Abstract
This Commentary discusses various aspects around the controversial issue of SARSCoV-2 and aerosol transmission, highlighting certain counter arguments and explaining why they are invalid.
Keywords: Severe acute respiratory syndrome coronavirus-2, SARS-CoV-2, Coronavirus disease 2019, Covid-19, Transmission, Aerosol, Airborne infection control, Epidemiology
An article by Cheng et al. (2020) on the effectiveness of universal masking in Hong Kong to control the spread of COVID-19 (coronavirus disease 2019) highlights the recent, very public change in guidance over the wearing of masks in public places by multiple agencies and countries. This runs in parallel with another, perhaps even larger ongoing debate – whether or not SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the cause of COVID-19, is transmitted via aerosols.
In this context, ‘aerosols’ mean fine droplets that can stay suspended in the air for long periods that are able to be carried over longer distances (greater than 2 m), but which can also transmit the virus over shorter distances (over 1 m or less). Traditionally, such fine droplets were classified to lie below a 5 μm diameter cut-off, but this is now considered to be only a relative threshold and much of the suspension and transport of such fine respiratory droplets (or ‘microdroplets’) will depend on the ambient airflow in each situation (Tellier et al., 2019).
Recent articles by various scientists, which have been covered extensively by various media, have highlighted the debate around this SARS-CoV-2 aerosol transmission issue - particularly with the WHO (World Health Organization) (Morawska et al., 2020; Morawska and Milton, 2020). The WHO have issued online guidance and tweets stating that SARS-CoV-2 is not airborne – except when certain so-called ‘AGPs’ (aerosol-generating procedures) are being performed. These are medical procedures, including bronchoscopy, intubation, nebulization, suctioning and tracheostomy (World Health Organization (WHO), 2020).
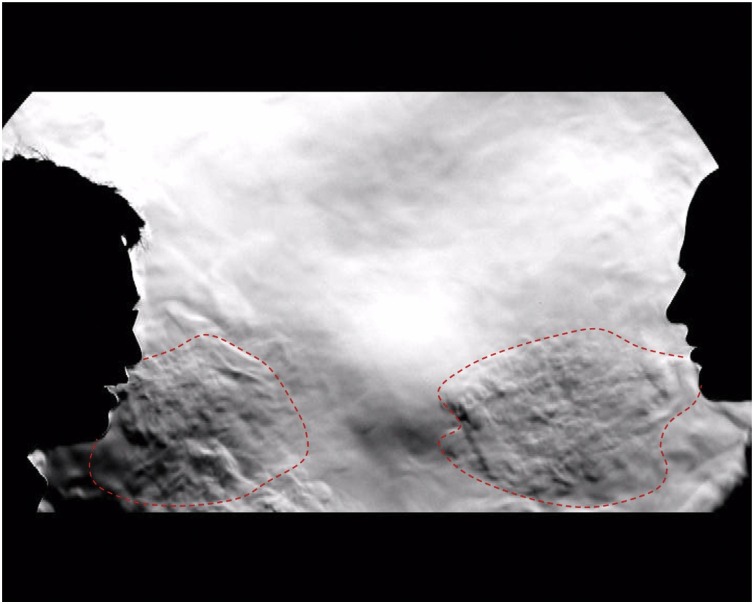
But surely are not the much more common, naturally produced human exhalations like breathing, talking, coughing, sneezing, laughing and singing also aerosol-generating procedures (Cheng et al., 2020; Tang et al., 2011) (Fig. 1 )? If not, why not? Here the reasoning and interpretation of the evidence becomes much more subjective and unclear – and many people are confused.
Fig. 1.
A schlieren image of two people talking approximately 1 m apart (adapted and annotated by the author, from reference (Tang et al. (2011))). Note the exhaled airflows produced by both individuals (indicated by dotted red lines), which quickly move across the gap between them, can be inhaled by the opposing individual. Essentially: ‘If I can smell the garlic on your breath, then I must be inhaling some of your exhaled air – and any viruses carried within it’. Full schlieren/shadowgraph videos of talking breathing, coughing, sneezing, laughing and singing are available at: https://www.youtube.com/watch?v=MaYcjyU6XT8&list=PL8pE_CuHoXJXZExcWwk_0tsqjT2Ydwsxg&index=1.
One of the main arguments against the aerosol transmission of SARS-CoV-2 is an example of ‘inaccurate comparison’, i.e. comparing apples with oranges.
The argument goes that if SARS-CoV-2 was spread via these naturally produced human aerosols, we should be seeing many more COVID-19 cases – like we do with measles and chickenpox, which are recognised as airborne infections with R0 values of 10–17 (Tang et al., 2006). The R0 or basic reproductive number is the number of secondary cases of a disease generated when a single index case arises in a population of uniformly distributed susceptible individuals. This has been discussed a lot in the press recently, especially in relation to when it might be safe to relax lockdown measures. If the R0 is less than 1, then it means that the epidemic is being brought under control, but if greater than 1, it means that the epidemic is continuing to spread.
There are a couple of problems with this argument. Firstly, with measles and chickenpox, these are systemic infections that produce a very distinct febrile rash illness with very few asymptomatic or mildly symptomatic cases. Therefore, secondary cases of these infections (as generated by an index case) are very easy to detect, count and isolate.
