Abstract
OBJECTIVES:
To examine whether the presence of significant depressive symptoms among postmenopausal women increases the risk of subsequent mild cognitive impairment and dementia.
DESIGN:
Prospective cohort study.
SETTING:
Thirty nine of the 40 Women’s Health Initiative (WHI) clinical centers that participated in a randomized clinical trial of hormone therapy.
PARTICIPANTS:
6376 postmenopausal women without cognitive impairment aged 65–79 years at baseline.
MEASUREMENTS:
Depressive disorders were assessed using an 8-item Burnam algorithm and followed annually for a mean period of 5.4 years. The presence of Mild Cognitive Impairment and Probable Dementia was classified by a central adjudication committee based on an extensive neuropsychiatric examination.
RESULTS:
Depressive symptoms above 0.06 cutpoint on Burnam algorithm were reported by 8.0% of postmenopausal women in this sample. Depressive disorder at baseline was associated with an increased risk of incident mild cognitive impairment (hazard ratio, 1.98; 95% confidence interval, 1.33–2.94), probable dementia (hazard ratio, 2.03; 95% confidence interval, 1.15–3.60), and either mild cognitive impairment or probable dementia (hazard ratio, 1.92; 95% confidence interval, 1.35–2.73), after controlling for sociodemographic characteristics, lifestyle and vascular risk factors, cardiovascular and cerebrovascular disease, antidepressant use and current and past hormone therapy status. These relationships were unaffected by assignment to hormone therapy and baseline cognitive function. Currently non-depressed women who endorsed a remote history of depression had a higher risk of developing dementia.
CONCLUSION:
Clinically significant depressive symptoms among women 65 years and older are independently associated with an increased incidence of mild cognitive impairment and probable dementia.
Keywords: Geriatric depression, cognitive decline, mild cognitive impairment, probable dementia, women
INTRODUCTION
Unless effective prevention and/or treatment interventions are discovered, an estimated 8.5–13 million individuals in the U.S. and 107 million persons globally will develop Alzheimer’s disease (AD) by year 2050.1 Further, an estimated 13–16% of individuals between 70 and 89 years of age have Mild Cognitive Impairment (MCI).2 MCI often is a prodrome of various types of dementias and approximately 10–15% of individuals with this syndrome progress to AD each year.3 The public health consequences of this are greater for women than men because they have a longer life expectancy and more often live long enough to develop these conditions.1 The Aging, Demographics and Memory Study (ADAMS) found that the overall prevalence of depression in persons 71 years and older is similar in men and women (roughly 11%).4
Several cross-sectional and longitudinal epidemiological studies have found an association between late-life depressive symptoms and subsequent cognitive decline, including MCI5, 6 and probable dementia.7–14 However, it remains uncertain whether the presence of depressive symptoms is a cause or a consequence of a pathological cognitive decline. A rigorously conducted meta-analytic study has suggested that a history of depression independently increases the risk of developing AD by approximately two-fold,15 supporting a causal factor hypothesis. In contrast, as the neurodegenerative changes seen in AD precede the clinical diagnosis by several years, depressive symptoms may be the earliest noncognitive manifestation of this neurodegenerative disease, suggesting a reverse causality hypothesis.8, 9 However, recent large prospective cohort studies have failed to show a relationship between depression and incidence of MCI16 and dementia.17, 18
Additional studies using large population-based samples are needed to clarify whether late-life depressive symptoms are associated with the future development of MCI and dementia in women. In the Baltimore Longitudinal Study of Aging and the Personnes Agée QUID study, the presence of premorbid depressive symptoms increased the risk of later development of dementia, especially AD, but only in men, suggesting a predisposition based on gender.19, 20 Although depressive symptoms are linked to poorer cognitive function in older women,21 longitudinal studies have not shown a relationship between depression and future risk of MCI and dementia among women.
The WHI randomized controlled trial (WHI-CT) a large, multi-site population-based study that assessed the risks and the benefits of hormone therapy in healthy postmenopausal women included evaluation of depressive symptoms at baseline.22, 23 The Women’s Health Initiative Memory Study (WHIMS), an ancillary study to the WHI-CT, was designed to examine the role of postmenopausal hormone therapy on cognition and memory in healthy women who were 65 years of age and older at study baseline. WHIMS found that the conjugated equine estrogen with and without medroxyprogesterone acetate substantially increased the risk of MCI and probable dementia, relative to placebo.24, 25 The WHI-CT and WHIMS studies permit us to prospectively evaluate the relationship between clinically significant depressive symptoms and cognitive and memory status in elderly women after controlling for several potentially confounding variables including cardiovascular disease and hormone therapy status.
The objective of this study was to determine prospectively whether clinically significant depressive symptoms independently increase the incidence of MCI and dementia of any type, among postmenopausal women.
METHODS
WHIMS Study Population
A total of 7479 community-dwelling postmenopausal women were enrolled in the WHIMS. The participants were women 65 to 79 years of age at enrollment and free of MCI and dementia as ascertained by the protocol previously published.24, 25 Thirty-nine of the 40 WHI clinical centers participated in the WHIMS. Written informed consent was obtained, and the WHI and WHIMS protocols were approved by the NIH and institutional review boards of the participating institutions. WHIMS study participants had initially met eligibility criteria, had provided informed consent for the WHI estrogen plus progestin (E+P) and estrogen-alone (E-alone) trials and were randomized to receive either hormone therapy or placebo. The study design, eligibility criteria, recruitment procedures and the results from the WHI estrogen plus progestin and estrogen alone trials are reported elsewhere.22–27
Analyses for this study were based on the 6376 (85%) WHIMS women who completed the six-item Center for Epidemiologic Studies Depression Scale (CES-D)28 and the two-item National Institute of Mental Health’s Diagnostic Interview Schedule (DIS)29 and who attended at least one follow-up visit. The mean follow-up of these women was 5.4 (SD=1.6) years, with times ranging between 1 and 8 years.
