Abstract
The most common liver disease in developing countries is non-alcoholic fatty liver disease (NAFLD). This involves the abnormal accumulation of lipids in the liver, the pathogenesis of the disease being related to dyslipidemia, obesity, insulin resistance and type 2 diabetes. Most often, the diagnosis of NAFLD is incidental, when performing routine blood tests or when performing a transabdominal ultrasound. The NAFLD spectrum ranges from simple forms of hepatic steatosis to the most advanced form of the disease, steatohepatitis (NASH), which in evolution can cause inflammation, fibrosis, cirrhosis of the liver and even liver cancer. For the evaluation of the prognosis and the clinical evolution, the most important parameter to define is the degree of liver fibrosis. Currently, the gold standard remains the liver biopsy, the differentiation between NAFLD and NASH being made only on the basis of histological analysis. However, liver biopsy is an invasive procedure, with numerous risks such as bleeding, lesions of the other organs and complications related to anesthesia, which significantly reduces its widespread use. Moreover, the risk of a false negative result and the increased costs of the procedure further limits its use in current practice. For this reason, non-invasive methods of evaluating the degree of liver fibrosis have gained ground in recent years. Imaging techniques such as elastography have shown promising results in evaluating and staging NAFLD. The aim of this article is to review the current status of the non-invasive tests for the assessment of NAFLD with a focus on the ultrasound-based elastography techniques.
Keywords: Fatty liver disease, NAFLD, elastography, ultrasound NAFLD
Introduction
Hepatic steatosis is defined as the accumulation of fat in hepatocytes, comprising more than 5% of the total weight of the liver [1].
As the global incidence of obesity is increasing, the incidence of liver steatosis has also increased, representing the most common cause of liver disease in Western countries [2].
In addition to obesity, this liver disease is closely linked to a wide range of metabolic comorbidities such as type 2 diabetes, dyslipidemia, high blood pressure.
At the same time, hepatic steatosis is also a common etiology for advanced liver diseases such as liver cirrhosis and hepatocellular carcinoma [3].
Depending on the risk factors, hepatic steatosis can be classified as: alcoholic fatty liver disease (AFLD) related to alcohol consumption and non-alcoholic fatty liver disease (NAFLD) related to obesity and metabolic syndrome [4].
NAFLD can be divided according to the histological appearance into 2 simplified subcategories: simple steatosis (presence of excess fat, no inflammation or cell damage) and steatohepatitis (NASH-presence of inflammation and cell damage, with or without fibrosis) [5].
NAFLD is an important cause of chronic liver injury with a prevalence of 20-50% worldwide [5].
As it affects approximately 30% of the population, accounting for almost 100 million people [6], it is important to estimate the degree of liver steatosis and fibrosis in order to determine the optimal treatment, as well as prognosis and surveillance of the disease.
At this moment, liver biopsy is still the only reliable “gold standard” for staging the severity of liver fibrosis.
However, this method has great costs, it is invasive and associated with possible risks and complications.
In addition, liver biopsy limitations include sampling error, as well as intra-observer and inter-observer variability [7].
These limitations have driven attention on the non-invasive imaging methods for the diagnose and staging of NAFLD.
Non-invasive alternatives include serum biomarkers, conventional ultrasound, ultrasound elastography, magnetic resonance elastography, and magnetic resonance-based fat quantitation techniques [8].
The first imaging modality used for the screening of suspected NAFLD and which can evaluate fatty liver is conventional ultrasound.
But in order to characterize liver stiffness, ultrasound elastography seems a promising non-invasive tool.
Ultrasound elastrography includes transient elastography and imaging-based elastography techniques.
Transient elastography (FibroScan) was the first ultrasound tool introduced to measure liver stiffness, mainly for chronic liver disease but recent studies have demonstrated increasing liver stiffness with advanced fibrosis for patients with NAFLD [9,10,11].
However, present limitations of transient elastography include ascites, whilst it is associated with unreliable results in obese patients as fat can attenuate elastic waves [12].
We summarize the non-imaging and ultrasound-based imaging methods for the non-invasive assessment of NAFLD.
Non-Invasive Tests
Serum markers
Routine biochemical tests
Serum transaminases, AST and ALT, are the most commonly used biomarkers as indicators of nonspecific hepatocellular injury [13].
Moderately elevated levels of ALT were identified in patients hospitalized with NASH, but not exceeding four times the normal value [14,15].
Regarding AST, it is considered that values twice as high as normal are an indicator of severity of liver fibrosis [16].
However, there is also a percentage of approximately 10% of patients with NASH with normal levels of AST and ALT [17].
Predictors of elevated ALT levels were identified as male gender, young age, increased abdominal circumference, alcohol consumption, and serum ALT levels and were positively correlated with triglycerides, fasting blood sugar, BMI, suggesting that there is a positive association with the signs and symptoms of the metabolic syndrome [18,19].
Also, the AST/ALT ratio is commonly used by clinicians to distinguish alcoholic hepatitis from NAFLD and to predict hepatic fibrosis.
It is considered that an AST/ALT ratio greater than 2 is suggestive for alcoholic hepatitis, while ALT levels above AST levels could be an indicator for NASH in the absence of cirrhosis [20].
The literature data on the link between NAFLD and the modified values of transaminases, in particular ALT, are contrasting.
Some studies find that ALT values cannot be used for the prediction or diagnosis of steatosis/fibrosis, as there is no clear link between ALT dynamics and histological changes (steatosis, inflammation, fibrosis) [21,22].
Several studies evaluated the percentage of patients with NAFLD who had normal transaminase values, the data being shown in Figure 1 [23,24,25,26].
Figure 1.
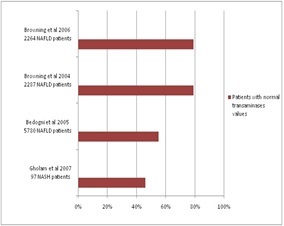
Studies that evaluated the percentage of patients with NAFLD with normal transaminase values
On the other hand, there are studies that support the link between the presence of steatohepatitis and the increased levels of ALT, whilst others suggest that this marker may independently predict liver fibrosis [27,28].
Studies in patients with type 2 diabetes or in obese patients have shown that elevated ALT values correlate with the risk of developing NAFLD, being the only variable that is independently associated with steatohepatitis [26,29,30].
Gamma-glutamyl transferase (GGT) may also have moderately elevated values simultaneously with increased serum transaminases.
A study of 193 non-diabetic patients with metabolic syndrome identified increased levels of GGT, alongside with increased levels of transaminases and triglycerides in patients identified with NAFLD [31].
Another study in a cohort of 90 patients with NAFLD proven by liver biopsy, found that a GGT value of over 96.5U/l is predictive for advanced fibrosis, with a sensitivity of 83% and a specificity of 69% [32].
