Abstract
Objective:
Early adversity is correlated with increased risk for negative outcomes including psychopathology and atypical neurodevelopment. The present study aimed to test the causal impact of an early parenting intervention (Attachment and Biobehavioral Catch-up; ABC) on children’s neural processing of parent cues and on psychosocial functioning in a longitudinal randomized clinical trial.
Method:
Participants (N = 68, Mage = 10.0 years) included 46 high-risk children whose parents were randomized to receive either ABC (n = 22) or a control intervention (n = 24) while children were infants, in addition to a comparison sample of low-risk children (n = 22). Children viewed pictures of their own mother and of a stranger during functional magnetic resonance imaging.
Results:
Children in the ABC condition showed greater maternal-cue-related activation than children in the control condition in clusters of brain regions including the precuneus, cingulate gyrus, and hippocampus, regions commonly associated with social cognition. Additionally, greater activity in these regions was associated with fewer total behavior problems. There was an indirect effect of early intervention group on middle childhood psychosocial functioning mediated through increased activity in brain regions in response to maternal cues.
Conclusions:
Results suggest that early parenting intervention (in this case, ABC) can enhance brain regions supporting children’s social cognitive development. In addition, findings highlight these brain effects as a possible neural pathway through which ABC may prevent future behavior problems among high-risk children, yielding psychosocial benefits that endure through at least middle childhood without a need to intervene with the child directly.
Keywords: randomized controlled trial, adversity, fMRI, attachment, parenting, prevention
Early adversity (e.g., childhood maltreatment) is associated with significantly elevated risk for negative developmental outcomes, including psychopathology and atypical neurobiological development (1, 2). One likely pathway through which early adversity may confer heightened risk for these problems is via attachment difficulties (3). In particular, it has been well documented that children who experience maltreatment are less likely to develop secure attachments to parents than nonmaltreated children (3, 4). Insecure or disorganized attachments, in turn, place children at greater risk for internalizing and externalizing behavior problems than secure attachments (5, 6). Findings from both human and non-human animal investigations have identified neural responses to parent cues during childhood as a candidate biological mechanism linking early caregiving experiences to attachment-related processes and mental health outcomes (7–12). However, although work with non-human animals has established the causal role of early parenting experiences on offspring reactivity to parent cues (12), such work with humans has been largely correlational (7–11). The present study leveraged a randomized clinical trial to test the causal impact of an early parenting intervention on human children’s neural processing of parent cues and on psychosocial functioning.
Caregiving that is attuned to the needs of the offspring is critical for the development of neural systems underlying social functioning in altricial mammals, including humans (10). In caring for offspring, mammalian mothers utilize a distinct subcortical network supporting maternal behavior (13), which in humans evolved to include cortical regions, such as the medial prefrontal cortex, (mPFC), anterior cingulate cortex (ACC), dorsolateral PFC, insula, inferior frontal and orbitofrontal cortex (OFC), and temporoparietal junction (14), that are implicated in social cognition, emotion regulation, and behavior. Relatively less is known about how human children respond to attachment cues at the level of brain activation, but recent findings indicate significant overlap with parents’ brain responses. For example, relative to listening to female control voices, when children listened to their own mother’s voice they exhibited greater activation in a host of brain regions including the mPFC, ACC, insula, OFC, precuneus, posterior cingulate cortex (PCC), angular gyrus, fusiform gyrus, and amygdala, with between-area connectivity during presentation of mothers’ voices predicting children’s social communication skills (11). This pattern of activation is not specific to auditory maternal cues, as many of the same brain areas (e.g., mPFC, PCC, fusiform gyrus, amygdala) have shown greater activation among children and adolescents while viewing pictures of their own mother relative to viewing pictures of a female control (7–9). An important remaining question is what factors contribute to the development of children’s responses (i.e., how these patterns of activation are transmitted across generations).
Perhaps unsurprisingly, evidence thus far points to sensitive parent-child interaction as critical for the development of typical attachment cue processing. For example, mother-child social synchrony during play has been associated with children’s cortical (i.e., fusiform gyrus, superior temporal sulcus, insula) theta and gamma oscillatory activity measured by magnetoencephalography in response to viewing own versus unfamiliar mother-child interactions (10). Moreover, children exposed to early caregiver deprivation (i.e., those with a history of previous institutional care) show less left amygdala discrimination between mother and stranger stimuli than children with no history of institutional care, with less amygdala discrimination being associated with older age-at-adoption and greater indiscriminate friendliness (indicating atypical attachment development) (8). Together, these findings suggest that brain areas implicated in social cognition and emotion processing are influenced by parenting and early adversity and that they may play a role in psychosocial outcomes, in line with the general view of attachment theory that children’s social representations of attachment figures influence their internal working models of the world (15). Importantly, however, most such studies to date have been correlational in nature and do not permit causal interpretations with regard to potential effects on brain development and subsequent behavior.
Interventions for Early Adversity
Early interventions that enhance parenting quality have been shown to improve atypical developmental trajectories associated with early adversity (16). Specifically, early parenting interventions can enhance parental responsiveness (4, 17, 18), improve infants’ attachment quality (4, 17–19), and physiological and behavioral regulation (20, 21). However, the neural mechanisms through which these early interventions improve psychosocial outcomes remain poorly understood.