Definition of Variables
Depression Assessment
Depression was measured using the Burnam screening algorithm30 that consists of six items from the 20-item Center for Epidemiologic Studies Depression Scale (CES-D)28 and two items from the National Institute of Mental Health’s Diagnostic Interview Schedule (DIS).29
As a part of CES-D questions, participants were asked about their feelings during the past week. The six items were:
You felt depressed (blue or down)
Your sleep was restless
You enjoyed life (reverse scored)
You had crying spells
You felt sad
You felt that people disliked you
Each item was scored as 0 (rarely or none of the time; less than 1 day), 1 (some or a little of the time; 1–2 days), 2 (occasionally or a moderate amount of time; 3–4 days), or 3 (most or all of the time; 5–7 days).
The two-item DIS was:
In the past year, have you had two weeks or more during which you felt sad, blue or depressed, or lost pleasure in things that you usually cared about or enjoyed? 0 (no); 1(yes)
Have you had two or more years in your life when you felt depressed or sad most days, even if you felt okay sometimes? 0 (no); 1 (yes). If yes, have you felt depressed or sad much of the time in the past year? 0 (no); 1 (yes)
The Burnam screen, initially developed for the Medical Outcomes Study30 uses a logistic regression algorithm and gives a composite score between 0 and 1. Burnam cutpoints of 0.06 and 0.009 are shown to have adequate psychometric properties to detect current depressive disorders.30 For the cutpoint of 0.06, this screening instrument has shown excellent sensitivity for detecting depressive disorder (major depressive disorder and dysthymia) in the past month in the primary care and mental health settings. For notational convenience and for purpose of this study, we will use the term depressive disorder for women who scores 0.06 or higher on the Burnam algorithm.
As in previous population-based studies in women,31, 32 we defined a score of 5 or more (of a possible 18) on the short-form CES-D as indicative of current depressive symptoms. If both DIS questions were answered “yes,” the participant was considered as having history of depressive symptoms. The history of the antidepressant and other medications use was ascertained directly from the pill bottles that the participants brought to the baseline clinic visit. Antidepressants included medications classified as tricyclics and tetracyclics, monoamine oxidase inhibitors, modified cyclics, selective serotonin reuptake inhibitors and other miscellaneous antidepressants.
Other Variables
Baseline data including demographic information, medical history and lifestyle variables were primarily obtained by self-report and clinical measurements using standardized study forms as detailed elsewhere.26 Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Physical exercise was defined as moderate or strenuous activity based on 20 minutes or more duration with the metabolic equivalent score of at least 4.0 as specified by Ainsworth and colleagues.33 Hormone treatment assignment variable was defined based on whether the women were randomized to the intervention arm (E-alone, E+P) or the control group (matching placebo) in the respective studies.24, 25
History of cardiovascular disease (CVD) was defined as a self-report of myocardial infarction, coronary bypass surgery, angioplasty, congestive heart failure, angina, carotid endarterectomy/angioplasty, cardiac catheterization, aortic aneurysm, atrial fibrillation, or cardiac arrest. Cerebrovascular disease was defined as self-report of transient ischemic attack or stroke. The level of vascular disease risk was calculated based on the number of risk factors and co-morbid vascular conditions the participant reported.
Cognitive function was measured using the Modified Mini-mental State (3MS) examination34 at baseline during the WHI screening visit and annually thereafter.24–26 The 3MS has good sensitivity and specificity for detecting cognitive impairment and the scores can range from 0 to 100 (higher scores reflect better cognitive function), and measures temporal and spatial orientation, immediate and delayed recall, executive functions, naming, verbal fluency, abstract reasoning, praxis, writing, and visuoconstructional abilities. For the first 16 months, participants who scored 72 or lower (and who had ≤ 8 years of education) or 76 or lower (who had ≥ 9 years of education) proceeded to phases 2 and 3. In order to increase the sensitivity, the cutoff points were changed to 80 or lower (for participants with <8 years of education) and 88 or lower (for individuals with > 9 years of education) after 16 months.24–26, 34 The 3MS was administered by a centrally trained and certified technician.
Main Outcome Measures
The WHIMS protocol for detecting MCI and probable dementia was previously described.24–26 WHIMS related data were collected by centrally trained and certified technicians who were recertified semiannually. The protocol was divided into four phases. In phase 1, 3MS was administered to all participants as a screening assessment of global cognitive functioning during either the second or third WHI baseline visit and then at annual follow-up visits. Women who advanced to phases 2 and 3 were scheduled for completion of phases 2 and 3 within three months of phase 1. In phase 2, the certified technician administered the modified Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological battery35, 36 and standardized interviews to assess for acquired cognitive and mental disorders.37, 38 Further, a standardized set of 36 questions that assessed the participant’s acquired cognitive and behavior changes (memory, language, orientation, personality/behavior, basic and instrumental activities of daily living, social functioning and judgment and problem solving was administered to the participant and reliable informant.26 The time between phase 2 and phase 3 assessment is within 1 month.
In phase 3, participants were evaluated by a local physician (i.e., geriatrician, neurologist, or geriatric psychiatrist) with experience in diagnosing dementia. After reviewing all available data and completing a structured medical history, and a physical and neuropsychiatric examination, the participants were classified as having probable dementia, MCI or no dementia, based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-1V) criteria.39 MCI was diagnosed based on the accepted criteria at the time when WHIMS was initiated,40 and was operationally defined as poor performance (10th or lower percentile based on CERAD norms41 on at least one CERAD test, some decline in the instrumental activities of daily living (IADL) due to cognitive impairment, intact basic activities of daily living (ADL) reported by a reliable informant, no evidence of medical or psychiatric disorders that can explain the decline in cognitive function, and the absence of adjudicated dementia.40 For the diagnosis of probable dementia, functional impairment also had to be secondary to cognitive impairment and could not be explained by medical etiologies.39
Women who were suspected of having probable dementia underwent phase 4 evaluation that included a noncontrast computed tomography brain scan and necessary laboratory blood tests to rule out possible reversible causes of cognitive decline and dementia. Subsequently, the physician was required to provide the most probable etiology of dementia based on the DSM-1V criteria for AD, vascular dementia, and other dementia related classifications.