Although the diagnosis of NAFLD/NASH cannot be formulated based solely on serum alkaline phosphatase (ALP) levels, it may be another biomarker associated with changes in steatohepatitis.
A study including 135 patients with liver biopsy proven NAFLD indicated that elevated ALP serum levels, associated with other risk factors for hepatic steatosis, could be an indicator of steatohepatitis [33].
Furthermore, a study in patients with type 2 diabetes suggested that increased levels of ALP are a risk factor for the development of liver fibrosis in patients with NASH [34].
Inflammatory markers
It is known that thrombocytopenia is common in advanced stages of chronic liver disease, most often in the stage equivalent to cirrhosis.
Fibrosis is also found in patients with advanced-stage NAFLD, a study from Japan that included 9 hepatology centers, showed that platelet values decrease as fibrosis lesions progress [35].
Moreover, they proposed as an optimum value, validated in multiple trials, 192 000 for stage 3 fibrosis with a sensitivity of 62.7% and a specificity of 76.3% and 153 000 for cirrhotic stage, with a sensitivity of 80.5% and a specificity of 88.8%. [35].
C-reactive protein (CRP) is an acute-phase reactant widely used for the diagnosis and evaluation of numerous pathologies.
This biomarker is mainly synthesized in the liver, but it also appears to occur in adipose tissue, a fact supported by the increased levels of CRP that are associated with obesity, particularly central type obesity, metabolic syndrome and diabetes [36].
In recent years, highly sensitive CRP (hs-CRP) has been evaluated in numerous studies as a diagnostic tool for the differentiation of NAFLD from NASH but also for the severity of liver fibrosis in patients with NASH [37,38].
Thus, several studies have demonstrated consistent increased hs-CRP levels in patients with NASH compared to those with simple steatosis [37,39,40].
Other authors also showed that patients with more severe NASH (Grade 2 and 3) have higher concentrations of hs-CRP than those with mild and moderate grades [41].
Regarding this marker, however, there is contradictory data in the literature, the hypothesis being contradicted by some authors who argue that hs-CRP is not useful in predicting histological changes in NAFLD or in clearly differentiating steatosis changes from steatohepatitis [42,43,44].
Although data on this biomarker is promising for steatohepatitis screening [41], it also has some drawbacks: a cut-off value for NAFLD has not been established and serum levels may be influenced by a number of factors such as age, sex, race, smoking and alcohol use [45,46].
Another biomarker that has been studied in an attempt to develop non-invasive diagnostic methods for NAFLD/NASH is serum ferritin.
It was observed that in patients with NAFLD, serum ferritin had elevated values in 20-50% of patients and transferrin in 5-10% of the cases [14].
One study evaluated 628 patients with NAFLD confirmed by liver biopsy and determined that any increase in serum ferritin above 300ng/mL in women and over 450ng/mL in men was associated with more severe histological changes in NAFLD and may represent an independent predictor for advanced fibrosis [47].
The results of the study thus indicate that serum ferritin is useful in identifying patients with NAFLD who are at risk for disease progression [47].
In another study, elevated serum ferritin levels have been found in patients with NAFLD, considering it a predictor of fibrosis, but with moderate specificity and sensitivity of 60% and 65%, respectively [48].
A study from Korea that included 25,597 participants, based on fatty liver score (NLFD) and hepatic steatosis index (HSI), attempted to identify the usefulness of serum ferritin in identifying patients with NAFLD. [49].
One of the findings of the study is that any increase in serum ferritin by 10ng/mL may increase the risk of developing NAFLD by up to 10% [49].
Data from the aforementioned studies is also supported by a recent meta-analysis that indicated that NAFLD is associated with increased levels of serum ferritin, which are higher in NASH as compared with simple steatosis [50].
A number of markers produced by adipose tissue have been studied for their potential correlation with NAFLD/NASH due to their role in inflammation and obesity.
These include adipocytokines, adiponectin and leptin.
Adipocytokines play a role in regulating inflammation, with some authors considering that they may influence the development of NAFLD [51], thus being considered as a potential index for advanced stages of NAFLD [52].
Adiponectin is a protein with anti-inflammatory properties [53], whose high values are associated with hepatic steatosis, while lower values have been identified in patients with steatohepatitis [54,55].
Leptin is another protein secreted by adipocytes that is thought to be associated with NAFLD.
Several studies have shown that increased levels of leptin can be found in patients with steatosis and steatohepatitis but are not associated with advanced lesions like fibrosis [56], whereas the data were contrasting in other studies that found no association between the two. [30,57].
Structural markers
In the pathogenesis of NAFLD, it is considered that hepatic cell apoptosis occurs in advanced stages, leading to the activation of some enzymes, including caspases, which in turn cleave different structures within the cell, cytokeratin-18 (CK18) being one of these [58].
CK18 and its fragments have been intensively studied and evaluated in recent years for the possible value they may have in screening patients with NAFLD and indirect prediction of NASH.
A multicenter study, which included 139 patients with biopsy-proven NAFLD, showed that cytokeratin-18 can be used in clinical practice as an indicator of NASH, with a specificity of over 90% or to exclude the presence NASH with a sensitivity of approximately 80% [59].
The data was supported by other studies that have shown an increased accuracy in differentiating patients with NASH from those with simple steatosis [60,61,62].
Although a biomarker validated in multiple studies, CK18 also has some limitations related to availability in clinical practice (it is not a routine laboratory test) and the cut-off values are not clearly established [63,64,65].
Another possible indicator of the histological lesions in patients with NAFLD is hyaluronic acid, a component of the extracellular matrix.
Studies investigating its role and correlation with NAFLD have focused on the serum hyaluronic acid levels in patients with steatohepatitis and fibrosis.
A recent study, which evaluated several potentially useful biomarkers in NASH prediction, identified hyaluronic acid as the most useful in predicting fibrosis, especially for advanced fibrosis (AUROC 0.77) [66].
The data was similar in other studies, hyaluronic acid being useful for estimating severe fibrosis with an AUROC of 0.90 [67] and 0.885 [68], respectively.
Ultrasound based imaging methods
Conventional ultrasound is the most commonly used imaging method for screening and initial assessment of NAFLD because it is accessible, inexpensive and noninvasive [69].
For the vast majority of patients with persistently high levels of serum transaminases, ultrasonography has an increased sensitivity for the diagnosis of hepatic steatosis if it is found in >30% of hepatocytes (moderate to severe steatosis) [70,71].
Some authors consider that the presence of over 33% liver fat is the optimal level for the ultrasonographic diagnosis of steatosis [72].
