Abstract
Background
Chronic kidney disease (CKD) and immunosuppression, such as in renal transplantation (RT), stand as one of the established potential risk factors for severe coronavirus disease 2019 (COVID-19). Case morbidity and mortality rates for any type of infection have always been much higher in CKD, haemodialysis (HD) and RT patients than in the general population. A large study comparing COVID-19 outcome in moderate to advanced CKD (Stages 3–5), HD and RT patients with a control group of patients is still lacking.
Methods
We conducted a multicentre, retrospective, observational study, involving hospitalized adult patients with COVID-19 from 47 centres in Turkey. Patients with CKD Stages 3–5, chronic HD and RT were compared with patients who had COVID-19 but no kidney disease. Demographics, comorbidities, medications, laboratory tests, COVID-19 treatments and outcome [in-hospital mortality and combined in-hospital outcome mortality or admission to the intensive care unit (ICU)] were compared.
Results
A total of 1210 patients were included [median age, 61 (quartile 1–quartile 3 48–71) years, female 551 (45.5%)] composed of four groups: control (n = 450), HD (n = 390), RT (n = 81) and CKD (n = 289). The ICU admission rate was 266/1210 (22.0%). A total of 172/1210 (14.2%) patients died. The ICU admission and in-hospital mortality rates in the CKD group [114/289 (39.4%); 95% confidence interval (CI) 33.9–45.2; and 82/289 (28.4%); 95% CI 23.9–34.5)] were significantly higher than the other groups: HD = 99/390 (25.4%; 95% CI 21.3–29.9; P < 0.001) and 63/390 (16.2%; 95% CI 13.0–20.4; P < 0.001); RT = 17/81 (21.0%; 95% CI 13.2–30.8; P = 0.002) and 9/81 (11.1%; 95% CI 5.7–19.5; P = 0.001); and control = 36/450 (8.0%; 95% CI 5.8–10.8; P < 0.001) and 18/450 (4%; 95% CI 2.5–6.2; P < 0.001). Adjusted mortality and adjusted combined outcomes in CKD group and HD groups were significantly higher than the control group [hazard ratio (HR) (95% CI) CKD: 2.88 (1.52–5.44); P = 0.001; 2.44 (1.35–4.40); P = 0.003; HD: 2.32 (1.21–4.46); P = 0.011; 2.25 (1.23–4.12); P = 0.008), respectively], but these were not significantly different in the RT from in the control group [HR (95% CI) 1.89 (0.76–4.72); P = 0.169; 1.87 (0.81–4.28); P = 0.138, respectively].
Conclusions
Hospitalized COVID-19 patients with CKDs, including Stages 3–5 CKD, HD and RT, have significantly higher mortality than patients without kidney disease. Stages 3–5 CKD patients have an in-hospital mortality rate as much as HD patients, which may be in part because of similar age and comorbidity burden. We were unable to assess if RT patients were or were not at increased risk for in-hospital mortality because of the relatively small sample size of the RT patients in this study.
Keywords: COVID-19, haemodialysis, kidney disease, mortality, renal transplantation
KEY LEARNING POINTS
What is already known about this subject?
increased prevalence and risk factors for mortality have been reported in several small and heterogeneous groups of chronic kidney disease (CKD) patients;
reports showed a very high risk of mortality among renal transplant (RT) recipients and maintenance haemodialysis (HD) patients; and
there is no comparative analysis among advanced CKD, RT and HD patients.
What this study adds?
Stages 3–5 CKD patients and HD patients hospitalized for coronavirus disease 2019 (COVID-19) had the highest mortality compared with RT patients and patients without kidney disease; and
mortality in RT patients appears to be lower than in HD and CKD patients, but higher than in patients without kidney disease.
What impact this may have on practice or policy?
the findings may have implications for organizing kidney services, including continuing RT activities during COVID-19.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), turned into a pandaemic after a group of patients with respiratory disease of unknown aetiology was reported in Wuhan, Hubei Province, China [1]. Chronic kidney disease (CKD) and immunosuppression, such as in renal transplantation (RT), stand as one of the established potential risk factors for severe COVID-19 [2]. Case morbidity and mortality rates for any type of infection have always been much higher in CKD, haemodialysis (HD) and RT patients than in the general population. Currently, little is known about the risk, presentation and outcomes of COVID-19 in these patients. There are some published case reports or case series with small numbers for CKD, dialysis or RT patients with COVID-19. The heterogeneity in these papers was too great to allow an ultimate conclusion to be reached, since patients were usually presented with atypical symptoms and had a high mortality rate compared with the general population. A large study that compares COVID-19 outcome in moderate to advanced CKD (Stages 3–5), HD and RT patients with a control group of patients is still lacking.