Attachment and Biobehavioral Catch-up (ABC) (22) is a well-characterized, evidence-based early parenting intervention that may permit researchers to investigate how children’s underlying neurobiology changes in response to early intervention delivered to their parents. ABC is delivered across 10 in-home sessions by trained parent coaches and has been shown to be efficacious in improving parent and child outcomes through multiple randomized controlled trials (RCTs) involving several vulnerable populations including children in the foster care system, children living with birth parents following involvement with Child Protective Services (CPS), and children who were adopted internationally. The intervention aims to increase rates of secure attachment (as well as reduce rates of disorganized attachment) and improve children’s behavioral and biological regulation by increasing parental nurturance when children are distressed, increasing parental sensitivity and positive regard when children are not distressed, and decreasing frightening and intrusive parental behavior. Parents randomly assigned to receive ABC have demonstrated greater sensitivity and positive regard, as well as lower intrusiveness and withdrawal, than parents who received a control intervention (23). The effects go beyond the parents; children whose parents received ABC demonstrated more adaptive patterns of autonomic regulation (24), more normative diurnal cortisol rhythms (20, 21), greater executive functioning skills (25, 26), stronger emotion regulation skills (27), and decreased disorganized attachment (23) than children of parents randomly assigned to the control intervention.
The Current Study
Given the centrality of parental influence in fostering the social brain and behavioral development, the present study aimed to test the causal impact of an early parenting intervention (ABC) on children’s neural processing of parent cues and children’s psychosocial functioning in middle childhood via a randomized clinical trial. It was hypothesized that, relative to high-risk children whose parents received a control intervention, high-risk children whose parents received ABC would 1) show greater neural responsivity to parent cues in cortical regions implicated in social/emotional processing, and 2) exhibit better psychosocial functioning as measured by parent report. In addition, if there were group differences in neural responsivity to parent cues, a secondary aim was to test whether such group differences mediated differences in children’s psychosocial functioning.
Method
Participants
Families (N = 212) were originally recruited as part of a randomized clinical trial (RCT; ClinicalTrials.gov Identifier: deleted for blind review) when children were infants in a major Mid-Atlantic city. As part of a city-wide initiative designed to redirect children from foster care, families were referred from CPS due to risk for abuse or neglect. Upon recruitment, enrolled families were randomly assigned to receive either ABC or a control intervention (described in more detail below). Families were not informed about their intervention group assignments. At pre-intervention, children across the intervention groups did not differ in age, race, or diurnal cortisol levels (20), and caregivers did not differ in age, educational attainment, race (19), parental sensitivity, or attachment-related representations (24). Of the 212 families enrolled in the RCT, 183 participated in initial post-intervention follow-up assessments and 112 participated in 8-year follow-up assessments (see CONSORT diagram in the supplement). A subset of families who participated in the 8-year follow-up assessments were invited to participate in this functional magnetic resonance imaging (fMRI) sub-study. To maximize chances of successful scans, children who successfully completed an electroencephalography (EEG) assessment as part of an 8-year follow-up visit were subsequently invited to participate in this fMRI sub-study. Ultimately, 54 high-risk children (ABC: n = 27, DEF: n = 27) aged 8.1 to 12.1 years participated in this fMRI sub-study (for demographic information, see Supplementary Table 1).
For comparison to the two high-risk groups (i.e., the ABC intervention group and the control intervention group), a new sample of 83 non-CPS-referred children who did not receive any intervention was recruited at age 8 through local community centers and schools. This sample was matched to the CPS-referred sample on race and gender. Families were ineligible for recruitment to the low-risk sample if they had any history of CPS involvement. Similar to the high-risk sample, comparison children who completed the 8-year EEG assessment were subsequently invited to participate in this fMRI sub-study. The fMRI low-risk comparison sample consisted of 26 children aged 9.1 to 11.0 years. Recruitment for the fMRI sub-study ended after a grand total of 80 children participated in the fMRI sub-study (ABC: n = 27, DEF: n = 27, low-risk: n = 26).
Experimental intervention.
ABC is a brief (10-session) home-based parenting intervention that promotes sensitive caregiving. ABC focuses on three main behavioral targets for parents: 1) increasing sensitivity to child signals, 2) increasing nurturance to child distress, and 3) decreasing frightening and harsh behaviors. In addition to manualized content, intervention sessions consist of parent coaches providing “in the moment” commenting and feedback to support parents in identifying their children’s signals and providing responsive care (22).
Control intervention.
Developmental Education for Families (DEF) is an adaptation of existing interventions (e.g., (28)) that have been shown to promote development of children’s motor skills, cognition, and language abilities. Components of the intervention related to parental sensitivity were removed for this study to help distinguish it from ABC.
Procedure
As noted above, families enrolled in the larger longitudinal study investigating the efficacy of ABC were invited to participate in this fMRI sub-study. After parents provided informed consent and children provided assent, children were acclimatized to the scanner using an MRI replica prior to the scanning session, which typically occurred within two weeks of the practice session. The protocol was approved by the institutional review board at the University of Delaware.
Questionnaires
Child Behavior Checklist.
Parents completed the Child Behavior Checklist (CBCL/6–18) (29) in the lab as part of a battery of measures. The CBCL asks about 113 emotional and behavioral problems rated from 0 (not true) to 2 (very true or often true). For the present study, two items related to suicidality and self-harm were removed from the questionnaires. Raw total scores were used in analyses as a measure of psychosocial functioning. In the present sample, the CBCL had excellent internal consistency (α = .94).
The Security Scale.