Finally, the clinical and test data were transmitted to the WHIMS CCC for review and central adjudication of MCI and probable dementia. The central adjudication committee consisted of 3 board-certified specialists (2 neurologists and 1 geriatric psychiatrist) with extensive experience in dementia. The adjudication process is described in detail in previous WHIMS publications.24–26 The breakdown of the types of dementias was previously reported: approximately 51% were classified as AD, 16% as mixed type, and 9% as vascular dementia.24, 25
Statistical Analysis
Baseline characteristics were compared between the significant depressive symptoms and no significant depressive symptoms groups using the Kruskal-Wallis test for continuous variables and chi-square test for categorical variables. We used Cox proportional hazards regression for unadjusted (single predictor) and adjusted (several predictors) analyses and plotted cumulative hazard functions to portray incidence distributions. Separate Cox models were fitted for the time to MCI, probable dementia and either MCI or probable dementia. The effect of depression was assessed via unadjusted and adjusted hazard ratio (HR) and significance was assessed with asymptotic Wald tests. Interactions between depressive symptoms and WHI treatment assignment (active versus placebo) were assessed. Due to high face validity of possible confounders, all of them were included in multivariable models regardless of their significance status. We also fitted parsimonious models for probable dementia, MCI and MCI/probable dementia with forward stepwise variable selection and the findings were not materially different from the fully adjusted models. The consistency of associations between WHI treatment assignments and for women grouped by baseline 3MS levels was assessed using test of interactions.
RESULTS
Of the 6376 women included in these analyses, 8.0% (N=508) met the depressive disorder cutoff at baseline. Women with depressive disorder were more likely to be African-American, widowed, separated or divorced; have lower education, income and global cognitive function; be on antidepressant therapy; have current history of smoking, a higher number of other cardiovascular disease factors, and a history of alcohol use; and were less likely to exercise. Regardless of the treatment arm, women in the E-alone trial were more likely to have depressive disorder compared to those in the WHI E+P trial (Table 1). 1103 (15%) of the WHIMS cohort was not included in our analyses, primarily due to missing data on depression scores. Compared to these women, women that were excluded due to missing data tended to have lower education, family incomes and 3MS scores, were less likely to be on current antidepressant therapy, and were more likely to have history of CVD and to be current smokers (all P<0.05).
Table 1.
Baseline Characteristics
| Variable | Depressive disorder (N=508, 8.0%) | No depressive disorder (N=5868, 92.0%) |
P Value*$ |
|---|---|---|---|
| Age (Mean (SD)) | 69.9 (3.8) | 70.1 (3.8) | 0.2582 |
| Ethnicity | 0.009 | ||
| White (not Hispanic) | 421 (82.9) | 5127 (87.4) | |
| Black | 51 (10.0) | 397 (6.2) | |
| Other/Unknown | 36 (7.1) | 344 (5.9) | |
| Education | <.0001 | ||
| -Not HS grad | 67 (13.3) | 394 (6.2) | |
| -High School/GED | 119 (23.7) | 1268 (19.6) | |
| -<4 years of college | 209 (41.6) | 2368 (37.2) | |
| -College 4 years or more | 108 (21.5) | 1825 (31.2) | |
| Family Income, ($) | <.0001 | ||
| <35,000 | 327 (64.4) | 3087 (52.6) | |
| 35,000–49,999 | 79 (15.6) | 1165 (19.8) | |
| >50,000 | 64 (12.6) | 1285 (21.9) | |
| Unreported | 38 (7.5) | 331 (5.6) | |
| Marital Status | <.0001 | ||
| Never married | 13 (2.6) | 206 (3.5) | |
| Separated/divorced | 74 (14.6) | 714 (12.2) | |
| Widowed | 204 (40.2) | 1753 (29.9) | |
| Married | 217 (42.7) | 3182 (54.4) | |
| Cognition (3MS) at baseline (mean (SD)) | 95.0 (4.7) | 96.0 (3.9) | <.0001 |
| Current Antidepressant therapy | <.0001 | ||
| Yes | 105 (20.7) | 353 (6.0) | |
| No | 403 (79.3) | 5515 (94.0) | |
| Alcohol Use | 0.0001 | ||
| Non-drinker | 66 (13.1) | 764 (13.1) | |
| Past drinker | 133 (26.4) | 1094 (18.8) | |
| Current drinker | 305 (60.5) | 3959 (68.1) | |
| Smoking | 0.0003 | ||
| Never | 250 (50.2) | 3106 (53.7) | |
| Past Smoker | 190 (38.2) | 2310 (39.9) | |
| Current Smoker | 58 (11.7) | 372 (6.4) | |
| BMI Category, (kg/m2) | 0.1279 | ||
| BMI <25 | 131 (25.8) | 1647 (28.1) | |
| BMI ≥25 and <30 | 160 (31.5) | 1992 (34.0) | |
| BMI ≥ 30 | 177 (34.8) | 1880 (32.0) | |
| Missing | 40 (7.9) | 349 (6.0) | |
| Exercise (times per week) | 0.0067 | ||
| None | 99 (19.5) | 1031 (17.8) | |
| <2 | 257 (50.7) | 2640 (45.0) | |
| 2–3 | 70 (13.8) | 934 (15.9) | |
| ≥4 | 81 (16.0) | 1256 (21.4) | |
| Coffee Consumption (cups/day) | 0.3958 | ||
| None | 79 (15.6) | 789 (13.4) | |
| 1–3 | 236 (46.5) | 2923 (49.8) | |
| 4 or more | 28 (5.5) | 338 (5.8) | |
| Missing | 165 (32.5) | 1818 (31.0) | |
| Hypertension | <.0001 | ||
| Yes | 240 (48.0) | 2235 (38.5) | |
| No | 260 (52.0) | 3571 (61.5) | |
| Diabetes | 0.0020 | ||
| Yes | 51 (10.0) | 379 (6.5) | |
| No | 457 (90.0) | 5489 (93.5) | |
| Dyslipidemia | 0.2788 | ||
| Yes | 101 (20.2) | 1054 (18.2) | |
| No | 400 (79.8) | 4735 (81.8) | |
| Heart Disease | 0.0022 | ||
| Yes | 111 (22.3) | 961 (16.6) | |
| No | 386 (77.7) | 4833 (80.4) | |
| Stroke/TIA | 0.6011 | ||
| Yes | 7 (1.4) | 99 (1.7) | |
| No | 501 (98.6) | 5769 (98.3) | |
| Vascular Factors (number) | <.0001 | ||
| 0 | 31 (6.1) | 560 (9.5) | |
| 1–3 | 218 (42.9) | 2878 (49.0) | |
| ≥4 | 259 (51.0) | 2430 (41.4) | |
| WHI Treatment Assignment | 0.0007 | ||
| E-alone (Intervention) | 122 (24.0) | 1108 (18.9) | |
| E-alone (Control) | 119 (23.4) | 1134 (19.3) | |
| E+P (Intervention) | 133 (26.2) | 1794 (30.6) | |
| E+P (Control) | 134 (26.4) | 1832 (31.2) | |
| Prior Hormone Therapy | 0.8295 | ||
| Ever | 36 (7.1) | 431 (7.4) | |
| Never | 472 (92.9) | 5436 (92.6) |
overall P Value for categorical variables based on Chi-Square test
P Value for continuous variables based on Kruskal-Wallis test
Abbreviations: SD: standard deviation; 3MS: modified mini-mental state exam; BMI: Body mass index; TIA: transient ischemic attack; HRTARM: hormone replacement treatment arm; E-alone: estrogen alone; E+P: Estrogen plus progestin.