In Turkey, after the pandaemic started, most nephrology clinics, like other clinics, were transformed into a pandaemic clinic and were involved in the follow-up and treatment of COVID-19 patients, other than those with kidney diseases. In addition, dialysis units related to these clinics were also reorganized and served HD patients who were hospitalized due to COVID-19, as well as HD patients without COVID-19 as outpatients.
In this multicentre, nationwide study in Turkey, we present clinical characteristics and outcomes of CKD, HD and RT patients hospitalized for COVID-19 and compare them with a control group without kidney disease.
MATERIALS AND METHODS
Study design and participants
This multicentre, retrospective, observational study was conducted on the basis of data collected from 47 centres in Turkey. The study was designed by the investigators and unconditionally supported by the Turkish Society of Nephrology. The study was approved by the Health Sciences University, Istanbul Haseki Training and Research Hospital Ethics Committee with the number 41-2020. Informed consent was waived due to the urgent need to collect data in the pandaemic time.
All hospitalized patients with a COVID-19 diagnosis in the participating centres were analysed from 17 April to 6 May 2020.
Diagnosis of COVID-19 was as follows: (i) COVID-19 was diagnosed by a positive result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) based on reverse transcriptase polymerase chain reaction (RT-PCR) testing of a nasopharyngeal swab. Patients whose first test was negative, but retests positive, were also accepted as confirmed cases. (ii) As the test may reveal false-negative results and/or may not be available for a significant portion of patients [3], patients with clinical symptoms were screened by a chest computed tomography (CT). If there was radiological evidence, the patient was diagnosed as ‘possible case of COVID-19’ according to the Ministry of Health ‘Covid-19 Diagnosis and Treatment Guideline’ [4], and included in the study.
In each centre, the next patient without known kidney disease that was hospitalized after a study patient, was included as the control group. Patients with a known diagnosis of CKD and having estimated glomerular filtration rates (eGFRs) <60 mL/min/1.73 m2 represented moderate to advanced CKD. Patients undergoing maintenance HD at least for 3 months or with a functioning RT represented HD and RT groups, respectively. Peritoneal dialysis patients were not included in the study. Patients who were <18 years of age, pregnant or who had acute kidney injury on admission, lacked hospital discharge information or survival data, were still hospitalized [except intensive care unit (ICU)] at the time of data collection and patients hospitalized for reasons other than COVID-19 were excluded from the study.
Data collection
All centres collected data by reviewing electronic health records of their hospital system. Admission data including demographic information, symptoms from onset to hospital admission and possible transmission sources of SARS-CoV-2 infection, comorbidities and medications, smoking habits, aetiology of kidney disease, initial laboratory data, including serum creatinine, eGFR calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [5], serum albumin, ferritin, C-reactive protein (CRP), haemoglobin, lymphocyte, platelet count, lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) were collected.
The dataset also included severity of COVID-19 (as mild to moderate, severe or critical), the same laboratory tests during hospitalization, treatment for COVID-19 at the hospital and living status. COVID-19 severity was defined as follows: mild disease defines patients who had mild clinical symptoms without sign of viral pneumonia on chest CT findings. Moderate disease relates to patients who had symptoms such as fever, cough, dyspnoea, fatigue, sore throat, anorexia, myalgia and respiratory system symptoms, etc., along with manifestation of viral pneumonia on chest CT findings. Severe disease refers to adults who met any of the following criteria: respiratory rate ≥30 breaths/min, oxygen saturation ≤93% at rest state; arterial PO2/oxygen concentration ≤300 mmHg. Patients with pulmonary lesion progression >50% within 24–48 h on radiologic imaging were treated as severe cases. According to national guidelines [4], critical disease was related to patients who met any of the following criteria: incident of respiratory failure needing mechanical ventilation; existence of shock; multiple organ dysfunction that requires monitoring; and treatment in the ICU.
Follow-up and outcomes
The follow-up period started from the date of hospitalization and ended the day of discharge, admission to the ICU or death. It was recorded as length of stay at the hospital. Study outcomes were in-hospital mortality and combined in-hospital outcome (mortality or admission to the ICU) in kidney disease groups (CKD, HD or RT) and in the control group.