According to a meta-analysis from 2011, the sensitivity and specificity of ultrasonography for NAFLD detection is between 73.3-90.5% and 69.5-85.2%, respectively [73], having lower values in patients with morbid obesity, 49% and 75% respectively [74].
Although sensitivity and specificity increase with the degree of steatosis, ultrasonography cannot differentiate steatosis from more advanced steatohepatitis lesions [75].
Although a normal image cannot rule out the diagnosis of hepatic steatosis, there are some suggestive ultrasonographic changes for the diagnosis of NAFLD: increased liver volume in varying degrees, diffuse hyperechoic ("bright liver") structure, echogenicity greater than that of the kidney, posterior attenuation [76,77] (Figures 2,3).
Figure 2.
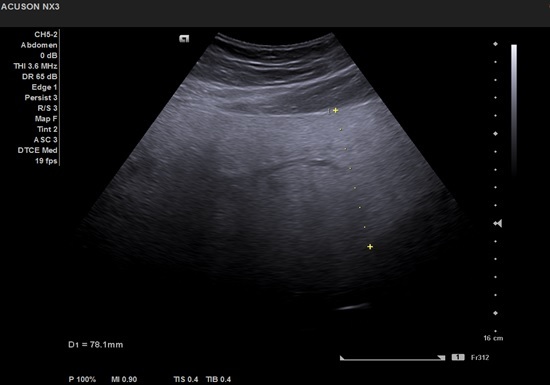
Posterior attenuation of the liver
Figure 3.
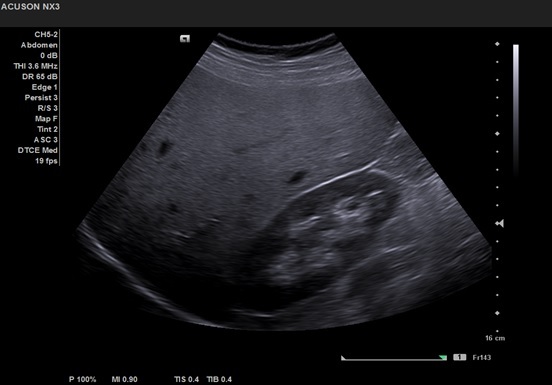
Liver echogenicity greater than that of the kidney
A group of 235 patients with NAFLD have been evaluated showing that the sensitivity, specificity, positive predictive value and negative predictive value for the diffuse hyperechoic structure were 91%, 93%, 89% and 94% respectively.
Also, when the aspect of "bright liver" and posterior attenuation were associated, the sensitivity, the specificity, the positive predictive value and the negative predictive value were 89.7%, 100%, 100% and 92.3% values respectively [71].
However, ultrasonography has certain limitations that cannot be ignored: [1] it is an operator-dependent procedure with considerable intra-and interobserver variability; [2] it cannot diagnose mild and moderate hepatic steatosis; [3] it cannot differentiate simple steatosis from steatohepatitis; [4] it cannot quantify steatosis; [5] it may have low sensitivity in patients with morbid obesity [72,78,79].
Quantitative and semiquantitative ultrasound assessment
As ultrasonographic diagnosis of NAFLD associates significant intra-and interobserver variability, an objective tool to quantify hepatic steatosis has been developed, with the aim of being operator-independent-hepato-renal index (HRI) [80].
This was calculated based on the relationship between the hepatic and the renal cortex using ultrasound histograms. IHR has proven useful in diagnosing mild steatosis as well, which could be omitted by ultrasonography. Thus, for the prediction of steatosis of more than 5%, HRI presented a sensitivity of 100% and a specificity of 91% as for more than 25% steatosis had a sensitivity and specificity of over 90% [80].
The validity of this index was also studied by other authors who confirmed its usefulness for the detection of patients with steatosis in a precise and easy way, whilst HRI has the potential to save 34% of patients to perform a useless liver biopsy [81,82,83].
One of the most studied techniques for liver fat quantification is controlled attenuation parameter (CAP), available on a vibration-controlled TE device (FibroScan, Echosens, Paris, France). CAP can estimate the attenuation of the ultrasound beam that crosses the liver tissues, reporting the results in units of decibel per meter (dB/m), values ranging from 100 to 400dB/m [84].
Several studies have evaluated the accuracy of this method to diagnose and distinguish hepatic steatosis grades, the cut-off values having no significant differences between different populations [85,86,87].
A recent prospective study identified steatosis as the only histopathological factor to influence CAP, with higher accuracy (over 80%) for steatosis grade 2 and 3 [87].
Studies that used liver biopsy to validate CAP have shown quite good accuracy for detecting any of the 3 grades of steatosis (S1 mild, S2 moderate, S3 severe).
One study included 261 patients with biopsy-proven NAFLD and established that a cut-off value of 310dB/m had a relatively good sensibility and specificity for S2, 79% and 71% respectively [88].
Another multicenter study which included 183 patients found similar accuracy in biopsy-proven NAFLD patients, with cut-off values of 247, 280 and 300dB/m for S1, S2 and S3 [85].
Regarding the probe used, studies found that there is similar accuracy for both XL and M probe, and thus, similar cut-off values can be used [89,90].
When compared to MRI imaging, CAP was inferior in detecting and grading steatosis, with higher AUROC values for MRI to identify S2 and S3 [91,92,93].
A recent meta-analysis which included 1297 patients with biopsy-proven NAFLD patients investigated the role of CAP as a substitute for liver biopsy.
The sensibility was over 80% for S1 and S2 with a specificity of 91% and 75% respectively, while severe steatosis had lower values, of 76% and 58% respectively, in contrast with earlier studies. [94].
The method is, however, dependent on the underlying disease, diabetes and BMI that may influence the results, thus limiting its clinical utility [95].
Another approach for objective quantification of hepatic steatosis is the use of convolutional neural networks (CNNs) for conventional ultrasonography imaging.
A recent study analyzing 550 images from 55 obese patients who were to undergo bariatric surgery, allowed extraction of features compatible with steatosis and then classified the images using deep learning.
All images were referenced by liver biopsy.
The results of the study showed that the approach using CNN is efficient and operator-independent, superior to other methods of ultrasound quantification (HRI, gray level co-occurrence matrix) [96].
Another study further evaluated the effectiveness of this method, validating its usefulness, thus obtaining a 90.6% accuracy for detecting liver steatosis [97].
Imaging elastography
Several techniques have been developed currently for the assessment of tissue elasticity, leading to an estimate of the stiffness degree, qualitatively or quantitatively.
Sonoelastographic techniques used for noninvasive evaluation of liver fibrosis have developed over the past 20 years, having a large spectrum of applications in numerous chronic liver diseases, including NAFLD.
