, Transplacental transfer of polycyclic aromatic hydrocarbons in paired samples of maternal serum, umbilical cord serum, and placenta in Shanghai, China, Environmental Pollution 222 (2017) 267–275. [DOI] [PubMed] [Google Scholar]
- [45].Henkler F, Stolpmann K, Luch A, Exposure to polycyclic aromatic hydrocarbons: Bulky DNA adducts and cellular responses, Molecular, Clinical and Environmental Toxicology, Springer; 2012, pp. 107–131. [DOI] [PubMed] [Google Scholar]
- [46].Shimada T, Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons, Drug metabolism and pharmacokinetics 21(4) (2006) 257–276. [DOI] [PubMed] [Google Scholar]
- [47].Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM, Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery, Nature medicine 23(3) (2017) 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, Blundell P, Harris RA, Frias AE, Grove KL, High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model, Nature Communications 5 (2014) 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pace RM, Prince AL, Ma J, Belfort BD, Harvey AS, Hu M, Baquero K, Blundell P, Takahashi D, Dean T, Modulations in the offspring gut microbiome are refractory to postnatal synbiotic supplementation among juvenile primates, BMC microbiology 18(1) (2018) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Suter MA, Aagaard KM, Coarfa C, Robertson M, Zhou G, Jackson BP, Thompson D, Putluri V, Putluri N, Hagan J, Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB), Biochemical and Biophysical Research Communications (2019).* * This study is the first report showing an association between PAH levels, DNA adducts, and modulation of endogenous metabolic pathways with preterm births in subjects residing near Superfund sites.
- [51].Martin EM, Fry RC, Environmental influences on the epigenome: Exposure-associated DNA methylation in human populations, Annual Review of Public Health 39 (2018) 309–333. [DOI] [PubMed] [Google Scholar]
- [52].Takiguchi M, Achanzar WE, Qu W, Li G, Waalkes MP, Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation, Experimental Cell Research 286(2) (2003) 355–365. [DOI] [PubMed] [Google Scholar]
- [53].Boeke CE, Baccarelli A, Kleinman KP, Burris HH, Litonjua AA, Rifas-Shiman SL, Tarantini L, Gillman M, Gestational intake of methyl donors and global LINE-1 DNA methylation in maternal and cord blood: Prospective results from a folate-replete population, Epigenetics 7(3) (2012) 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kippler M, Engström K, Mlakar SJ, Bottai M, Ahmed S, Hossain MB, Raqib R, Vahter M, Broberg K, Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight, Epigenetics 8(5) (2013) 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Herbstman JB, Tang D, Zhu D, Qu L, Sjödin A, Li Z, Camann D, Perera FP, Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA Adducts, and genomic DNA methylation in cord blood, Environmental Health Perspectives 120(5) (2012) 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Perera F, Tang W.-y., Herbstman J, Tang D, Levin L, Miller R, Ho S.-m., Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma, PloS One 4(2) (2009) e4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Miller RL, Yan Z, Maher C, Zhang H, Gudsnuk K, McDonald J, Champagne FA, Impact of prenatal polycyclic aromatic hydrocarbon exposure on behavior, cortical gene expression, and DNA methylation of the Bdnf gene, Neuroepigenetics 5 (2016) 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fazeli M, Hassanzadeh P, Alaei S, Cadmium chloride exhibits a profound toxic effect on bacterial microflora of the mice gastrointestinal tract, Human & Experimental Toxicology 30(2) (2011) 152–9. [DOI] [PubMed] [Google Scholar]
- [59].Breton J, Daniel C, Dewulf J, Pothion S, Froux N, Sauty M, Thomas P, Pot B, Foligne B, Gut microbiota limits heavy metals burden caused by chronic oral exposure, Toxicology Letters 222(2) (2013) 132–8. [DOI] [PubMed] [Google Scholar]
- [60].Van de Wiele T, Vanhaecke L, Boeckaert C, Peru K, Headley J, Verstraete W, Siciliano S, Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites, Environ Health Perspect 113(1) (2005) 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jašarević E, Bale TL, Prenatal and postnatal contributions of the maternal microbiome on offspring programming, Frontiers in Neuroendocrinology (2019) 100797. [DOI] [PubMed] [Google Scholar]
- [62].Li Y, Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring, Frontiers in Genetics 9(342) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kadawathagedara M, Maternal diet during pregnancy and early growth: Focus on diet duality and food chemicals exposure, Food and Nutrition, Université Paris-Saclay, 2018. [Google Scholar]
- [64].Pevsner-Fischer M, Zmora N, Braverman S, Elinav E, Epigenetics and the microbiome, Handbook of Nutrition, Diet, and Epigenetics (2019) 79–103. [Google Scholar]