Statistical analysis
The analyses were performed using the IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were expressed as numbers and percentages for categorical variables and as mean, standard deviation, median, minimum–maximum and 25th–75th percentile for numerical variables. The conformity of variables to normal distribution was assessed using visual (histogram and probability graphs) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk tests). For multiple group comparisons of categorical variables, the Chi-square test was used. In multiple group comparisons of numerical variables, the analysis of variance test was used for normally distributed numerical variables, and the Kruskal–Wallis test was used for non-normally distributed numerical variables. In post hoc analysis, the Mann–Whitney U test with Bonferroni correction was used for subgroup analysis of non-normally distributed variables, and the Chi-square test with Bonferroni correction was used for subgroup analysis of categorical variables. The survival of the patients during their follow-up until discharge was evaluated using Kaplan–Meier method, and comparisons of survival between diagnostic groups were analysed using log-rank test. Since the follow-up data for evaluating 28-day mortality with a logistic regression model was not present for most of the cases with shorter length of hospitalization, the independent predictors of mortality were evaluated by time-to-event approach using multivariate Cox regression analyses. The Cox models included demographic and clinical parameters that suggested a potential effect on the mortality in univariate analyses and the statistically significant independent predictors of mortality were determined by the Cox regression using enter method (P < 0.05). For attaining a balanced distribution of basal demographic and clinical characteristics in diagnostic groups, a case-to-case propensity score matching (PSM) analysis was performed to match the groups for age, gender, presence of diabetes, hypertension, cardiovascular disease, chronic obstructive pulmonary disease (COPD) and eGFR. eGFR was not used when the HD group was matched with any other groups, and the CKD group was matched with the control group, as calculation of eGFR for HD patients was not applicable, and eGFR defines the CKD and the control groups. The PSM was performed as pairwise matching of the diagnostic groups, since matching all groups should significantly decrease the sample size, and also the clinical interest was in comparing pairwise comparisons of particular groups to each other. Following matching of the diagnostic groups, survival analyses were performed between them. The missing data were considered as listwise missing in the analyses and were not imputed in the study. The statistical significance level was set at P < 0.05.
RESULTS
Demographic and clinical characteristics
A total of 1246 hospitalized patients with COVID-19 from 47 different centres were included. After excluding 36 patients due to a variety of exclusion criteria (Figure 1), the final analysis was performed in 1210 patients [median age, 61 (48–71) years, female 551 (45.5%)]. The four groups were composed as HD group (n = 390), RT group (n = 81), CKD group (n = 289) and control group (n = 450).
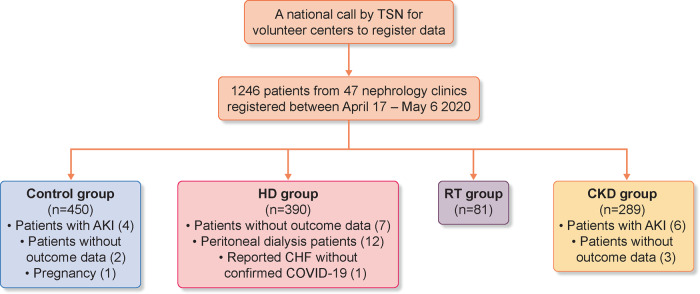
FIGURE 1.
Consort of the study illustrating population selection. Control group: four patients with acute kidney injury (AKI), two patients without outcome data, one patient due to pregnancy. HD group: 12 peritoneal dialysis patients, 5 patients still at the hospital, 2 patients without outcome data, 1 patient reported congestive heart failure without confirmed COVID-19. CKD group: six patients with AKI, three patients without outcome data.
Table 1 shows the baseline demographic and clinical characteristics and laboratory values of the patients. The most common comorbidities were hypertension (745/1187, 62.8%) and diabetes mellitus (394/1178, 33.4%), and these were significantly higher in kidney patients than in the control group (P < 0.05). Cardiovascular diseases were significantly higher in the HD and CKD groups.
Table 1.