This is not the case with COVID-19, where there is substantial evidence with many reports of asymptomatic or mild illness that may be missed and not counted, making under-diagnosis a significant problem (Long et al., 2020). Various epidemiological models that have been developed during the COVID-19 pandemic have recognised and included this as an unknown and potentially rather large variable in their estimates (Imperial College London COVID-19 Response Team, 2020). Even serosurveys are likely underestimating the full extent of COVID-19 cases because it is emerging that many infected cases may not produce antibodies, those that do may not retain them for very long (Long et al., 2020), and the various serology assays (particularly the rapid tests) are of widely differing sensitivities (Ong et al., 2020).
Thus the R0 value may be much larger than the currently accepted value of around 1.5–2.0, based on the numbers of confirmed COVID-19 cases.
Yet, even with a lower current R0 value this does not exclude the short-range (‘conversational’) aerosol route of infection, which brings us to the second point. Those who are advocating the aerosol transmission for SARS-CoV-2 are not saying that this necessarily indicates that longer-range transmission is predominant, and this is not the pattern that we should necessarily be expecting to see.
Even short-range aerosol transmission (i.e. within a typical 1 m conversational distance) is possible and very likely more common in everyday social interactions. This is where the benefits of surgical masks and face coverings becomes manifest. Although these physical barriers will not block all SARS-CoV-2 aerosols, if they are worn by everyone, they act to contain the virus at source in those infected (including in the asymptomatic and undiagnosed), as well as blocking a fraction of incoming aerosols towards those who are susceptible (Makison Booth et al., 2013). Thus, whilst masking will not reduce exposure to zero, it has incremental benefits that, in combination with other measures, will reduce further reduce the risk of infection.
A recent article has highlighted some of the thinking used to counter the belief that SARS-CoV-2 is transmitted by aerosols (Morawska and Milton, 2020), where much hinges around various forms of the phrase “insufficient evidence to demonstrate airborne transmission”, without going into detail about exactly how much evidence and in what form would be sufficient. This has been a problem throughout this COVID-19 pandemic where those stating that “no evidence exists for ….” do not then define the nature of the evidence they will accept to then recommend any specific intervention, such as the use of face masks. This lack of clarity has hampered and delayed the release, supply and use of such interventions early in the pandemic, which would have protected thousands of healthcare workers looking after COVID-19 patients.
As the SARS-CoV-2/COVID-19 pandemic continues into the influenza season, the biggest fear now is that healthcare teams will need to cope with patients being admitted with one or both of these viral infections (UK Government, 2020a)– a proportion of whom will be requiring intensive care beds. This SARS-CoV-2 aerosol transmission debate will no doubt rage on, but now we are seeing Western healthcare teams requiring to mask in all clinical areas, which will improve their protection whilst caring for COVID-19 patients (UK Government, 2020b). Even if institutions like the WHO do not formally admit that SARS-CoV-2 is airborne, the very public discussions around this mean that such hospital teams are now better informed on how to best to protect their staff and patients, with the available resources.
Author’s statement
The sole author, Julian W Tang, conceived, wrote and submitted this article as Corresponding author. The Fig. 1 shows an image from one of his earlier studies, as described and referenced in the Figure legend.
References
- Cheng V.C., Wong S., Chuang V.W., et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020;81(1):107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial College London COVID-19 Response Team . 2020. Report 4: Severity of 2019-novel Coronavirus (nCoV)https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-02-10-COVID19-Report-4.pdf 10 February 2020. [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020 doi: 10.1038/s41591-020-0965-6. Jun 18. [DOI] [PubMed] [Google Scholar]
- Makison Booth C., Clayton M., Crook B., Gawn J.M. Effectiveness of surgical masks against influenza bioaerosols. J. Hosp. Infect. 2013;84(1):22–26. doi: 10.1016/j.jhin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa939. 6 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Tang J.W., Bahnfleth W., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D.S.Y., de Man S.J., Lindeboom F.A., Koeleman J.G.M. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.05.028. Jun 2:S1198-743X(20)30305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Nicolle A.D., Pantelic J., et al. Qualitative real-time schlieren and shadowgraph imaging of human exhaled airflows: an aid to aerosol infection control. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Li Y., Eames I., Chan P.K., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006;64(2):100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Government . 2020. Health Matters: Delivering the Flu Immunisation Programme During the COVID-19 Pandemic. 29 September 2020. https://www.gov.uk/government/publications/health-matters-flu-immunisation-programme-and-covid-19/health-matters-delivering-the-flu-immunisation-programme-during-the-covid-19-pandemic (Accessed 21 October, 2020) [Google Scholar]
- UK Government . 2020. Face Masks and Coverings to Be Worn by All NHS Hospital Staff and Visitors. 5 June 2020. https://www.gov.uk/government/news/face-masks-and-coverings-to-be-worn-by-all-nhs-hospital-staff-and-visitors (Accessed 21 October, 2020) [Google Scholar]
- World Health Organization (WHO) 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. 29 March 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (Accessed July 5, 2020) [Google Scholar]