Missing data: For actors for which data were missing for >100 participants, separate categories were created to denote missing data. Missing data on other factors were ignored, which excluded some participants from covariate models: N=18 for education, N=63 for marital status; N=55 for alcohol use; N=90 for smoking; N=8 for exercise; N=86 for dyslipidemia; N=85 for heart disease; and N=1 for prior hormone therapy.
As shown in Table 2, a total of 216 participants (3.4%) developed MCI and 102 women (1.6%) developed dementia of any type. A total of 285 (4.5%) women were classified as MCI or probable dementia at least one time during follow-up (i.e. 33 women who initially developed MCI subsequently converted to probable dementia during follow-up). Figure1 portrays the distributions of times until the first incidence these events. In the unadjusted analysis, depressive disorder was associated with an increased risk of incident MCI (HR, 2.14; 95% CI, 1.47–3.09), probable dementia (HR, 2.18; 1.28–3.71) and either MCI/probable dementia (HR, 2.01; 95% CI, 1.44–2.80) (Table 2). Supporting information (Table S1) shows the scores on the CERAD measures for depressed and non-depressed women grouped by incident MCI, probable dementia, and MCI/probable dementia.
Table 2.
Unadjusted Hazard Ratio (95% Confidence Interval) of Developing MCI, Probable Dementia and Either by Depressive Disorder Status
| Variable (N) | (MCI) N | Developed MCI (N =216, 3.4%) HR (CI) | P | (Dementia) N | Developed Dementia (N=102, 1.6%) HR (CI) | P | (MCI/Dementia) N | Developed MCI/Dementia (N=285, 4.5%) HR (CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| Depressive Disorder (N=508) | 33 | 2.14 (1.47, 3.09) | <0.0001 | 16 | 2.18 (1.28, 3.71) | 0.0043 | 41 | 2.01 (1.44, 2.80) | <0.0001 |
| No Depressive Disorder (N=5868) | 183 | 1.00 | - | 86 | 1.00 | - | 244 | 1.00 | - |
HR: Hazard Ratio; CI: Confidence Interval; MCI: mild cognitive impairment
Figure 1.

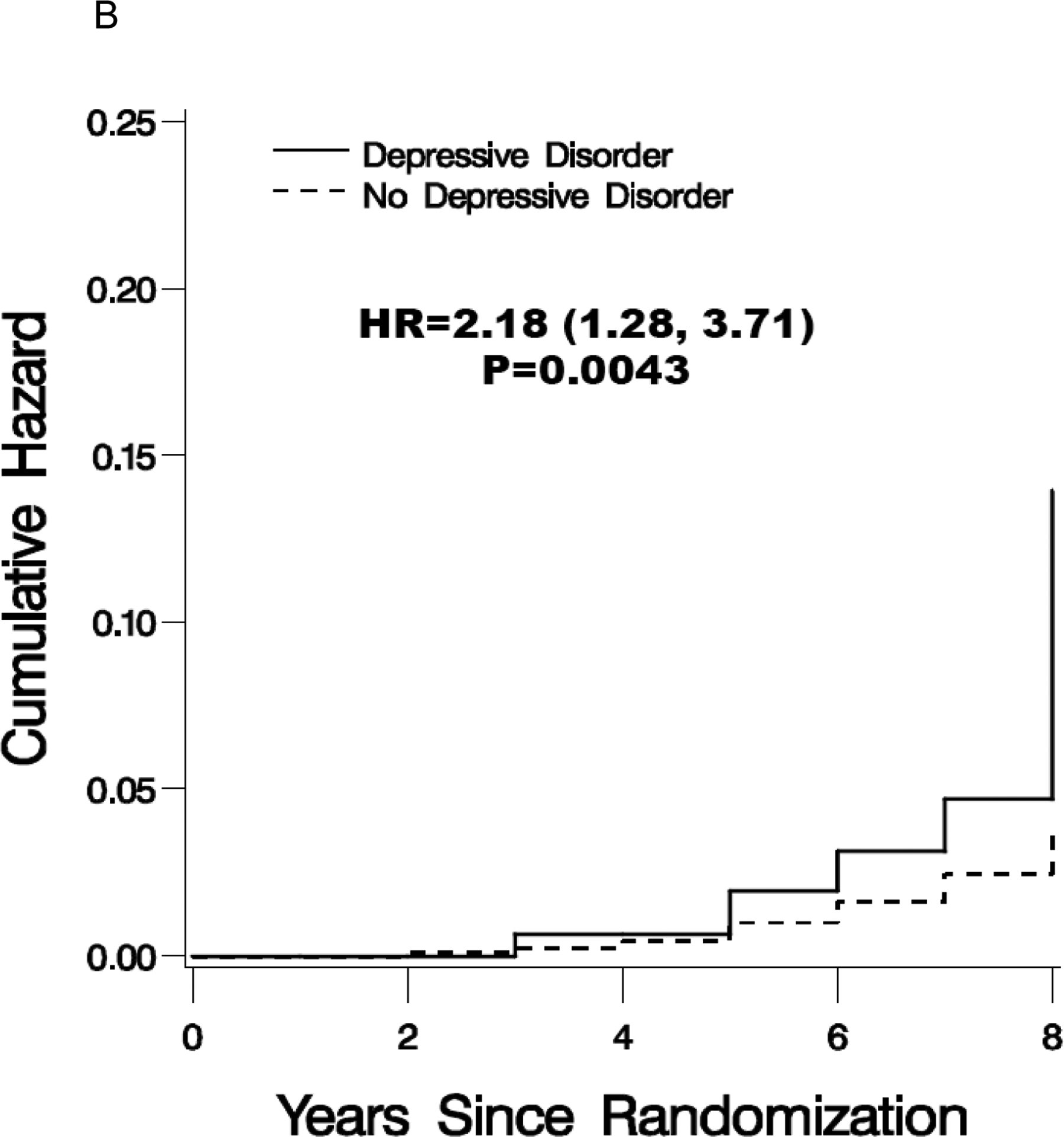

Cumulative hazard estimates for the incidence of mild cognitive impairment (A), probable dementia (B) and mild cognitive impairment/probable dementia (C) for women grouped by baseline depressive disorder (Burnam cutpoint >0.06). Included are the hazard ratio and 95% confidence interval.
Four models were fitted with adjustment for: demographic variables (Model 1), lifestyle variables and vascular risk factors (Model 2), co-morbid cardiovascular and cerebrovascular conditions (Model 3), and all demographic and lifestyle variables, co-morbid vascular disease, WHIMS hormone therapy assignment, 3MS at baseline and antidepressant therapy (Model 4). The findings remained significant in all models (Table 3). In the fully adjusted models, the fitted hazard ratios were 1.98 (95% CI, 1.33–2.94) for MCI, 2.03 (1.15 – 3.60) for