Baseline characteristics and tests of patients according to disease groups
| Characteristic | All patients | Control group | HD group | RT group | CKD group |
|---|---|---|---|---|---|
| (N = 1210) | (N = 450) | (N = 390) | (N = 81) | (N = 289) | |
| Demographic information | |||||
| Gender, n (%) | |||||
| Female, n (%) | 551 (45.5) | 204 (45.3) | 189 (48.5) | 33 (40.7) | 125 (43.3) |
| Age (years), median (Q1–Q3) | 61 (48–71) | 51 (38–63) | 64 (55–71) | 48 (38–56) | 71 (63–79) |
| RTT duration, years, median (Q1–Q3) | – | NA | 3.4 (1–6) | 5 (3–9) | NA |
| Coexisting disorder, n/N (%) | |||||
| Diabetes mellitus | 394/1178 (33.4) | 68/440 (15.5) | 185/383 (48.3) | 20/79 (25.3) | 121/276 (43.8) |
| Hypertension | 745/1187 (62.8) | 132/440 (30) | 302/383 (78.9) | 57/79 (72.2) | 254/285 (89.1) |
| Ischaemic heart disease | 341/1134 (30.1) | 40/431 (9.3) | 161/355 (45.4) | 13/76 (17.1) | 127/272 (46.7) |
| Heart failure | 179/1134 (15.8) | 19/436 (4.4) | 89/354 (25.1) | 2/78 (2.6) | 69/266 (25.9) |
| COPD | 156/1143 (13.6) | 44/436 (10.1) | 50/361 (13.9) | 5/77 (6.5) | 57/269 (21.2) |
| Cancer | 60/1149 (5.2) | 20/436 (4.6) | 20/369 (5.4) | 2/77 (2.6) | 18/267 (6.7) |
| Chronic liver disease | 10/1156 (0.9) | 4/437 (0.9) | 5/370 (1.4) | 0/77 (0) | 1/272 (0.4) |
| Smoking, n/N (%) | |||||
| Never smoked | 490/836 (58.6) | 190/301 (63.1) | 152/284 (53.5) | 33/47 (70.2) | 115/204 (56.4) |
| Former smoker | 238/836 (28.5) | 60/301 (19.9) | 96/284 (33.8) | 13/47 (27.7) | 69/204 (33.8) |
| Current smoker | 108/836 (12.9) | 51/301 (16.9) | 36/284 (12.7) | 1/47 (2.1) | 20/204 (9.8) |
| Medicationsa, n/N (%) | |||||
| ACE inhibitors | 199/1082 (18.4) | 43/431 (10) | 72/334 (21.6) | 14/78 (17.9) | 70/239 (29.3) |
| ARBs | 166/1075 (15.4) | 39/431 (9) | 31/329 (9.4) | 12/77 (15.6) | 84/238 (35.3) |
| Calcium channel blockers | 410/1105 (37.1) | 61/432 (14.1) | 162/349 (46.4) | 42/76 (55.3) | 145/248 (58.5) |
| Beta-blockers | 358/1093 (32.8) | 47/433 (10.9) | 162/341 (47.5) | 25/77 (32.5) | 124/242 (51.2) |
| Other antihypertensives | 136/1045 (13) | 28/428 (6.5) | 38/314 (12.1) | 8/76 (10.5) | 62/227 (27.3) |
| Insulin | 233/1089 (21.4) | 23/434 (5.3) | 131/343 (38.2) | 14/76 (18.4) | 65/236 (27.5) |
| Oral antidiabetics | 131/1080 (12.1) | 48/437 (11) | 17/325 (5.2) | 6/76 (7.9) | 60/242 (24.8) |
| Statins | 152/1061 (14.3) | 25/430 (5.8) | 52/327 (15.9) | 7/74 (9.5) | 68/230 (29.6) |
| Antiaggregant or anticoagulants | 448/1099 (40.8) | 70/431 (16.2) | 194/340 (57.1) | 36/75 (48) | 148/253 (58.5) |
| Primary kidney disease, n/N (%) | |||||
| Amyloidosis | 12/653 (1.8) | NA | 9/343 (2.6) | 3/58 (5.2) | 0/250 (0) |
| Diabetic nephropathy | 265/653 (40.6) | NA | 163/343 (47.5) | 10/58 (17.2) | 91/250 (36.4) |
| Hypertensive nephrosclerosis | 274/653 (42.0) | NA | 122/343 (35.6) | 18/58 (31) | 133/250 (53.2) |
| ADPCKD | 19/653 (2.9) | NA | 13/343 (3.8) | 4/58 (6.9) | 2/250 (0.8) |
| Primary glomerular disease | 40/653 (6.1) | NA | 16/343 (4.7) | 12/58 (20.7) | 12/250 (4.8) |
| Urologic diseases | 24/653 (3.7) | NA | 7/343 (2) | 9/58 (15.5) | 8/250 (3.2) |
| Other | 19/653 (2.9) | NA | 13/343 (3.8) | 2/58 (3.4) | 4/250 (1.6) |
| Laboratory findings, median (Q1–Q3) | |||||
| Creatinine (mg/dL) | 1.6 (0.9–5.3) | 0.8 (0.7–0.93) | 6.7 (5.2–8.57) | 1.48 (1.1–2.05) | 1.8 (1.41–2.63) |
| Albumin (g/dL) | 3.61 (3.2–4) | 3.9 (3.59–4.2) | 3.5 (3.04–3.81) | 3.74 (3.3–4) | 3.4 (3–3.8) |