- [65].Cortese R, Lu L, Yu Y, Ruden D, Claud EC, Epigenome-microbiome crosstalk: A potential new paradigm influencing neonatal susceptibility to disease, Epigenetics 11(3) (2016) 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Prince AL, Pace RM, Dean T, Takahashi D, Kievit P, Friedman JE, Aagaard KM, The development and ecology of the Japanese macaque gut microbiome from weaning to early adolescence in association with diet, American Journal of Primatology (2019) e22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Maher SE, O’Brien EC, Moore RL, Byrne DF, Geraghty AA, Saldova R, Murphy EF, Van Sinderen D, Cotter PD, McAuliffe FM (2020) The association between the maternal diet and the maternal and infant gut microbiome: a systematic review. BJN. 4: 1–29 [DOI] [PubMed] [Google Scholar]
- [68].Estarlich M, Ballester F, Davdand P, Llop S, Esplugues A, Fernández-Somoano A, Lertxundi A, Guxens M, Basterrechea M, Tardón A, Exposure to ambient air pollution during pregnancy and preterm birth: A Spanish multicenter birth cohort study, Environmental Research 147 (2016) 50–58. [DOI] [PubMed] [Google Scholar]
- [69].Padula AM, Noth EM, Hammond SK, Lurmann FW, Yang W, Tager IB, Shaw GM, Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth, Environmental Research 135 (2014) 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Licht TR, Bahl MI, Impact of the gut microbiota on chemical risk assessment, Current Opinion in Toxicology 15 (2019) 109–113. [Google Scholar]
- [71].Amaral-Zettler LA, Rocca JD, Lamontagne MG, Dennett MR, Gast RJ, Changes in microbial community structure in the wake of Hurricanes Katrina and Rita, Environmental Science & Technology 42(24) (2008) 9072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sinigalliano CD, Gidley M, Shibata T, Whitman D, Dixon T, Laws E, Hou A, Bachoon D, Brand L, Amaral-Zettler L, Impacts of Hurricanes Katrina and Rita on the microbial landscape of the New Orleans area, Proceedings of the National Academy of Sciences 104(21) (2007) 9029–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bloom E, Grimsley LF, Pehrson C, Lewis J, Larsson L, Molds and mycotoxins in dust from water-damaged homes in New Orleans after hurricane Katrina, Indoor Air 19(2) (2009) 153–8. [DOI] [PubMed] [Google Scholar]
- [74].Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, Muilenberg ML, Thorne PS, Dearborn DG, Morley RL, Mold and endotoxin levels in the aftermath of Hurricane Katrina: A pilot project of homes in New Orleans undergoing renovation, Environ Health Perspect 114(12) (2006) 1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ravikrishna R, Lee HW, Mbuligwe S, Valsaraj KT, Pardue JH, Air quality during demolition and recovery activities in post-Katrina New Orleans, Environmental Toxicology and Chemistry 29(7) (2010) 1438–44. [DOI] [PubMed] [Google Scholar]
- [76].Noe R, Cohen AL, Lederman E, Gould LH, Alsdurf H, Vranken P, Ratard R, Morgan J, Norton SA, Mott J, Skin disorders among construction workers following Hurricane Katrina and Hurricane Rita: An outbreak investigation in New Orleans, Louisiana, Archives of Dermatology 143(11) (2007) 1393–8. [DOI] [PubMed] [Google Scholar]
- [77].Seybold U, White N, Wang YF, Halvosa JS, Blumberg HM, Colonization with multidrug-resistant organisms in evacuees after Hurricane Katrina, Infect Control Hosp Epidemiol 28(6) (2007) 726–9. [DOI] [PubMed] [Google Scholar]
- [78].Rath B, Young EA, Harris A, Perrin K, Bronfin DR, Ratard R, Vandyke R, Goldshore M, Magnus M, Adverse respiratory symptoms and environmental exposures among children and adolescents following Hurricane Katrina, Public Health Repository 126(6) (2011) 853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bera G, Camargo K, Sericano J, Liu Y, Sweet S, Horney J, Jun M, Chiu W, Rusyn I, Wade T, Baseline data for distribution of contaminants by natural disasters: Results from a residential Houston neighborhood during Hurricane Harvey flooding, Heliyon 5(11) (2019) e02860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Horney JA, Casillas GA, Baker E, Stone KW, Kirsch KR, Camargo K, Wade TL, McDonald TJ, Comparing residential contamination in a Houston environmental justice neighborhood before and after Hurricane Harvey, PloS One 13(2) (2018) e0192660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mendez-Figueroa H, Chauhan SP, Tolcher MC, Shamshirsaz AA, Sangi-Haghpeykar H, Pace RM, Chu DM, Aagaard K, Peripartum outcomes before and after hurricane Harvey, Obstetrics & Gynecology 134(5) (2019) 1005–1016.* * This is an in-depth report of maternal and neonatal data from more than 3,800 gravid women and their offspring exposed to Hurricane Harvey landfall who were found to significantly be more likely to have adverse outcomes as were their neonates compared with women delivering before the storm’s landfall.