probable dementia, and 1.92 (1.35–2.73) for the first occurrence of either MCI or probable dementia. In the adjusted models, the depression-pathological cognitive decline relationship observed in unadjusted analyses did not significantly change (Tables 2 and 3). There was no evidence that these relationships varied by WHI treatment assignment (tests of interaction p>0.70 for each of MCI, probable dementia, and MCI/dementia) or baseline cognitive function (p>0.50).
Table 3.
Adjusted Hazard Ratio (95% Confidence Interval) of Developing MCI, Probable Dementia, and Either By Depressive Disorder at Baseline
| Variable | MCI | Dementia | MCI/Dementia | |||
|---|---|---|---|---|---|---|
| Depressive Disorder vs no Depression | HR (CI) | P | HR (CI) | P | HR (CI) | P |
| Model 1 adjusted for demographic variables1 | 1.91 (1.31, 2.78) | 0.0007 | 1.98 (1.15, 3.39) | 0.0135 | 1.83 (1.31, 2.55) | 0.0004 |
| Model 2 adjusted for lifestyle variables2 | 1.97 (1.34, 2.88) | 0.0005 | 2.17 (1.25, 3.75) | 0.0056 | 1.88 (1.34, 2.64) | 0.0003 |
| Model 3 adjusted for co-morbid vascular diseases3 | 2.08 (1.42, 3.05) | 0.0002 | 2.09 (1.21, 3.62) | 0.0085 | 1.98 (1.40, 2.78) | <.0001 |
| Model 4 fully adjusted4 | 1.98 (1.33, 2.94) | 0.0008 | 2.03 (1.15, 3.60) | 0.0150 | 1.92 (1.35, 2.73) | 0.0003 |
Model 1: Demographic Variables: Age, Ethnicity, Education, Family Income, Marital Status
Model 2: Lifestyle Variables: Alcohol Use, Smoking, Exercise, BMI, Hormone Use; Vascular Risk Factors: Cholesterol requiring pills, Hypertension, Diabetes (treated or not).
Model 3: Cardiovascular Co-morbid Conditions: cardiovascular disease, cardiac arrest, congestive heart failure, Cardiac catheterization, coronary artery bypass graft, coronary angioplasty, carotid stenosis, atrial fibrillation, Angina, aortic aneurysm; Cerebrovascular co-morbid conditions: Stroke/TIA.
Model 4: Fully adjusted: all variables in models 1–3, hormone therapy assignment in WHIMS, 3MS at baseline, current antidepressant therapy.
The Relationship Between Current and History of Depressive Symptoms and Pathological Cognitive Decline
Further analysis was performed using other depressive symptom variables that included (1) current depressive symptoms versus the non-depressed cohort, and (2) history of depressive symptoms versus no history of depressive symptoms as the reference group. We also examined whether the history of depressive symptoms in women without current symptoms was a predictor for MCI or probable dementia. Current depressive symptoms were related to the risk of MCI both without (HR, 1.38; 95% CI, 1.05–1.80) and with (HR, 1.33; 95% CI 1.01–1.77) full covariate adjustment. In the fully adjusted analysis, similar relationships were also observed between the history of depressive symptoms and MCI (HR, 1.38; 95% CI, 1.03–1.85) (Table 4).