| Ferritin (ng/mL) | 394 (142–878) | 174 (85–380) | 850 (487–1643) | 316 (135–865) | 316 (140–590) |
| Haemoglobin (g/dL) | 11.72 ± 2.31 | 13.1 ± 1.87 | 10.4 ± 1.97 | 11.6 ± 2.27 | 11.41 ± 2.19 |
| Lymphocyte count (/mm3) | 1050 (600–1580) | 1305 (890–1860) | 885 (490–1290) | 690 (390–1100) | 1000 (520–1550) |
| Platelet count (×1000/mm3) | 210 (165–270) | 221 (177–266) | 185.5 (149–264) | 198 (164–239) | 227 (176–286) |
| CRP, n/N (%) (> × upper limit) | |||||
| Normal | 194/1210 (16) | 131/450 (29.1) | 32/390 (8.2) | 7/81 (8.6) | 24/289 (8.3) |
| 1–5 | 345/1210 (28.5) | 158/450 (35.1) | 96/390 (24.6) | 18/81 (22.2) | 73/289 (25.3) |
| 5–10 | 193/1210 (16) | 60/450 (13.3) | 65/390 (16.7) | 20/81 (24.7) | 48/289 (16.6) |
| 10–20 | 224/1210 (18.5) | 57/450 (12.7) | 85/390 (21.8) | 18/81 (22.2) | 64/289 (22.1) |
| >20 | 254/1210 (21) | 44/450 (9.8) | 112/390 (28.7) | 18/81 (22.2) | 80/289 (27.7) |
HD group: erythropoiesis-stimulating agents 65/354 (18.4%), intravenous iron 54/354 (15.3%), active vitamin D and analogues 52/354 (13.3%), and phosphorus binder 56/354 (15.8%); RT group: corticosteroids 78 (96.3%), tacrolimus 65 (80.2%), cyclosporine-A 7 (8.6%), mycophenolate/mycophenolic acid 67 (82.7%), mammalian target of rapamycin inhibitors 8 (9.9%) and azathioprine 6 (7.4%).
ADPCKD, autosomal dominant polycystic kidney disease; ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers; NA, not applicable; RRT, renal replacement therapy.
In the HD group, the median (interquartile range) duration of dialysis was 3.4 years (1–6), and the most common vascular access was arteriovenous (A-V) fistula (267/382, 69.9%). The median (interquartile range) time after transplantation was 5 years (3–9), tacrolimus, and mycophenolic acid derivatives and corticosteroids were used as immunosuppressants in 80% of the RT patients.
Clinical presentation, diagnosis, treatment regiments for COVID-19, ICU requirement and survival data were shown in Table 2. COVID-19 was diagnosed with a nasopharyngeal swab for SARS-CoV-2 by RT-PCR assay in 56.4% of patients. This positive test rate was significantly lower in the HD group (175/390, 44.9%). On the other hand, diagnostic CT confirmation was used in >90% in all kidney patient groups.
Table 2.
Clinical presentation, diagnosis and treatments given for COVID-19, ICU requirement and survival data
| Characteristic | All patients | Control group | HD group | RT group | CKD group |
|---|---|---|---|---|---|
| (N = 1210) | (N = 450) | (N = 390) | (N = 81) | (N = 289) | |
| Time between first symptom and diagnosis (days), median (Q1–Q3) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 4 (3–7) | 3 (2–5) |
| Clinical presentation, n/N (%) | |||||
| Mild–moderate disease | 797/1205 (66.1) | 377/448 (84.2) | 225/387 (58.1) | 48/81 (59.3) | 147/289 (50.9) |
| Severe–critical disease | 408/1205 (33.9) | 71/448 (15.8) | 162/387 (41.9) | 33/81 (40.7) | 142/289 (49.1) |
| COVID-19 confirmed by RT-PCR, n/N (%) | 682/1210 (56.4) | 291/450 (64.7) | 175/390 (44.9) | 53/81 (65.4) | 163/289 (56.4) |
| Radiological confirmation n/N (%) | 1069/1210 (88.3) | 364/450 (80.9) | 363/390 (93.1) | 75/81 (92.6) | 267/289 (92.4) |
| Possible source of COVID-19, n/N (%) | |||||
| Family–home environment | 256/644 (39.8) | 105/282 (37.2) | 60/184 (32.6) | 18/33 (54.5) | 73/145 (50.3) |
| Workplace/nursing home etc. | 16/644 (2.5) | 9/282 (3.2) | 1/184 (0.5) | 1/33 (3.0) | 5/145 (3.4) |
| Social life | 196/644 (30.4) | 102/282 (36.2) | 34/184 (18.5) | 8/33 (24.2) | 52/145 (35.9) |