Children completed the Kerns’ Security Scale (30) which was used to measure their perceived security to their mothers. The Security Scale consists of 15 items divided into three subscales mapping onto parent responsivity and/or availability, reliability during times of stress, and interest in communicating with the parent. Higher scores indicate greater feelings of security in the mother-child relationship. The three subscales were collapsed for analyses. In the present sample, the Security Scale had moderate internal consistency (α = .68), possibly due to the relatively small number of items.
Imaging
Parent/stranger fMRI task.
In the scanner, children completed a parent/stranger task (8, 9) in which they were presented with eight alternating blocks (28 seconds each, for a total task time of 4m 54s) of color photographs of their own mother or another child’s mother (i.e., stranger; matched to parent for ethnicity, age, and body type) exhibiting smiling and neutral facial expressions. Only mother-child dyads were included in this study, so parent and stranger sex was always female. Each alternating block consisted of either images of the child’s parent or images of the stranger. To ensure attention to the task, participants were instructed to respond with a button press to only the smiling stimuli; however, smiling and neutral trials were collapsed together for analyses. Additionally, due to the nature of a block design, analyses of each emotion were not possible.
Image acquisition.
Images were acquired with a Siemens Prisma 3T MRI scanner (Siemens Corp., Erlangen, Germany). A whole-brain, high-resolution, T1-weighted anatomical scan (magnetization prepared rapid gradient echo; 256 × 256 in-plane resolution, 256-mm field of view, 192 × 1-mm sagittal slices) was used for transformation and localization of each participant’s functional data into Montreal Neurological Institute 152 (MNI152) space. For the parent/stranger functional task, T2*-weighted echo-planar images (34 slices) were acquired using an oblique angle of ~30° from each participant’s position, 4-mm slice thickness (skip = 0), repetition time 2000 ms, echo time 30 ms, flip 90°, matrix 64 × 64.
fMRI preprocessing.
Functional imaging data were preprocessed and analyzed with the FMRIB Software Library (FSL v6.0.1) software package (31). Preprocessing, single-subject statistics, and higher-level analyses were performed using FSL’s fMRI Expert Analysis tool (FEAT) (32). Preprocessing steps included slice-timing correction, motion correction (with FMRIB’s linear registration tool (MCFLIRT) (33), image registration to the first volume, smoothing with an anisotropic 6-mm Gaussian kernel (full width at half maximum), time series normalization, and transformation into MNI152 space. Eight explanatory variables were included in the regression model (six motion parameters and the two stimulus types: mother and stranger). Volumes with excessive framewise motion (>0.9 mm from adjacent volume) were censored (34), and participants with >30% total volumes censored were excluded from analysis. From the low-risk group, one participant was excluded due to excessive motion, two were excluded due to image registration problems, and one did not complete the parent-stranger task (see CONSORT diagram in the supplement for high-risk group exclusion details). The final sample consisted of 68 children (ABC = 22, DEF = 24, low-risk = 22) included in analyses. There were no significant group differences in age (F(2,65) = 0.602, p = .551) or sex (χ2(2, N = 68) = 0.123, p = .940) in this final sample.
Statistical Analysis
Whole-brain analyses were performed to test the within-subject effect of stimulus type (mother vs stranger) on activity in cortical and subcortical brain regions, as well as possible group differences in this stimulus effect via a series of planned comparisons. The FLAME 1 mixed effects model was used with automatic outlier de-weighting. Clusters of blood-oxygen-level-dependent (BOLD) activation were considered significant if Z > 2.3 with a corrected cluster significance threshold of p = .05. In addition, due to the number of group comparisons, the family-wise error rate was further controlled with FSL’s “randomise” function with threshold-free cluster enhancement. Brain structure labels were estimated probabilistically using the Harvard-Oxford cortical and subcortical structural atlases in FSL using the “autoaq” function. Lastly, causal mediation analysis (35) was performed in R (version 3.6.1) using the “mediation” package (36) to determine whether intervention group differences in mother-specific BOLD reactivity mediated the relationship between intervention group assignment and psychosocial outcomes.
Results
Behavior
To test the main effects and interactions of stimulus type and group, 2 (stimulus type: mother vs stranger) X 3 (group: ABC vs DEF vs low-risk) analyses of variance were performed for hit rate, false alarm rate, hit reaction time (RT), and false alarm RT. There were no significant main or interaction effects for any of these behavioral measures (all ps > .05). These effects remained non-significant when controlling for child age and child sex (all ps > .05).
Imaging
In order to verify that the parent/stranger task elicited the expected neural responses, whole-brain analysis was performed comparing parent and stranger trials across all participants (see Figure 1). Compared to viewing the stranger photographs, viewing pictures of one’s own mother was associated with greater activation in clusters including the bilateral amygdala, hippocampus, thalamus, and frontal orbital cortex (pcluster < .025; for a complete list of brain regions, see Table 1). Conversely, viewing pictures of the stranger was associated with greater activation in clusters including the precentral and postcentral gyri (p < .05) relative to viewing pictures of one’s own mother.
Figure 1.

Grand average Mother > Stranger contrast. Positive Z-values indicate Mother > Stranger. All ps < .05. Montreal Neurological Institute coordinates X = −25, Y = −6, Z = 13.
Table 1.