There are two types of sonelastographic techniques: techniques based on tissue compression and deformation by internal or external compression-strain elastography (SE) and techniques based on measuring the propagation of a shear wave emitted by the ultrasonography probe-transient elastography (TE (Fibroscan®)), point shear wave (pSWE) elastography and 2D shear wave elastography (2D-SWE) [98].
Transient elastography (Fibroscan® Echosens, Paris, France) is the first and most widely used technique for the non-invasive liver fibrosis assessment in many chronic liver diseases but also in NAFLD [99].
The principle for quantifying liver fibrosis is as follows: the probe contains both an ultrasound transducer and a mechanical vibrating device. TE has been successfully used to identify fibrosis in patients with chronic hepatitis B and C [100,101,102], having a good sensitivity and specificity for the detection of advanced fibrosis, especially for cirrhosis [103].
Due to the possibility of differentiating early and advanced fibrosis, the method has been used for other chronic diseases as well, to evaluate the prognosis in autoimmune liver disease but also in NAFLD, to differentiate simple steatosis from steatohepatitis [104].
Several studies have also evaluated the accuracy of the method in NAFLD. In a study that included 67 patients with NAFLD, the cut-off value for F3 was 8kPa with a negative predictive value of 95.6%, whilst for F4 the negative predictive value increased to 100% for a cut-off value of 17 kPa [105].
In another study with 246 patients with NAFLD, the sensitivity and specificity for a cut-off value of 7.9 kPa for F3 were 91%, and 75%, respectively, and the negative predictive value to exclude F3 was up to 97% [11].
Two meta-analyzes validated the value of TE for advanced fibrosis detection in NAFLD. First one included 1047 patients from 9 studies, obtaining a very good accuracy for grade 3 and 4 fibrosis diagnosis, with sensitivity and specificity increasing with advanced fibrosis up to 92%, but with moderate accuracy for F2 with a sensitivity of 79% and a specificity of 75% [106].
Another recent meta-analysis that included 2697 patients from 14 studies showed that the cut-off value for advanced fibrosis ranged from 7.6 to 9kPa with a sensitivity of 83-89% and a specificity of 77-78% [107].
According to the results of these studies, the European Society of Ultrasonography in Medicine and Biology (EFSUMB), recommends, in the updated guideline on the clinical utility of liver elastography, that TE should be used in patients with NAFLD only to exclude cirrhosis [108].
Although it is an objective, easy and fast method, which can be performed at the bedside and in primary care, TE has some limitations, mainly related to obesity. An increased BMI and abdominal circumference could lead to impossibility of measurements or to results that cannot be interpreted. In this regard, standard M probes have been replaced with XL probes that offer lower cut-off values of 1.5-2kPa, with a sensitivity and specificity of over 70% [109].
Other limitations of the method include extrahepatic cholestasis, limited operator experience and presence of ascites [11,109].
Point shear wave elastography (pSWE), a newer method for the non-invasive evaluation of liver fibrosis, uses acoustic radiation force impulse imaging (ARFI) to induce tissue dislocation.
Compared to strain elastography, pSWE does not measure tissue dislocation, but the velocity of shear waves perpendicular to the excitation plane that resulted from the conversion of longitudinal waves generated by ARFI [110].
Devices that incorporated pSWE include Virtual TouchTM Tissue Quantification (Siemens Healthcare, Erlangen, Germany) or ElastPQTM (EPIQ7 ultrasound system, Philips Healthcare, Bothell, WA, USA).
Several studies have evaluated the utility of pSWE in the estimation of fibrosis in patients with NAFLD/NASH, the results being promising, with an accuracy comparable to that of Fibroscan [111] (Figure 4).
Figure 4.
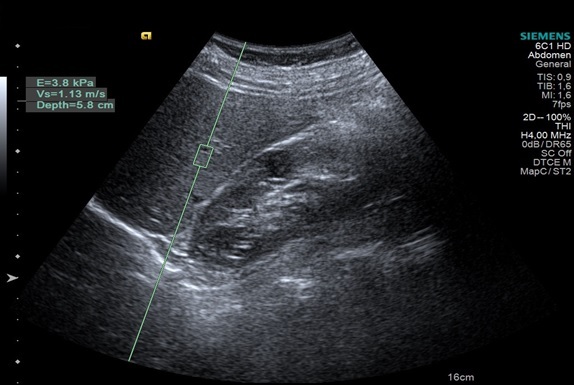
pSWE elastography. The pSWE value is 1.13m/s, suggesting no significant fibrosis
An initial study tested the accuracy of pSWE in 54 patients with NAFLD, the sensitivity and specificity being 100% and 91% respectively for a cut-off of 1.77m/s for stage 3 fibrosis [112].
Similarly, other authors demonstrated in a group of 135 patients with NAFLD that pSWE was able to distinguish high-grade and low-grade fibrosis with a sensitivity of 90% and specificity of 90% [113].
However, a systematic review indicated a moderate accuracy of pSWE in detecting advanced fibrosis in patients with NAFLD, with 80% sensitivity [114], while a recent meta-analysis demonstrated similar accuracy between pSWE and Fibroscan for advanced fibrosis and cirrhosis [115].
Due to the low accuracy to identify lower levels of fibrosis, this method is not useful for the follow-up of patients with NAFLD [116], the World Society of Ultrasonography in Medicine and Biology (WFUMB) recommending the use of pSWE only to exclude advanced fibrosis in patients with NAFLD and for the selection of patients requiring further evaluation [117].
In accordance with these recommendations we assessed 40 patients with NAFLD using elastrography on a Siemens Acuson S3000 machine.
The values obtained were consistent with steatosis, excluding advanced fibrosis, ranging from 1.8 to 3.7kPa.
The patients were prior diagnosed with NAFLD using B-mode ultrasound combined with serum markers.
All patients signed a consent to participate, and the study was approved by the Ethical Comitee of U.M.F. of Craiova.
The advantage of this method over Fibroscan is that a conventional ultrasonography probe can be used and the operator can select the area to be examined and is less influenced by obesity or ascites as it is using shear waves located inside the liver [118,119].
2D shear wave elastography (2D-SWE) is the newest sonelastographic method that uses shear waves, but with the advantage over pSWE that it quickly interrogates multiple focal areas with the possibility of real-time viewing of 2D shear waves with a color elastogram [108,119].
The technology was first developed by Supersonic Imagine (France) and included in the Aixplorer system, and subsequently other companies have adopted similar techniques (Shear Wave Elastography, LOGIQ E9, GE Healthcare, WI, USA; ElastQ, Phillips Healthcare, Netherlands; Aplio 500 i-series, Canon Medical Systems, Japan).
To date, data from the literature has confirmed the value of this method for evaluating the degree of fibrosis in patients with viral chronic liver disease [120,121].