| Travel | 17/644 (2.6) | 10/282 (3.5) | 1/184 (0.5) | 2/33 (6.1) | 4/145 (2.8) |
| Healthcare centre | 159/644 (24.7) | 56/282 (19.9) | 88/184 (47.8) | 4/33 (12.1) | 11/145 (7.6) |
| Drug treatments, n/N (%) | |||||
| Hydroxychloroquine | 1173/1199 (97.8) | 444/448 (99.1) | 369/383 (96.3) | 81/81 (100) | 279/287 (97.2) |
| Oseltamivir | 776/1122 (69.2) | 306/426 (71.8) | 228/358 (63.7) | 49/80 (61.3) | 193/258 (74.8) |
| Macrolides | 950/1155 (82.3) | 379/434 (87.3) | 280/370 (75.7) | 53/80 (66.3) | 238/271 (87.8) |
| Lopinavir–ritonavir | 49/925 (5.3) | 7/336 (2.1) | 6/313 (1.9) | 10/71 (14.1) | 26/205 (12.7) |
| Favipiravir | 360/1013 (35.5) | 95/362 (26.2) | 105/331 (31.7) | 37/75 (49.3) | 123/245 (50.2) |
| Glucocorticoids | 92/925 (9.9) | 14/338 (4.1) | 12/316 (3.8) | 42/76 (55.3) | 24/195 (12.3) |
| Tocilizumab | 27/921 (2.9) | 8/338 (2.4) | 6/316 (1.9) | 9/74 (12.2) | 4/193 (2.1) |
| Convalescent plasma | 5/916 (0.5) | 1/334 (0.3) | 1/316 (0.3) | 3/75 (4) | 0/191 (0) |
| Canakinumab/anakinra | 6/918 (0.7) | 0/336 (0) | 2/316 (0.6) | 3/75 (4) | 1/191 (0.5) |
| Any side effects related to these drugs, n/N (%) | 49/1092 (4.5) | 11/417 (2.6) | 11/337 (3.3) | 8/75 (10.7) | 19/263 (7.2) |
| Laboratory tests during hospitalization, n/N (%) | |||||
| Leukopaenia (<4000/mm3) | 220/1190 (18.5) | 69/438 (15.8) | 80/383 (20.9) | 30/81 (37) | 41/288 (14.2) |
| Lymphopaenia (<1500/mm3) | 662/1186 (55.8) | 179/436 (41.1) | 233/382 (61) | 59/81 (72.8) | 191/287 (66.6) |
| Anaemia (<10 g/dL) | 406/1187 (34.2) | 47/439 (10.7) | 190/381 (49.9) | 25/81 (30.9) | 144/286 (50.3) |
| Thrombocytopaenia (<150 × 103/mm3) | 205/1189 (17.2) | 45/438 (10.3) | 85/383 (22.2) | 13/81 (16) | 62/287 (21.6) |
| LDH (>2× upper limit of normal) | 295/1145 (25.8) | 69/423 (16.3) | 90/361 (24.9) | 24/80 (30) | 112/281 (39.9) |
| AST (>2× upper limit of normal) | 198/1170 (16.9) | 57/432 (13.2) | 43/379 (11.3) | 10/73 (13.7) | 88/286 (30.8) |
| Highest value of CRP level during follow-up, n/N (%) (> x of upper normal value) | |||||
| Normal | 163/1143 (14.3) | 118/425 (27.8) | 26/357 (7.3) | 8/79 (10.1) | 11/282 (3.9) |
| 1–5 | 260/1143 (22.7) | 124/425 (29.2) | 73/357 (20.4) | 13/79 (16.5) | 50/282 (17.7) |
| 5–10 | 160/1143 (14) | 52/425 (12.2) | 49/357 (13.7) | 19/79 (24.1) | 40/282 (14.2) |
| 10–20 | 213/1143 (18.6) | 64/425 (15.1) | 79/357 (22.1) | 10/79 (12.7) | 60/282 (21.3) |
| >20 | 347/1143 (30.4) | 67/425 (15.8) | 130/357 (36.4) | 29/79 (36.7) | 121/282 (42.9) |
| Length of stay at hospital, day, median (Q1–Q3) | 9 (6–14) | 8 (6–12) | 9 (6–14) | 9 (6–13) | 10 (7–15) |
| ICU admission, n/N (%) | 266/1210 (21.9) | 36/450 (8.0) | 99/390 (25.4) | 17/81 (21.0) | 114/289 (39.4) |
| Mechanical ventilation in ICU, n/N (%) | 198/251 (77.0) | 20/34 (58.8) | 73/94 (77.7) | 14/17 (82.4) | 91/112 (81.3) |
| Outcome | |||||
| Dead | 172/1210 (14.2) | 18/450 (4) | 63/390 (16.2) | 9/81 (11.1) | 82/289 (28.4) |
| Still in ICU | 32/1210 (2.6) | 5/450 (1.1) | 12/390 (3.1) | 2/81 (2.5) | 13/289 (4.5) |
| Discharged | 988/1210 (81.7) | 423/450 (94) | 308/390 (79) | 69/81 (85.2) | 188/289 (65.1) |
| Transfer to another centre | 18/1210 (1.5) | 4/450 (0.9) | 7/390 (1.8) | 1/81 (1.2) | 6/289 (2.1) |
As a possible source of infection, transmission in the health institution was significantly higher in HD patients (88/184, 47.8%), whereas the family–home environment was significantly higher in RT and CKD groups.