Table 4:
Association Between Current and History of Depressive Symptoms at Baseline and Subsequent MCI, Probable Dementia and MCI/Probable Dementia
| Unadjusted Hazard Ratio (95% Confidence Interval) of Developing MCI, Probable Dementia and Either by Current Depressive Symptom, History of Depressive Symptom Status at Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | (MCI) N | MCI HR (CI) | P Value | (Dementia) N | Dementia HR (CI) |
P Value | (MCI/ Dementia) N | MCI/Dementia HR (CI) | P Value |
| Current Depressive Symptoms | |||||||||
| Yes (N= 2363) | 97 | 1.38 (1.05, 1.80) | 0.0190 | 47 | 1.43 (0.97, 2.11) | 0.0714 | 128 | 1.38 (1.09, 1.74) | 0.0066 |
| No (N=4013) | 119 | 1.00 | 55 | 1.00 | 157 | 1.00 | |||
| History of Depressive Symptoms | |||||||||
| Yes (N=2066) | 83 | 1.32 (1.00, 1.74) | 0.0480 | 47 | 1.80 (1.22, 2.66) | 0.0031 | 117 | 1.48 (1.16, 1.87) | 0.0012 |
| Adjusted Hazard Ratio (95% Confidence Interval) of MCI, Probable Dementia and Either by Current Depressive Symptoms, History of Depressive Symptoms Status at Baseline | |||||||||
| Variable | MCI HR (CI) | P Value | Dementia HR (CI) | P Value | MCI/Dementia HR (CI) | P Value | |||
| Current Depressive Symptoms | |||||||||
| Yes | 1.33 (1.01, 1.77) | 0.0494 | 1.39 (0.92, 2.11) | 0.1188 | 1.34 (1.05, 1.72) | 0.0200 | |||
| No | |||||||||
| History of Depressive Symptoms | |||||||||
| Yes | 1.38 (1.03, 1.85) | 0.0317 | 1.98 (1.31, 3.01) | 0.0013 | 1.62 (1.26, 2.08) | 0.0002 | |||
| No | |||||||||
HR: Hazard Ratio; CI: Confidence Interval; MCI: mild cognitive impairment.
In the adjusted model, current depressive symptoms were not associated with PD (HR, 1.39; 95% CI 0.92–2.11) whereas history of depressive symptoms was associated with subsequent development of PD (HR, 1.98; 95% CI 1.31–3.01) (Table 4). With the same covariate adjustment as in Table 4, the remote (no current) history of depressive symptoms were significantly associated with probable dementia (HR=2.08; 95% CI 1.15–3.78, p=0.02), but not with MCI (HR=1.03; 95% CI 0.66–1.63, p=0.89) or MCI/probable dementia (HR=1.34; 95% CI 0.92–1.96, p=0.12).
DISCUSSION
In a cohort of postmenopausal women aged 65 to 79 years of age at study baseline who met screening cutoff for depressive disorder, we found an increased risk of subsequent MCI and incident dementia after a mean follow-up of 5.4 years. These findings remained significant after adjusting for multiple potential confounding variables including demographics, lifestyle variables, cardiovascular risk factors, the presence of cardiovascular and cerebrovascular disease, antidepressant use, baseline cognitive function (as measured by 3MS), and prior use and current prescription of hormone therapy. The participants who were depressed had approximately twice the hazard for developing MCI and probable dementia. To our knowledge, this is the single largest sample of postmenopausal women in which the existence of an independent link was examined between depression and MCI or dementia.
Relationship between depression and MCI
Our findings are consistent with the previous prospective studies5,6 that showed an association between depressive symptoms and incident MCI. The Cardiovascular Health Study Cognition Study (CHS-CS) that assessed 2220 participants for depression at baseline using the 10-item CES-D scale found a significant association between more depressive symptoms and the development of MCI over a 6-year period.5 The significance of relationship between depressive symptoms and MCI did not change when adjusted for demographics and underlying vascular disease measures similar to our findings. In a smaller study of 840 cognitively normal, non-depressed elderly subjects at baseline, the individuals who developed depression during the median follow-up period of 3.5 years showed an increased risk of subsequent MCI. This association was stronger among men than women and a synergistic interaction between apolipoprotein E genotype and depressive symptoms was observed.6 However, in the Italian Longitudinal study on Aging (ILSA), depressive symptoms were not associated with an increased risk of incident MCI16 and with the rate of MCI conversion to dementia.18 Moreover, chronic psychological distress and not depressive symptoms were associated with increased incidence of MCI in another study.42
Depressive disorder and probable dementia
Previously, both case-control and cohort studies have found an association between depressive symptoms and future risk of probable dementia including AD.7, 8, 12, 20 However, the association between depressive symptoms and dementia including AD occur primarily in the elderly individuals who have cognitive deficits at baseline.7, 8 These findings are also not universal. In the CHS-CS for instance, although the presence of depressive symptoms was linked with MCI,5 the association of depressed mood with incident dementia was not evident.17 The observed depression-MCI link in the CHS-CS study was therefore thought to occur from the depression related cognitive dysfunction being severe enough to meet MCI criteria.5, 17 In contrast, our study supports the hypothesis that if depression is a risk factor for dementia and MCI is a prodromal phase of dementia, depression is also associated with incident MCI in women.5, 6 Although both CHS-CS and WHIMS are large population based studies that used similar clinical assessments, the former recruited both men and women whereas our sample included only women that may have led to the differences in findings. In contrast to previous studies, we found an association between depressive disorder and dementia independent to baseline cognitive function (p>0.50).
There are several strengths to our study. This is the largest cohort of postmenopausal women that has been studied with regards to cognitive function and we were able to control for a large number of potentially confounding variables. The participants underwent extensive screening prior to enrollment and completed several standardized measures including the Burnam screening algorithm,30 a highly sensitive and specific screening tool for current depressive disorders. The identification of MCI and probable dementia were made on the basis of extensive neuropsychiatric examinations and the cases were centrally adjudicated. More importantly, all MCI cases were determined based on clinical diagnosis after these women appeared impaired to a specialist. In addition, as compared to other studies, WHIMS had a much larger and multi-ethnic sample size,6–9, 16–19 had a longer duration of follow-up6, 7, 16 and the participants were recruited from 39 different communities across the United States, thus providing significant heterogeneity to the study population.5–8, 17, 20 Finally, this is the first study to our knowledge that showed a relationship between clinically significant depressive symptoms and the future development of MCI and dementia in the same study cohort.