Significant Task-Related Activations (Collapsed Across Groups)
| Mother > Stranger Contrast | |||||||
|---|---|---|---|---|---|---|---|
| Cluster | Cluster Size (voxels) |
Center of mass | Peak Z statistic |
Hemisphere | Regions | ||
| x | y | z | |||||
| 6 | 8377 | −26.2 | −6.04 | −9.03 | 5.07 | Left | Insular cortex, superolateral prefrontal cortex, medial/inferior prefrontal cortex, precentral gyrus, superolateral temporal cortex, medial/inferior temporal cortex, anterior/posterior cingulate gyrus, parahippocampal gyrus, medial/inferior occipital cortex, L/R thalamus, L/R caudate, L/R putamen, L/R pallidum, L/R hippocampus, L/R amygdala, cerebellum |
| 5 | 2291 | 44.7 | 10.5 | −21.3 | 4.75 | Right | Insular cortex, superolateral prefrontal cortex, medial/inferior prefrontal cortex, superolateral temporal cortex, medial/inferior temporal cortex, parahippocampal gyrus, putamen, pallidum, hippocampus, amygdala |
| 4 | 2122 | −5.37 | 49.8 | 26.1 | 5.05 | Bilateral | Superolateral prefrontal cortex, medial/inferior prefrontal cortex, anterior/posterior cingulate gyrus |
| 3 | 1179 | −48.2 | −57 | 18.2 | 4.38 | Left | Superolateral temporal cortex, superolateral parietal cortex, superolateral occipital cortex |
| 2 | 1156 | 52 | −48 | 10.4 | 4.19 | Right | Superolateral temporal cortex, medial/inferior temporal cortex, superolateral parietal cortex superolateral occipital cortex, hippocampus |
| 1 | 821 | −3.01 | −55.1 | 28.7 | 3.63 | Bilateral | Superolateral parietal cortex, posterior cingulate gyrus, medial/inferior parietal cortex, medial/inferior |
| Stranger > Mother Contrast | |||||||
| Cluster | Cluster size (voxels) |
Center of mass | Peak Z statistic |
Hemisphere | Regions | ||
| x | y | z | |||||
| 3 | 1032 | −11.7 | −9.33 | 56.4 | 4.11 | Bilateral | Superolateral prefrontal cortex, medial/inferior prefrontal cortex, precentral gyrus, postcentral gyrus, anterior/posterior cingulate gyrus, medial/inferior parietal lobe |
| 2 | 786 | 21.5 | −44.9 | 56.6 | 3.74 | Right | Precentral gyrus, postcentral gyrus, superolateral parietal cortex, superolateral occipital cortex, posterior cingulate gyrus, medial/inferior parietal cortex |
| 1 | 750 | −18.6 | −44.1 | 52.4 | 4.78 | Left | Precentral gyrus, postcentral gyrus, superolateral parietal cortex, superolateral occipital cortex, anterior/posterior cingulate gyrus, medial/inferior parietal cortex |
Note. Unless otherwise specified, regions listed correspond to the hemisphere(s) noted for the given cluster.
Whole-brain analysis comparing the three groups’ responsivity to mother faces vs stranger faces revealed significant differences between the two high-risk groups. Specifically, children whose parents received ABC exhibited greater relative activation to mother (vs stranger) images than children whose parents received DEF. These effects were observed in clusters including the precuneus and cuneal cortex, PCC, middle temporal gyrus, lateral occipital cortex, angular gyrus, and hippocampus (pcorrected < .05; see Figure 2; for a complete list of brain regions with significant group differences, see Table 2). The observed intervention effects remained significant when controlling for child age and child sex (pcorrected < .05). The ABC group also exhibited somewhat greater relative mother activation than the low-risk group in the bilateral precuneus and bilateral cingulate gyrus; however, this group difference did not survive correction for multiple group comparisons (puncorrected < .001, pcorrected = .421). There were no significant whole-brain group differences between the DEF and low-risk groups.