Regarding NAFLD, there are no guideline recommendations to use this method, as studies were limited, but interest has increased over the last 3 years.
A recent comparative study including 291 patients with liver biopsy-proven NAFLD, compared TE and 2D-SWE, demonstrating that cut-off values for fibrosis prediction did not vary significantly, with sensitivity exceeding 90% for F2-F4 stages [122].
A more recent study comparing the efficacy of TE, 2D-SWE and elasto-RM found similar efficacy for all 3 techniques in diagnosing advanced fibrosis and slightly lower for significant fibrosis (F2) [123].
A 2018 meta-analysis evaluated the accuracy of the method for staging liver fibrosis, demonstrating a good accuracy for the prediction of cirrhosis and advanced fibrosis (F3-F4).
Thus, the cutoff values for F2-F4 fibrosis in the literature ranged from 6 to 18.1kPa, with a probability of diagnosing F2, F3 and F4 of 80.1%, 85.7% and 91.8%, respectively [124].
The results are similar to another meta-analysis that proposed a cutoff value of>7.1kPa for significant fibrosis (F≥2) [121].
Contrast enhanced ultrasound (CEUS)
CEUS use for patients with NAFLD is limited, with just a few studies investigating its role, mostly evaluating fibrosis by taking into consideration the changes in the intrahepatic blood flow.
A study proposed that CEUS can be used to differentiate none or mild from severe fibrosis in patients with biopsy proven NAFLD.
The parameters taken into consideration to indicate fibrosis were hepatic vein arrival time (HV), difference between the hepatic and portal vein(ΔHV-PV) which were shorter for severe fibrosis and the difference in arrival time between the portal vein and hepatic artery (ΔPV-HA) which was longer in more advanced fibrosis [125].
Another study combined transient elastography and CEUS in order to stage NAFLD, evaluating the percentage of maximal contrast activity, time to peak, regional blood volume, regional blood flow and mean transit time while Fibroscan was performed 24-48 hours after CEUS [126].
The data from the study was similar with other studies that analyzed liver blood flow with CEUS in patients with cirrhosis, with a reduced vascular compliance in the liver which appears before the onset of fibrosis [127,128].
The increased intrahepatic resistance (hepatic vascular resistance to portal blood flow) before the onset of inflammation and fibrosis was demonstrated in previous studies as well [129,130].
The study concluded that CEUS could be a useful instrument to quantify the functional vascular liver changes before the fibrotic stage [126].
However, the studies included a small number of patients, further studies are needed to confirm the value of CEUS for the evaluation and staging of NAFLD.
In our experience, the only clinical application seems to be the clear diagnosis/differentiation of focal sparing in a fatty liver or a fatty focal change in a normal liver, as in both situations the uptake of contrast is similar between the “focal lesion” and the surrounding parenchyma (Figures 5,6).
Figure 5.
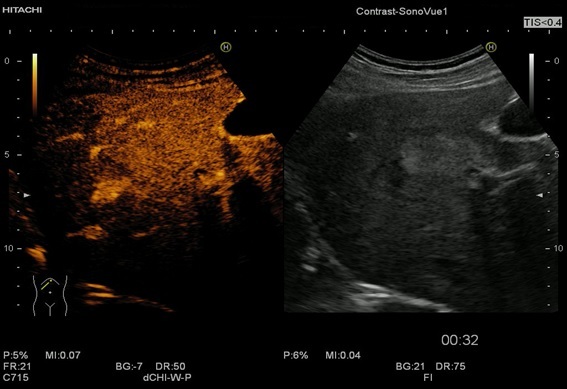
In the arterial phase, the CEUS appearance of focal fatty sparing shows iso-enhancement with the surrounding liver
Figure 6.
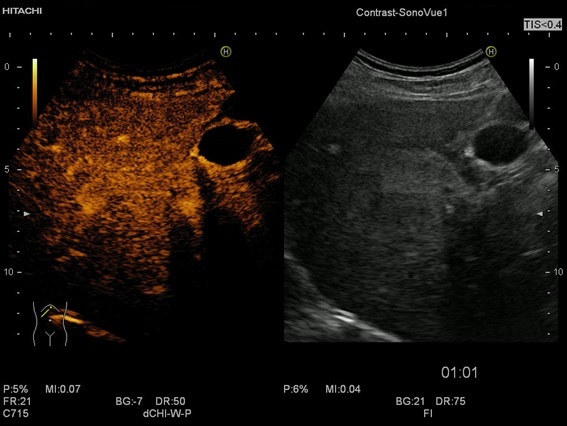
The CEUS appearance of the focal fatty sparing is iso-enhancement as well in the portal venous phase
CEUS is as sensitive as contrast enhanced computed tomography, with over 90% in one study, providing even more information on the vasculature and enhancement pattern [131].
Conclusions
NAFLD is a major health issue, with an increasing prevalence worldwide.
Consequently, the non-invasive assessment of hepatic steatosis and especially fibrosis has encountered a significant growth during the past decade.
Quantitative ultrasound-based methods, especially shear wave elastography (SWE), are indeed promising for a correct diagnosis and precise evaluation of severity of NAFLD and accompanying fibrosis.
Conflict of interests
None to declare.
Acknowledgments
Acknowledgments
Codruta Constantinescu and Larisa Săndulescu share equal contributions to this work.