Various pathophysiological mechanisms are suggested as a link between depression and cognitive decline.10 First, cerebrovascular disease and vascular risk factors such as hypertension are postulated as the potential link between geriatric depression and dementia19, 43 but recent studies including ours have not supported this theory.5, 14 Second, the excessive stress hormone release during a depressive episode may result in neurotoxic damage and atrophy of the hippocampus, similar to MCI and AD.44–46 We are currently examining whether the depressed women show reductions in the regional brain volumes and increases the ischemic lesion volumes. Finally, amyloid plaques and neurofibrillary tangles and the presence of the APOE e4 genotype in the depressed elderly may be the mechanistic link between geriatric depression and cognitive decline.6, 47–49
Our findings that the currently non-depressed women with a remote depressive symptom history were more likely to progress to dementia supports the causal factor hypothesis at least in some cases, and these results are consistent with the literature.15 Majority of the women in the WHIMS cohort who developed dementia had Alzheimer’s, vascular or mixed type etiologies.24, 25 We therefore speculate that the women with a remote depression history had more underlying pathology and transitioned more quickly into the above types of dementias. Another possibility is that these women who were previously exposed to excessive stress were subjected to glucocorticoid hypersecretion-related hippocampal atrophy and accelerated cognitive decline.10, 15, 49 However, we did not find similar relationship with MCI. It is plausible that the remote depression history-incident MCI relationship may exist only in women with Alzheimer’s disease or vascular pathologies. WHIMS classification of MCI required abnormality in any cognitive domain and therefore, our MCI sample most likely included women with both amnestic and non-amnestic MCI subtypes. Non-amnestic MCI subtypes are associated with a higher risk of non-AD type dementias.3 Future research is therefore essential to elucidate the various pathophysiological mechanisms that link the history of depression and pathological cognitive decline relationships.
Our study is not without limitations. At baseline, depressive disorders were not assessed in the WHIMS cohort using standardized interviews such as the Structured Clinical Interview for DSM-1V disorders (SCID). Although the Burnam algorithm has excellent sensitivity, the positive predictive value for detecting syndromal depression is low (20–50%) when compared to the SCID.50 Despite this, the Burnam algorithm using both 0.06 and 0.009 cutpoints (latter findings not reported here) was more strongly related (i.e. had higher hazard ratios) to the risk MCI and dementia, when compared with the short form of CES-D cut-off of ≥ 5. This is important as previous epidemiological studies that have addressed similar associations have utilized various versions of the CES-D to evaluate depressive symptoms and this may have contributed to the discrepant findings.5, 17, 42, 49 Self-report measures were used to ascertain the history of vascular risk factors and a medical history of cardiovascular and cerebrovascular diseases, which may have resulted in an inaccurate estimation of these variables. In addition, the 3MS, a relatively insensitive measure of cognitive impairment was used for cognitive function screening at baseline similar to prior epidemiological studies.5, 17 15% of the WHIMS cohort was not included in our analyses due to missing scores on depression measures. 15% of the WHIMS cohort was not included in our analyses due to missing scores on depression measures, thus our findings may not generalize fully to the entire study cohort. Finally, the operational definition of MCI has continued to evolve since WHIMS was initiated. Since the diagnosis of MCI in the WHIMS cohort required abnormality only in any cognitive domain, the women classified as MCI may include individuals with both amnestic and non-amnestic MCI. This is significant as there is an increasing body of evidence supporting the concept that amnestic and nonamnestic MCI subtypes have different prognoses and although a significant majority may progress to AD and non-AD dementias, some may revert back to normal.3 Re-analysis of the data based on the currently accepted MCI classification may provide further insights into this issue.
In conclusion, we report here the largest multi-site cohort of postmenopausal women where depressive disorder at baseline increased the future risk of developing MCI and more than doubled the chances of probable dementia with average follow-up of 5.4 years. Our findings were robust to adjustment for confounding variables. We found an association between late-life depressive disorder and incidence of MCI and probable dementia among postmenopausal women. If depression is a risk factor for dementia as suggested by our findings, then adequate treatment of depression may prevent future development of dementia in women.
Supplementary Material
Funding/Support:
The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The active study drug and placebo were supplied by Wyeth-Ayerst Research Laboratories, Philadelphia, Pennsylvania. The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals as an ancillary study to the WHI. Wyeth Pharmaceuticals did not participate in the design and conduct of the studies, in the collection, analysis, and interpretation of the data, or in preparation, review or approval of this manuscript.
APPENDIX
SHORT LIST OF WHI INVESTIGATORS
Program Office:
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center:
(Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers:
(Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Women’s Health Initiative Memory Study:
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Conflict of Interest Disclosures: Below is a checklist for all authors to complete and attach to their papers during submission.
| Elements of Financial/Personal Conflicts | *Author 1 Joseph Goveas | Author 2 Mark Espeland | Author 3 Nancy Woods | Author 4 Sylvia Wassertheil-Smoller | ||||
|---|---|---|---|---|---|---|---|---|
| No | ||||||||
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
| Elements of Financial/Personal Conflicts | *Author 5 Jane Kotchen | Author 6 | Author 7 | Author | ||||
| No | ||||||||
| Employment or Affiliation | X | |||||||
| Grants/Funds | X | |||||||
| Honoraria | X | |||||||
| Speaker Forum | X | |||||||
| Consultant | X | |||||||
| Stocks | X | |||||||
| Royalties | X | |||||||
| Expert Testimony | X | |||||||
| Board Member | X | |||||||
| Patents | X | |||||||
| Personal Relationship | X | |||||||
Authors can be listed by abbreviations of their names.
For “yes” x mark(s): give brief explanation below:
Footnotes
Category: Clinical Investigations
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article: Table S1.