COVID-19 precautions, clinical course and treatments
The HD shift was changed in 62 (15.9%) patients, dialysis was performed in an isolated room in 248 (63.6%) patients and 50 (12.8%) patients underwent dialysis in ICU. No change was made in the dialysis sessions of the remaining 30 (7.7%) HD patients. Immunosuppressive therapy change (discontinuation, drug or dose modifications) was made in 71/79 (97.5%) of RT patients.
The time between the onset of the first symptoms of the COVID-19 and diagnosis was significantly shorter in HD patients as compared with other groups. Severe–critical disease was more frequent in kidney patients 408/1205 (33.9%) as compared with the control group (Table 2). Frequency of lymphopaenia, anaemia and thrombocytopaenia, and increased serum LDH levels were significantly lower in the control group, whereas AST levels were higher in the CKD group. The rate of serum CRP increase was significantly lower in the control group than the other groups.
Almost all (1173/1199, 97.8%) patients were administered hydroxychloroquine. The rates of oseltamivir (776/1122, 69.2%) and macrolide antibiotics (950/1155, 82.3%) use were also high, as suggested in the previous versions of the Turkish National COVID-19 treatment guideline (Table 2). Favipiravir was used at a higher rate in RT (37/75, 49.3%) and CKD groups (123/245, 50.2%) than other groups (P < 0.001). Tocilizumab use was significantly higher in the RT group (9/74, 12.2%). The rate of reported side effects due to any of these drugs was 4.5% (49/1092).
Clinical outcomes
Over one-fifth of the whole group (266/1210, 21.9%) was admitted to ICU. A total of 172/1210 (14.2%) patients died. ICU admission and mortality rates were significantly lower in the control group than in the all kidney disease groups, and higher in the CKD group than in the other groups (Table 2 and Figure 2). Patients who died were older and had higher comorbidities, with male predominance (Table 3). These patients also had severe clinical presentation, worse initial and in-hospital lab values and increased inflammatory parameters. Antiviral drug use was significantly higher in this group.
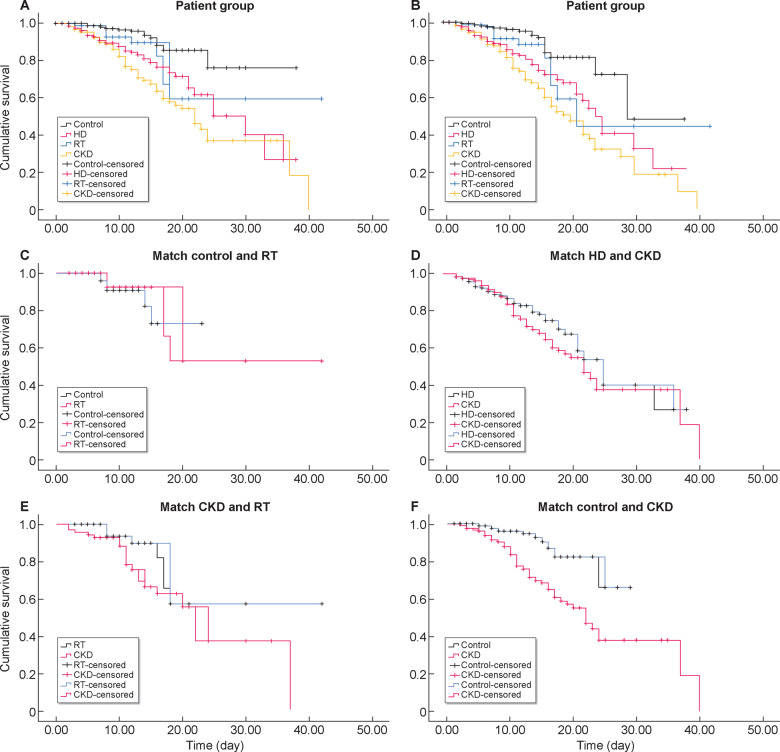
FIGURE 2.