References
- 1.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin LiverDis. 2001;21(1):3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 2.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Scott Harmsen JL, Charatcharoenwitthaya P, Enders FB, Therneau T, Angulo P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567–1573. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 6.Rinella ME. Nonalcoholic fatty liver disease: a systematic review [published correction appears in JAMA. 2015 Oct 13;314(14):1521] JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 7.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(2):475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10(8):530–542. doi: 10.4254/wjh.v10.i8.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takemoto R, Nakamuta M, Aoyagi Y, Tatsuya F, Yasutake K, Koga K. Validity of fibroscan values for predicting hepatic fibrosis stage in patients wit chronic HCV infection. Journal Diagnosis Disease. 2009;10(2):145–148. doi: 10.1111/j.1751-2980.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H. Noninvasive assessment of liver fibrosis by measurement of stifness in patients with nonalcoholic fatty liver disease (NAFLD) Digestive and Liver Disease. 2008;40(5):371–378. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Wong V, Vergnoil J, Wong G, Foucher J, Chan H, Le Bail B. Diagnosis of Fibrosis and Cirrhosis Using Liver Stiffness Measurement in Nonalcoholic Fatty Liver Disease. Hepatology. 2010;51(2):454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 12.Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18(4):411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Díez-Vallejo J, Comas-Fuentes Á. Asymptomatic hypertransaminasemia in patients in Primary Care. Rev EspEnferm Dig (Madrid) 2011;103(10):530–535. doi: 10.4321/s1130-01082011001000005. [DOI] [PubMed] [Google Scholar]
- 14.Adams LA, Talwalkar JA. Diagnostic evaluation of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(Suppl 1):S34–S38. doi: 10.1097/01.mcg.0000168642.38945.f1. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53(4):686–692. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 16.Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific. J Gastroenterol Hepatol. 2007;22(6):794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver (EASL) . European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101(1):76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 19.Esteghamati A, Jamali A, Khalilzadeh O, Noshad S, Khalili M, Zandieh A, Morteza A, Nakhjavani M. Metabolic syndrome is linked to a mild elevation in liver aminotransferases in diabetic patients with undetectable non-alcoholic fatty liver disease by ultrasound. DiabetolMetabSyndr. 2010;2:65–65. doi: 10.1186/1758-5996-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basaranoglu M, Neuschwander-Tetri BA. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol Hepatol (N Y) 2006;2(4):282–291. [PMC free article] [PubMed] [Google Scholar]
- 21.Shi JP, Xun YH, Hu CB, Zhang L, Liu H, Lou GQ, Fan JG. Clinical and histological features of non-alcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2009;17(11):812–816. [PubMed] [Google Scholar]
- 22.Charatcharoenwitthaya P, Lindor KD, Angulo P. The spontaneous course of liver enzymes and its correlation in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57(7):1925–1931. doi: 10.1007/s10620-012-2098-3. [DOI] [PubMed] [Google Scholar]
- 23.Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44(2):466–471. doi: 10.1002/hep.21248. [DOI] [PubMed] [Google Scholar]
- 24.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 25.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 26.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102(2):399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 27.Díez-Vallejo J, Comas-Fuentes A. Asymptomatic hypertransaminasemia in patients in primary care. Rev Esp Enferm Dig. 2011;103(10):530–535. doi: 10.4321/s1130-01082011001000005. [DOI] [PubMed] [Google Scholar]
- 28.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52(3):913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, Shah SR, Rathi PM, Joshi AS, Thakkar H. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- 30.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 31.Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramirez JC, Castro MG. Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24(7):805–810. doi: 10.1097/MEG.0b013e328354044a. [DOI] [PubMed] [Google Scholar]
- 32.Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology. 2008;55(85):1433–1438. [PubMed] [Google Scholar]
- 33.Pantsari MW. Nonalcoholic fatty liver disease presenting with an isolated elevated alkaline phosphatase. Journal of Clinical Gastroenterology. 2006;40(7):633–635. doi: 10.1097/00004836-200608000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Kocabay G, Telci A, Tutuncu Y. Alkaline phosphatase: can it be considered as an indicator of liver fibrosis in non‐alcoholic steatohepatitis with type 2 diabetes. Bratisl Lek Listy. 2010;112(11):626–629. [PubMed] [Google Scholar]
- 35.Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46(11):1300–1306. doi: 10.1007/s00535-011-0436-4. [DOI] [PubMed] [Google Scholar]
- 36.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ. 2000;321(7255):199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42(7):573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 38.Foroughi M, Maghsoudi Z, Khayyatzadeh S, Ghiasvand R, Askari G, Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;5:28–28. doi: 10.4103/2277-9175.176368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring) 2008;16(6):1394–1399. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 40.Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: A case-control study on Asian Indians in North India. PLoS ONE. 2013;8(1):e49286–e49286. doi: 10.1371/journal.pone.0049286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchihara M, Izumi N. High-sensitivity C-reactive protein (hs-CRP): A promising biomarker for the screening of non-alcoholic steatohepatitis (NASH) Nihon Rinsho. 2006;64(6):1133–1138. [PubMed] [Google Scholar]
- 42.Zimmermann E, Anty R, Tordjman J, Verrijken A, Gual P, Tran A, Iannelli A, Gugenheim J, Bedossa P, Francque S. C-reactive protein levels in relation to various features of non-alcoholic fatty liver disease among obese patients. J Hepatol. 2011;55(3):660–665. doi: 10.1016/j.jhep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44(6):1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Oruc N, Ozutemiz O, Yuce G, Akarca US, Ersoz G, Gunsar F. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: A case control study. BMC Gastroenterol. 2009;9:16–16. doi: 10.1186/1471-230X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K. High-sensitivity C-reactive protein as a serum predictor of nonalcoholic fatty liver disease based on the Akaike Information Criterion scoring system in the general Japanese population. J Gastroenterol. 2009;44(4):313–321. doi: 10.1007/s00535-009-0002-5. [DOI] [PubMed] [Google Scholar]
- 46.Das I. Raised C-reactive protein levels in serum from smokers. Clin Chim Acta. 1985;153(1):9–13. doi: 10.1016/0009-8981(85)90133-0. [DOI] [PubMed] [Google Scholar]
- 47.Kowdley KV, Belt P, Wilson LA. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(1):77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Nakeeb N, Saleh SA, Massoud YM, Hussein A, Hamed R. Serum ferritin as a non-invasive marker in the prediction of hepatic fibrosis among Egyptian patients with non-alcoholic fatty liver disease. JGH Open. 2017;1(3):112–119. doi: 10.1002/jgh3.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung JY, Shim J-J, Park SK. Serum ferritin level is associated with liver steatosis and fibrosis in Korean general population. Hepatol Int. 2019;13(2):222–233. doi: 10.1007/s12072-018-9892-8. [DOI] [PubMed] [Google Scholar]