REFERENCES
- 1.Alzheimer’s Association. 2009 Alzheimer’s disease facts and figures. Alzheimers Dement 2009;5:234–270. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RO, Geda YE, Knopman DS et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier S, Reisberg B, Zaudig M et al. ; International Psychogeriatric Association Expert Conference on mild cognitive impairment. Mild cognitive impairment. Lancet 2006;367:1262–1270. [DOI] [PubMed] [Google Scholar]
- 4.Steffens DC, Fisher GG, Langa KM et al. Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. Int Psychogeriatr 2009; 21:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes DE, Alexopoulos GS, Lopez OL et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry 2006;63:273–279. [DOI] [PubMed] [Google Scholar]
- 6.Geda YE, Knopman DS, Mrazek DA et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol 2006;63:435–440. [DOI] [PubMed] [Google Scholar]
- 7.Devanand DP, Sano M, Tang MX et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry 1996; 53:175–182. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry 1993;150:1693–1699. [DOI] [PubMed] [Google Scholar]
- 9.Chen P, Ganguli M, Mulsant BH et al. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry 1999;56:261–266. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry 2001;35:776–781. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Barnes LL, Mendes de Leon CF et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002; 59:364–370. [DOI] [PubMed] [Google Scholar]
- 12.Green RC, Cupples LA, Kurz A et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol 2003;60:753–759. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Arnold SE, Beck TL et al. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry 2008;65:439–445. [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger JA, Honig LS, Tang MX et al. Depressive symptoms, vascular risk factors, and Alzheimer’s disease. Int J Geriatr Psychiatry 2008;23:922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ownby RL, Crocco E, Acevedo A et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006;63:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panza F, D’Introno A, Colacicco AM et al. Italian Longitudinal Study on Aging Working Group. Depressive symptoms, vascular risk factors and mild cognitive impairment. The Italian longitudinal study on aging. Dement Geriatr Cogn Disord 2008;25: 336–346. [DOI] [PubMed] [Google Scholar]
- 17.Becker JT, Chang YF, Lopez OL et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry 2009;17:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panza F, Capurso C, D’Introno A et al. Impact of depressive symptoms on the rate of progression to dementia in patients affected by mild cognitive impairment. The Italian Longitudinal Study on Aging. Int J Geriatr Psychiatry 2008;23:726–734. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrer R, Dufouil C, Dartigues JF; PAQUID Study. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc 2003; 51:1055–1063. [DOI] [PubMed] [Google Scholar]
- 20.Dal Forno G, Palermo MT, Donohue JE et al. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol 2005;57:381–387. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe K, Blackwell T, Gore R et al. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry 1999;56:425–430. [DOI] [PubMed] [Google Scholar]
- 22.Rossouw JE, Anderson GL, Prentice RL et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 23.Wassertheil-Smoller S, Hendrix SL, Limacher M et al. ; WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 2003;289:2673–2684. [DOI] [PubMed] [Google Scholar]
- 24.Shumaker SA, Legault C, Rapp SR et al. ; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662. [DOI] [PubMed] [Google Scholar]
- 25.Shumaker SA, Legault C, Kuller L et al. ; Women’s Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2947–2958. [DOI] [PubMed] [Google Scholar]
- 26.Shumaker SA, Reboussin BA, Espeland MA et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 1998;19: 604–621. [DOI] [PubMed] [Google Scholar]
- 27.Anderson GL, Limacher M, Assaf AR et al. ; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1997; 1: 385–401. [Google Scholar]
- 29.Robins LN, Helzer JE, Croughan J et al. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry 1981;38:381–389. [DOI] [PubMed] [Google Scholar]
- 30.Burnam MA, Wells KB, Leake B et al. Development of a brief screening instrument for detecting depressive disorders. Med Care 1988;26:775–789. [DOI] [PubMed] [Google Scholar]
- 31.Borhani NO, Applegate WB, Cutler JA et al. Systolic Hypertension in the Elderly Program (SHEP). Part 1: Rationale and design. Hypertension 1991;17:II2–15. [DOI] [PubMed] [Google Scholar]
- 32.Wassertheil-Smoller S, Shumaker S, Ockene J et al. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI). Arch Intern Med 2004;164:289–298. [DOI] [PubMed] [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Leon AS et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 34.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 35.Morris JC, Heyman A, Mohs RC et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer disease. Neurology 1989; 39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 36.Reitan RM, Wolfson D. Conventional intelligence measurements and neuropsychological concepts of adaptive abilities. J Clin Psychol 1992;48: 521–529. [DOI] [PubMed] [Google Scholar]
- 37.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol 1991;4:173–178. [DOI] [PubMed] [Google Scholar]
- 38.Spitzer RL, Williams JB, Kroenke K et al. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA 1994;272:1749–1756. [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 40.Petersen RC, Doody R, Kurz A et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 41.Ganguli M, Belle S, Ratclliff G et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol 1993;48:M152–M161. [DOI] [PubMed] [Google Scholar]
- 42.Wilson RS, Schneider JA, Boyle PA et al. Chronic distress and incidence of mild cognitive impairment. Neurology 2007;68:2085–2092. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS, Meyers BS, Young RC et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997;54:915–922. [DOI] [PubMed] [Google Scholar]
- 44.Steffens DC, Byrum CE, McQuoid DR et al. Hippocampal volume in geriatric depression. Biol Psychiatry 2000;48:301–309. [DOI] [PubMed] [Google Scholar]
- 45.Bell-McGinty S, Butters MA, Meltzer CC et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry 2002;159:1424–1427. [DOI] [PubMed] [Google Scholar]
- 46.Devanand DP, Pradhaban G, Liu X et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 2007; 68: 828–836. [DOI] [PubMed] [Google Scholar]
- 47.Butters MA, Klunk WE, Mathis CA et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord 2008; 22:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irie F, Masaki KH, Petrovitch H et al. Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia Aging Study. Arch Gen Psychiatry 2008;65:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steffens DC, Otey E, Alexopoulos GS et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry 2006; 63:130–138. [DOI] [PubMed] [Google Scholar]
- 50.Tuunainen A, Langer RD, Klauber MR et al. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Res 2001;103:261–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