Figure 2.
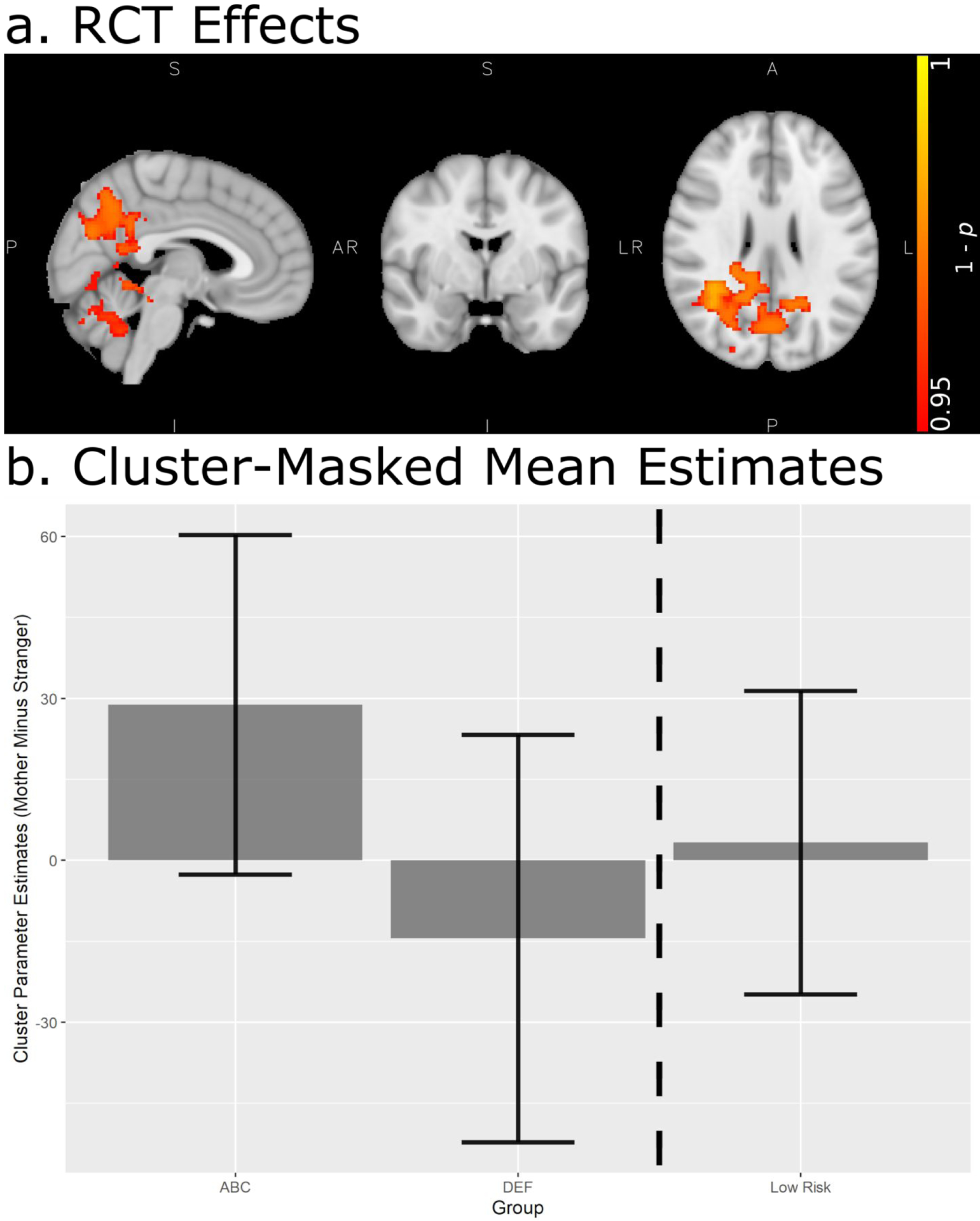
(a) Randomized clinical trial (RCT) group differences in Mother > Stranger contrast from whole-brain analysis after adjusting for multiple group comparisons. Colored regions indicate areas where experimental intervention > control intervention (there were no significant differences where control intervention > experimental intervention). Montreal Neurological Institute coordinates X = 4, Y = −2, Z = 27. (b) Cluster-masked mean voxel-wise statistics from the voxels highlighted in the panel above. Note that these parameter estimates were extracted from voxels that were already identified (via whole-brain analysis) to reflect an ABC > DEF group difference and are plotted here to illustrate the group means at these voxels. Error bars show +/− 1 SD. ABC = Attachment and Biobehavioral Catch-up (experimental intervention). DEF = Developmental Education for Families (control intervention).
Table 2.
Significant ABC > DEF Group Differences (Mother > Stranger Contrast)
| Cluster | Cluster size (voxels) | Center of mass | Peak 1-p statistic | Hemisphere | Regions | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 7 | 7484 | 9.05 | −55.2 | 9.55 | .986 | Bilateral | Superolateral temporal cortex, medial/inferior temporal cortex, postcentral gyrus, superolateral parietal cortex, medial/inferior parietal cortex, superolateral occipital cortex, anterior/posterior cingulate gyrus, parahippocampal gyrus, medial/inferior occipital cortex, L/R thalamus, brain-stem, L/R hippocampus, R caudate, R putamen, L/R cerebellum |
| 6 | 28 | −7.79 | −38 | 58.9 | .952 | Bilateral | Precentral cyrus, postcentral gyrus, posterior cingulate gyrus, medial/inferior parietal cortex |
| 5 | 23 | −21.5 | −34 | 48.3 | .957 | Left | Precentral gyrus, postcentral gyrus, superolateral parietal cortex |
| 4 | 20 | 21.8 | −41.5 | −43.9 | .953 | Right | Brain-stem, cerebellum |
| 3 | 8 | −4 | −26.3 | 57.5 | .951 | Left | Precentral gyrus, postcentral gyrus, superolateral parietal cortex |
| 2 | 4 | 8 | −33 | 57 | .951 | Right | Precentral gyrus, postcentral gyrus, posterior cingulate cortex, medial/inferior parietal cortex |
| 1 | 1 | 2 | −38 | 58 | .950 | Left | Precentral gyrus, postcentral gyrus, medial/inferior parietal cortex |
Note. Unless otherwise specified, regions listed correspond to the hemisphere(s) noted for the given cluster. All p-values adjusted for multiple comparisons. There were no significant differences where DEF > ABC. ABC = Attachment and Biobehavioral Catch-up (experimental intervention). DEF = Developmental Education for Families (control intervention).
Additionally, based on previous literature (8, 9), we performed amygdala region-of-interest (ROI) analyses with separate left and right amygdala ROIs based on the Harvard-Oxford Subcortical Structural Atlas. There were no significant group differences in the left amygdala (mother only: F(2,65) = 0.117, p = .890, η2 = .004; stranger only: F(2,65) = 0.800, p = .454, η2 = .024; mother vs stranger: F(2,65) = 1.711, p = .189, η2 = .050) or right amygdala (mother only: F(2,65) = 0.529, p = .592, η2 = .016; stranger only: F(2,65) = 1.804, p = .173, η2 = .053; mother vs stranger: F(2,65) = 2.371, p = .101, η2 = .068). These amygdala group effects remained non-significant when controlling for child age and child sex (all ps > .05).