- 50.Du SX, Lu LL, Geng N. Association of serum ferritin with non-alcoholic fatty liver disease: a meta-analysis. Lipids Health Dis. 2017;16(1):228–228. doi: 10.1186/s12944-017-0613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):179–185. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 52.Kashyap SR, Diab DL, Baker AR, Yerian L, Bajaj H, Gray-McGuire C, Schauer PR, Gupta M, Feldstein AE, Hazen SL. Triglyceride levels and not adipokine concentrations are closely related to severity of nonalcoholic fatty liver disease in an obesity surgery cohort. Obesity (Silver Spring) 2009;17(9):1696–1701. doi: 10.1038/oby.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin. Hepatology. 2004;40(1):46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 54.Aller R, de Luis DA, Fernandez L, Calle F, Velayos B, Olcoz JL, Izaola O, Sagrado MG, Conde R, Gonzalez JM. Influence of insulin resistance and adipokines in the grade of steatosis of nonalcoholic fatty liver disease. Dig Dis Sci. 2008;53(4):1088–1092. doi: 10.1007/s10620-007-9981-3. [DOI] [PubMed] [Google Scholar]
- 55.Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, Tsutsumi M, Takase S. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol. 2007;102(9):1931–1938. doi: 10.1111/j.1572-0241.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 56.Procaccini C, Galgani M, De Rosa V, Carbone F, La Rocca C, Ranucci G, Iorio R, Matarese G. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr Pharm Des. 2010;16(17):1902–1912. doi: 10.2174/138161210791208884. [DOI] [PubMed] [Google Scholar]
- 57.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134(6):1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 58.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 59.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta M, Duseja A, Mitra S, Das A, Taneja S, Dhiman RK, Chawla YK. Cytokeratin-18 (CK-18) Is A Useful Biomarker in Differentiating Between NASH and No-NASH Amongst Patients With Nonalcoholic Fatty Liver Disease (NAFLD) Journal of Clinical and Experimental Hepatology. 2017;7:S44–S44. [Google Scholar]
- 61.Joka D, Wahl K, Moeller S. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology. 2012;55(2):455–464. doi: 10.1002/hep.24734. [DOI] [PubMed] [Google Scholar]
- 62.Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, Feldstein AE. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6(11):1249–1254. doi: 10.1016/j.cgh.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 64.Maher MM, Ibrahim WA, Saleh SA, Shash L, Gabal HA, Tarif M, Gamal M. Cytokeratin 18 as a non invasive marker in diagnosis of NASH and its usefulness in correlation with disease severity in Egyptian patients. Egyptian Journal of Medical Human Genetics. 2015;16(1):41–46. [Google Scholar]
- 65.Kawanaka M, Nishino K, Nakamura J, Urata N, Oka T, Goto D, Suehiro M, Kawamoto H, Yamada G. Correlation between serum cytokeratin-18 and the progression or regression of non-alcoholic fatty liver disease. Ann Hepatol. 2015;14(6):837–844. doi: 10.5604/16652681.1171767. [DOI] [PubMed] [Google Scholar]
- 66.Malik R, Chang M, Bhaskar K, Nasser I, Curry M, Schuppan D, Byrnes V, Afdhal N. The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24(4):564–568. doi: 10.1111/j.1440-1746.2008.05731.x. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki A, Angulo P, Lymp J. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non‐alcoholic fatty liver disease. Liver Int. 2005;25(4):779–786. doi: 10.1111/j.1478-3231.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- 68.Palekar NA, Naus R, Larson SP. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26(2):151–156. doi: 10.1111/j.1478-3231.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 69.Hernaez R, Lazo M, Bonekamp S. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hultcrantz R, Gabrielsson N. Patients with persistent elevation of aminotransferases: investigation with ultrasonography, radionuclide imaging and liver biopsy. J Intern Med. 1993;233(1):7–12. doi: 10.1111/j.1365-2796.1993.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 71.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, Torella R, Persico M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38(7):485–489. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 72.Saadeh S, Younossi ZM, Remer EM. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 73.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21(1):87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mottin CC, Moretto M, Padoin AV. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obesity Surgery. 2004;14(5):635–637. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 75.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Seminars in Liver Disease. 2008;28(4):386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 76.Chartampilas E. Imaging of nonalcoholic fatty liver disease and its clinical utility. Hormones (Athens) 2018;17(1):69–81. doi: 10.1007/s42000-018-0012-x. [DOI] [PubMed] [Google Scholar]
- 77.Badea R. In: Tratat de ultrasonografieclinica. Badea R, Dudea SM, Mircea PA, Stamataian F, editors. Bucuresti: EdituraMedicala; 2000. Ficatul; pp. 105–175. [Google Scholar]
- 78.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. American Journal of Roentgenology. 2007;189(6):W320–W323. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 79.De Moura Almeida A, Cotrim HP, Barbosa DB, de Athayde LG, Santos AS, Bitencourt AG. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14(9):1415–1418. doi: 10.3748/wjg.14.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Webb M, Yeshua H, Zelber-Sagi S. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR. 2009;192(4):909–914. doi: 10.2214/AJR.07.4016. [DOI] [PubMed] [Google Scholar]
- 81.Marshall RH, Eissa M, Bluth EI, Gulotta PM, Davis NK. Hepatorenal index as an accurate, simple, and effective tool in screening for steatosis. Am J Roentgenol. 2012;199(5):997–1002. doi: 10.2214/AJR.11.6677. [DOI] [PubMed] [Google Scholar]
- 82.Isaksen VT, Larsen MA, Goll R, Florholmen JR, Paulssen EJ. Hepatic steatosis, detected by hepatorenal index in ultrasonography, as a predictor of insulin resistance in obese subjects. BMC Obes. 2016;3:39–39. doi: 10.1186/s40608-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.von Volkmann HL, Havre RF, Løberg EM, Haaland T, Immervoll H, Haukeland JW, Hausken T, Gilja OH. Quantitative measurement of ultrasound attenuation and Hepato-Renal Index in Non-Alcoholic Fatty Liver Disease. Med Ultrason. 2013;15(1):16–22. doi: 10.11152/mu.2013.2066.151.hlv1qmu2. [DOI] [PubMed] [Google Scholar]
- 84.Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29(7):1470–1476. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 85.Lee HW, Park SY, Kim SU. Discrimination of Nonalcoholic Steatohepatitis Using Transient Elastography in Patients with Nonalcoholic Fatty Liver Disease. PLoS One. 2016;11(6):e0157358–e0157358. doi: 10.1371/journal.pone.0157358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim do Y. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34(1):102–109. doi: 10.1111/liv.12282. [DOI] [PubMed] [Google Scholar]
- 87.Lupsor-Platon M, Feier D, Stefanescu H, Tamas A, Botan E, Sparchez Z. Diagnostic accuracy of controlled attenuation parameter measured by transient elastography for the non-invasive assessment of liver steatosis: a prospective study. J Gastrointestin Liver Dis. 2015;24(1):35–42. doi: 10.15403/jgld.2014.1121.mlp. [DOI] [PubMed] [Google Scholar]
- 88.de Lédinghen V, Wong GL, Vergniol J, Chan HL, Hiriart JB, Chan AW, Chermak F, Choi PC, Foucher J, Chan CK, Merrouche W, Chim AM, Le Bail B, Wong VW. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31(4):848–855. doi: 10.1111/jgh.13219. [DOI] [PubMed] [Google Scholar]
- 89.Chan WK, Nik Mustapha NR, Wong GL, Wong VW, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterology Journal. 2017;5(1):76–85. doi: 10.1177/2050640616646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chan WK, Nik Mustapha NR, Mahadeva S, Wong VW, Cheng JY, Wong GL. Can the same controlled attenuation parameter cut-offs be used for M and XL probes for diagnosing hepatic steatosis. J Gastroenterol Hepatol. 2018;33(10):1787–1794. doi: 10.1111/jgh.14150. [DOI] [PubMed] [Google Scholar]