Further, there were no significant associations between task behavior and task-related BOLD activation in the left or right amygdala or in the clusters of brain regions that differentiated the ABC and DEF groups (primarily precuneus and cingulate gyrus; see above) in any of the contrasts of interest (i.e., mother vs stranger, mother only, stranger only; all ps > .05).
Questionnaires
For descriptive statistics and correlations among questionnaire measures, see tables in the supplement. There were no significant group differences in CBCL total score (F(2,64) = 0.473, p = .625, η2 = .015) or Security Scale total score (F(2,65) = 0.391, p = .678, η2 = .012). Group effects on questionnaire measures remained non-significant when controlling for child age and child sex (all ps > .05). See Supplementary Table 1 for descriptive statistics of subscale scores. However, greater activity in the clusters differentiating the ABC and DEF groups (mother vs stranger) was associated with lower CBCL total scores (r(65) = −0.27, p = .030; see Figure 3), indicating that greater relative mother (versus stranger) activation in these areas was associated with fewer parent-reported behavior problems. For associations between Security Scale scores and task-related BOLD measures, see Supplementary Table 2.
Figure 3.
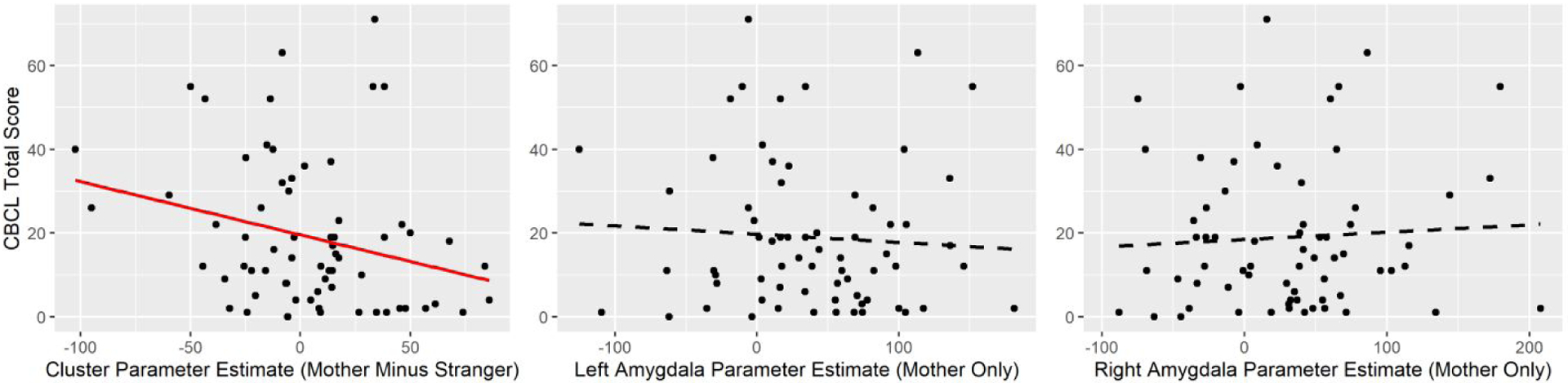
Scatterplots depicting relationships between neural activation during the parent/stranger task and the Child Behavior Checklist (CBCL) The solid red regression line indicates a significant correlation (p < .05) whereas dashed black regression lines indicate non-significant correlations.
Mediation Analysis
In order to test for potential indirect effects of intervention group on psychosocial outcomes within the high-risk sample, causal mediation analysis (35, 36) was performed with 10,000 permutations using intervention group assignment as the predictor, BOLD reactivity during the parent/stranger task as the mediator, and total CBCL score as the outcome. Specifically, the mediator consisted of the average beta weights from the mother-stranger contrast cluster that significantly differentiated the ABC and DEF groups. Although there was no significant direct effect of intervention on CBCL score (p > .05), there was a significant indirect effect of intervention) on CBCL score (average mediation estimate = −7.453, 95% CI [−16.773 −0.320], p = .037). This estimate indicates the average decrease in total CBCL scores that was attributable to ABC’s impact on BOLD reactivity during the parent/stranger task. See Supplementary Table 3 for additional mediation models involving CBCL subscale scores and Security Scale scores.
Discussion
The present study aimed to test the causal impact of an early intervention that enhances parenting on children’s neural processing of maternal cues and on their psychosocial functioning during middle childhood in a randomized clinical trial. To date, most previous work in this area has been correlational and, thus, vulnerable to numerous threats to internal validity. It was hypothesized that, relative to high-risk children of mothers randomized to receive the control intervention, high-risk children of mothers randomized to receive the ABC intervention would show greater neural responsivity to maternal cues in areas implicated in social processing (e.g., amygdala and cortical regions such as the OFC, PCC, insula, temporal fusiform cortex, and precuneus cortex). When looking across both high-risk and low-risk children, consistent with prior child neuroimaging studies involving presentation of maternal cues (9, 11), mother-specific activation was observed across a wide variety of brain regions, including frontal and sensory cortices as well as subcortical structures. With regard to this study’s main hypothesis, children whose parents received the ABC intervention exhibited greater responsivity to maternal cues in clusters of brain regions including the precuneus and cuneal cortex, PCC, middle temporal gyrus, temporal fusiform cortex, lateral occipital cortex, angular gyrus, hippocampus, and others. The ABC group also exhibited somewhat greater responsivity to maternal cues in a subset of these brain regions (i.e., precuneus and PCC) compared to the low-risk comparison group, but this effect did not survive correction for multiple group comparisons. Somewhat contrary to what was predicted, there were no significant group differences in amygdala activation; however, given that the amygdala is a relatively small structure with low MR signal, it is possible that the present study was underpowered to detect group differences in amygdala activation. Nevertheless, clear intervention effects were observed among the high-risk sample, allowing causal interpretations of the effects of a parenting intervention on neural reactivity to attachment cues among human children. Results suggest that ABC may enhance children’s brain development despite the fact that it targets parental sensitivity rather than intervening with the child directly.
Many of the brain areas whose maternal-cue-related activation was augmented by ABC are also implicated in aspects of social cognition such as theory of mind and other aspects of social representation (37), suggesting that ABC could be enhancing brain regions supporting children’s social cognitive development. Cortical midline structures (CMS), which include the precuneus and PCC, have been implicated in understanding “complex psychological aspects of others” (p. 156), such as their attitudes (38). The precuneus, in particular, is a major node of the CMS thought to be involved in elaborating highly integrated and associative information such as maintaining self-other representations across multiple domains, and has direct connections to the mirror neuron system (MNS) involved in imitative behavior and social cognition (38). The MNS, which also includes regions whose maternal-cue-related activity was augmented by ABC (e.g., superior parietal lobule, inferior occipital cortex), is especially sensitive to self-other mappings such that its level of activation tracks the degree of schematic overlap between the self and a perceived other (39). Although based on reverse-inference, these findings, when taken together, point to a possible interpretation wherein ABC enhances children’s relational representation of their mothers, resulting in heightened activation of CMS and MNS brain regions while viewing pictures of the caregiver. However, because the control (i.e., stranger) face was unfamiliar to the participant, further study is needed to determine whether the observed effect of the parenting intervention on facial processing is specific to maternal cues or generalizable to familiar others. This would help clarify the extent to which intervention effects are limited to reactivity to parent cues versus having a more global impact on social processing.
Given that ABC has been associated with improvements in executive functioning and emotion regulation skills that endure through at least early childhood (25–27), we also hypothesized that children whose parents received ABC might exhibit better psychosocial functioning as late as middle childhood compared to children whose parents received the control intervention. Although we did not find a significant direct effect of intervention on CBCL scores at the age of scanning (i.e., 8.1 to 12.1 years), there was a significant indirect effect of intervention on total CBCL scores mediated through maternal-specific activation of the clusters of brain regions that significantly differentiated the ABC group from the control intervention group (e.g., precuneus, PCC, superior parietal lobule, inferior occipital cortex). A potential limitation of this mediation model is that because the mediator and outcome were measured at the same time point, temporal precedence of the mediator over the outcome cannot be established. However, because there was no significant direct effect of intervention group on CBCL scores, an alternative model in which the association between intervention group and task-related BOLD reactivity is mediated by psychosocial functioning can be ruled out. If it is indeed the case that the mediator reflects enhancement of children’s parent-child relational representations, the results of the significant mediation model would be consistent with the view that attachment figures influence children’s internal working model of the social world, which, in turn, influences children’s psychosocial functioning (15).
In addition to the limitations mentioned earlier, it should be noted that due to a lack of detailed CPS referral information, the high-risk group (consisting of families who received ABC or the control intervention) combined children who are likely to have experienced neglect, abuse, or both. Although children with substantiated and unsubstantiated allegations of maltreatment are at similar risk for negative behavioral and developmental outcomes (40), it is not unreasonable to suppose that an intervention aimed at enhancing parental sensitivity may have a differential impact as a function of the type of early adversity or maltreatment a child experienced; however, the fact that significant intervention effects emerged in a relatively small sample despite this potential heterogeneity highlights the value of early intervention.
Overall, the significant indirect effects of ABC revealed by mediation analysis suggest that, in addition to ABC causing greater mother-specific activation of the empirically-identified brain regions (perhaps suggesting enhancement of the child’s parent-child relational representation), this pattern of activation may be indicative of improved parent-child relationship factors that are 1) enhanced by ABC, and 2) associated with better psychosocial outcomes. In other words, results suggest a possible neural pathway through which an early parenting intervention–in this case, ABC–may prevent future behavior problems among high-risk children, yielding psychosocial benefits that endure through at least middle childhood without a need for additional intervention.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Mental Health, Grand/Award Numbers: R01MH074374 (to M. D.) and R01MH091864 (to N. T.).
Footnotes
All authors report no financial relationships with commercial interests.