- 91.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH, Valasek MA, Rizo E, Richards L, Brenner D, Sirlin CB, Loomba R. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152(3):598–607. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, Nederveen AJ, Beuers U, Stoker J. MR Spectroscopy-derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic Steatosis. Radiology. 2018;286(2):547–556. doi: 10.1148/radiol.2017162931. [DOI] [PubMed] [Google Scholar]
- 93.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, Ajmera V, Bettencourt R, Collier S, Hooker J, Sy E, Rizo E, Richards L, Sirlin CB, Loomba R. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67(4):1348–1359. doi: 10.1002/hep.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pu K, Wang Y, Bai S. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver. disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):51–51. doi: 10.1186/s12876-019-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 96.Byra M, Styczynski G, Szmigielski C. Transfer learning with deep convolutional neural network for liver steatosis assessment in ultrasound images. Int J Comput Assist Radiol Surg. 2018;13(12):1895–1903. doi: 10.1007/s11548-018-1843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.IEEE Xplore, 2018, A Novel Computer-Aided Diagnosis Framework Using Deep Learning for Classification of Fatty Liver Disease in Ultrasound Imaging. Available from: https://ieeexplore.ieee.org/document/8531118[Accessed 08.03.2020]
- 98.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7(5):1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garra BS. Elastography: history, principles, and technique comparison. Abdominal imaging. 2015;40(4):680–697. doi: 10.1007/s00261-014-0305-8. [DOI] [PubMed] [Google Scholar]
- 100.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Ziol M, Handra‐Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 102.Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Ledinghen V. Non‐invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29(2):242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 103.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55(3):403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dhyani M, Anvari A, Samir AE. Ultrasound elastography: liver. Abdom Imaging. 2015;40(4):698–708. doi: 10.1007/s00261-015-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoneda M, Yoneda M, Fujita K. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD) [published correction appears in Gut. 2007 Dec;56(12):1800. Yoneda, M [corrected to Yoneda, Masato]; Yoneda, Masashi[added]; Fujita, K [corrected to Fujita, Koji]; Inamori, M [corrected to Inamori, Masahiko]; Tamano, M [corrected to Tamano, Masaya]; Hiraishi, H [corrected to Hiriishi, Hideyuki]; Nakajima, A [correc] Gut. 2007;56(9):1330–1331. doi: 10.1136/gut.2007.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwok R, Tse YK, Wong GL. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease-the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39(3):254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 107.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66(5):1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 108.Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version) Ultraschall Med. 2017;38(4):e16–e47. doi: 10.1055/s-0043-103952. [DOI] [PubMed] [Google Scholar]
- 109.Castéra L, Foucher J, Bernard P‐H, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: A 5‐year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 110.Nightingale K. Acoustic Radiation Force Impulse (ARFI) Imaging: a Review. Current medical imaging reviews. 2011;7(4):328–239. doi: 10.2174/157340511798038657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J. Liver Fibrosis in Viral Hepatitis: Noninvasive Assessment with Acoustic Radiation Force Impulse Imaging versus Transient Elastography. Radiology. 2009;252(2):595–604. doi: 10.1148/radiol.2523081928. [DOI] [PubMed] [Google Scholar]
- 112.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256(2):640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 113.Palmeri ML, Wang MH, Rouze NC. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55(3):666–672. doi: 10.1016/j.jhep.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu H, Fu J, Hong R. Acoustic Radiation Force Impulse Elastography for the Non-Invasive Evaluation of Hepatic Fibrosis in Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review & Meta-Analysis. PLoS One. 2015;10(7):e0127782–e0127782. doi: 10.1371/journal.pone.0127782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang W. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: a meta-analysis. BMJ open. 2018;8(8):e021787–e021787. doi: 10.1136/bmjopen-2018-021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(5):1264–1281. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferraioli G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound in Medicine and Biology. 2018;44(12):2419 – 2440. doi: 10.1016/j.ultrasmedbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 118.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. Journal of viral hepatitis. 2012;19(2):e212–219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 119.Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 3: Liver. Ultrasound in medicine & biology. 2015;41(5):1161–1179. doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Ferraioli G, Tinelli C, Dal Bello B. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology. 2012;56(6):2125–2133. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 121.Herrmann E, de Lédinghen V, Cassinotto C. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67(1):260–272. doi: 10.1002/hep.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63(6):1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 123.Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. American Journal of Roentgenology. 2020;214(1):W20–W26. doi: 10.2214/AJR.19.21267. [DOI] [PubMed] [Google Scholar]
- 124.Zhang W, Zhu Y, Zhang C, Ran H. Diagnostic accuracy of 2-dimensional shear wave elastography for the staging of liver fibrosis: a meta-analysis. . J Ultrasound Med. 2019;38(3):733–740. doi: 10.1002/jum.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nasr P, Hilliges A, Thorelius L, Kechagias S, Ekstedt M. Contrast-enhanced ultrasonography could be a non-invasive method for differentiating none or mild from severe fibrosis in patients with biopsy proven non-alcoholic fatty liver disease. Scandinavian Journal of Gastroenterology. 2016;51(9):1126–1132. doi: 10.3109/00365521.2016.1172336. [DOI] [PubMed] [Google Scholar]
- 126.Cocciolillo S, Parruti G, Marzio L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J Hepatol. 2014;6(7):496–503. doi: 10.4254/wjh.v6.i7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin LW, Duan XJ, Wang XY, Xue ES, He YM, Gao SD, Yu LY. Color Doppler velocity profile and contrast-enhanced ultrasonography in assessment of liver cirrhosis. Hepatobiliary Pancreat Dis Int. 2008;7(1):34–39. [PubMed] [Google Scholar]
- 128.Ridolfi F, Abbattista T, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound evaluation of hepatic microvascular changes in liver diseases. World J Gastroenterol. 2012;18(37):5225–5230. doi: 10.3748/wjg.v18.i37.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Francque S, Laleman W, Verbeke L, Van Steenkiste C, Casteleyn C, Kwanten W, Van Dyck C, D’Hondt M, Ramon A, Vermeulen W. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab Invest. 2012;92(10):1428–1439. doi: 10.1038/labinvest.2012.103. [DOI] [PubMed] [Google Scholar]
- 130.Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7(4):e32785–e32785. doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Janica J, Ustymowicz A, Lukasiewicz A. Comparison of contrast-enhanced ultrasonography with grey-scale ultrasonography and contrast-enhanced computed tomography in diagnosing focal fatty liver infiltrations and focal fatty sparing. Adv Med Sci. 2013;58(2):408–418. doi: 10.2478/ams-2013-0027. [DOI] [PubMed] [Google Scholar]