References
- 1.Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology. 2016;41:177–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goff B, Tottenham N. Early-life adversity and adolescent depression: Mechanisms involving the ventral striatum. CNS Spectrums. 2015;20:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer JC, Martinez CD. Child maltreatment and insecure attachment: A meta-analysis. Journal of Reproductive and Infant Psychology. 2006;24:187–197. [Google Scholar]
- 4.Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18:623–649. [DOI] [PubMed] [Google Scholar]
- 5.Fearon RP, Bakermans-Kranenburg MJ, van IJzendoorn MH, Lapsley A-M, Roisman GI. The significance of insecure attachment and disorganization in the development of children’s externalizing behavior: A meta-analytic study. Child Development. 2010;81:435–456. [DOI] [PubMed] [Google Scholar]
- 6.Groh AM, Roisman GI, van IJzendoorn MH, Bakermans-Kranenburg MJ, Fearon RP. The significance of insecure and disorganized attachment for children’s internalizing symptoms: A meta-analytic study. Child Development. 2012;83:591–610. [DOI] [PubMed] [Google Scholar]
- 7.Callaghan BL, Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N. Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: An examination across 3 years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2019;4:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsavsky AK, Telzer EH, Shapiro M, Humphreys KL, Flannery J, Goff B, Tottenham N. Indiscriminate amygdala response to mothers and strangers after early maternal deprivation. Biological Psychiatry. 2013;74:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tottenham N, Shapiro M, Telzer EH, Humphreys KL. Amygdala response to mother. Developmental Science. 2012;15:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt M, Goldstein A, Feldman R. Child brain exhibits a multi-rhythmic response to attachment cues. Social cognitive and affective neuroscience. 2018;13:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrams DA, Chen T, Odriozola P, Cheng KM, Baker AE, Padmanabhan A, Ryali S, Kochalka J, Feinstein C, Menon V. Neural circuits underlying mother’s voice perception predict social communication abilities in children. Proceedings of the National Academy of Sciences. 2016;113:6295–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. [DOI] [PubMed] [Google Scholar]
- 13.Numan M, Young LJ. Neural mechanisms of mother–infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior. 2016;77:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM. Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain research. 2014;1580:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowlby J: Attachment and Loss: Vol. 1. Attachment London, Hogarth Press and the Institute of Psycho-Analysis; 1969. [Google Scholar]
- 16.Scott S Parenting quality and children’s mental health: Biological mechanisms and psychological interventions. Current Opinion in Psychiatry. 2012;25:301–306. [DOI] [PubMed] [Google Scholar]
- 17.Letarte MJ, Normandeau S, Allard J. Effectiveness of a parent training program “Incredible Years” in a child protection service. Child Abuse and Neglect. 2010;34:253–261. [DOI] [PubMed] [Google Scholar]
- 18.Swenson CC, Schaeffer CM, Henggeler SW, Faldowski R, Mayhew AM. Multisystemic therapy for child abuse and neglect: A randomized effectiveness trial. Journal of Family Psychology. 2010;24:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, Carlson E. Enhancing attachment organization among maltreated children: Results of a randomized clinical trial. Child Development. 2012;83:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard K, Dozier M, Bick J, Gordon MK. Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Development and Psychopathology. 2015;27:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard K, Hostinar CE, Dozier M. Intervention effects on diurnal cortisol rhythms of child protective services–referred infants in early childhood: Preschool follow-up results of a randomized clinical trial. JAMA Pediatrics. 2015;169:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dozier M, Bernard K: Coaching Parents of Vulnerable Infants: The Attachment and Biobehavioral Catch-up Approach. New York, Guilford Press; 2019. [Google Scholar]
- 23.Yarger HA, Bernard K, Caron EB, Wallin A, Dozier M. Enhancing parenting quality for young children adopted internationally: Results of a randomized controlled trial Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53; 2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabachnick AR, Raby KL, Goldstein A, Zajac L, Dozier M. Effects of an attachment-based intervention in infancy on children’s autonomic regulation during middle childhood. Biological Psychology. 2019;143:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis-Morrarty E, Dozier M, Bernard K, Terracciano SM, Moore SV. Cognitive flexibility and theory of mind outcomes among foster children: Preschool follow-up results of a randomized clinical trial. Journal of Adolescent Health. 2012;51:S17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lind T, Lee Raby K, Caron EB, Roben CK, Dozier M. Enhancing executive functioning among toddlers in foster care with an attachment-based intervention. Development and Psychopathology. 2017;29:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind T, Bernard K, Ross E, Dozier M. Intervention effects on negative affect of CPS-referred children: Results of a randomized clinical trial. Child Abuse and Neglect. 2014;38:1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramey CT, Yeates KO, Short EJ. The plasticity of intellectual development: insights from preventive intervention. Child Development. 1984;55:1913–1925. [PubMed] [Google Scholar]
- 29.Achenbach TM, Rescorla L: Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-Informant Assessment, ASEBA Burlington, VT:; 2001. [Google Scholar]
- 30.Kerns KA, Klepac L, Cole A. Peer relationships and preadolescents’ perceptions of security in the child-mother relationship. Developmental Psychology. 1996;32:457. [Google Scholar]
- 31.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 32.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–1386. [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 34.Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human brain mapping. 2014;35:1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Zheng C, Kim C, Van Poucke S, Lin S, Lan P. Causal mediation analysis in the context of clinical research. Annals of Translational Medicine. 2016;4:425–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R Package for Causal Mediation Analysis. 2014.
- 37.Saxe R, Powell LJ. It’s the thought that counts: Specific brain regions for one component of theory of mind. Psychological Science. 2006;17:692–699. [DOI] [PubMed] [Google Scholar]
- 38.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11:153–157. [DOI] [PubMed] [Google Scholar]
- 39.Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: An event-related fMRI study. NeuroImage. 2005;25:926–935. [DOI] [PubMed] [Google Scholar]
- 40.Hussey JM, Marshall JM, English DJ, Knight ED, Lau AS, Dubowitz H, Kotch JB. Defining maltreatment according to substantiation: Distinction without a difference? Child Abuse Negl. 2005;29:479–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