Kaplan–Meier plots of patient survival. (A) All patient groups were compared in terms of mortality. The median duration for the mortality was 30 days in the HD group, >20 days in the RT group, 22 days in the CKD group and >30 days in the control group. The mean duration for mortality of both HD and CKD groups was significantly shorter than that of the control group (log-rank P < 0.01 for both). There was no significant difference between RT and the control group (log-rank P = 0.053). (B) All groups were compared in terms of composite outcome (mortality or ICU admission). Median time to composite outcome was 24 days in the HD group, 21 days in the RT group, 20 days in the CKD group and 29 days in the control group. Mean survival durations of both the HD and CKD groups were statistically significantly shorter than that of the control group (log-rank P < 0.01 for both). There was no significant difference between RT and the control group (log-rank P = 0.052). (C) New matched groups created by PSM from the control and RT groups were compared. The median survival duration was >16 days in the matched RT group and >18 days in the matched control group. The mean survival duration of the matched RT group was similar to that of the matched control group (log-rank P = 0.37). (D) New matched groups created by PSM from HD and CKD groups were compared. Median survival duration was 25 days in the matched HD group and 22 days in the matched CKD group. Mean survival duration of the matched HD group was not significantly different from that of the matched CKD group (log-rank P = 0.21). (E) New matched groups created by PSM from CKD and RT groups were compared. Median survival duration was 23 days in the matched CKD group and >20 days in the matched RT group. Mean survival duration of the matched CKD group was not significantly different than that of the matched RT group (log-rank P = 0.077). (F) New matched groups created by PSM from CKD and control groups were compared. Median survival duration was 23 days in the matched CKD group and >24 days in the matched control group. Mean survival duration of the matched CKD group was significantly different from that of the matched control group (log-rank P < 0.001).
Table 3.
Comparative analysis of characteristics and laboratory values obtained during hospitalization of patients according to survival
| Characteristic | All patients | Dead | Discharged | P-value |
|---|---|---|---|---|
| N = 1160 | N = 172 | N = 988 | ||
| Age (years), median (Q1–Q3) | 60.5 (47–71) | 70 (60–78.5) | 59 (46–69) | <0.001 |
| Gender, n/N (%) | ||||
| Female | 533/1160 (45.9) | 63/172 (36.6) | 470/988 (47.6) | 0.008 |
| Male | 627/1160 (54.1) | 109/172 (63.4) | 518/988 (52.4) | – |
| Coexisting disorder, n/N (%) | ||||
| Diabetes mellitus | 374/1135 (33) | 78/168 (46.4) | 296/967 (30.6) | <0.001 |
| Hypertension | 706/1140 (61.9) | 134/168 (79.8) | 572/972 (58.8) | <0.001 |
| Ischaemic heart disease | 319/1088 (29.3) | 78/158 (49.4) | 241/930 (25.9) | <0.001 |
| Heart failure | 169/1091 (15.5) | 51/161 (31.7) | 118/930 (12.7) | <0.001 |
| Ischaemic heart disease or heart failure | 363/1092 (33.2) | 87/161 (54.0) | 276/931 (29.6) | <0.001 |
| COPD | 142/1100 (12.9) | 35/159 (22) | 107/941 (11.4) | <0.001 |
| Cancer | 57/1109 (5.1) | 12/162 (7.4) | 45/947 (4.8) | 0.157 |
| Chronic liver disease | 10/1114 (0.9) | 3/165 (1.8) | 7/949 (0.7) | 0.174 |
| Smoking, n/N (%) | ||||
| Never smoked | 479/811 (59.1) | 64/111 (57.7) | 415/700 (59.3) | 0.417 |
| Former smoker | 228/811 (28.1) | 36/111 (32.4) | 192/700 (27.4) | – |
| Current smoker | 104/811 (12.8) | 11/111 (9.9) | 93/700 (13.3) | – |
| Primary kidney disease, n/N (%) | ||||
| Amyloidosis | 12/620 (1.9) | 3/137 (2.2) | 9/483 (1.9) | 0.171 |
| Diabetic nephropathy | 252/620 (40.6) | 63/137 (46) | 189/483 (39.1) | – |
| Hypertensive nephrosclerosis | 258/620 (41.6) | 59/137 (43.1) | 199/483 (41.2) | – |
| ADPCKD | 18/620 (2.9) | 0/137 (0) | 18/483 (3.7) | – |
| Primary glomerular disease | 38/620 (6.1) | 6/137 (4.4) | 32/483 (6.6) | – |
| Urologic diseases | 24/620 (3.9) | 3/137 (2.2) | 21/483 (4.3) | – |
| Other | 18/620 (2.9) | 3/137 (2.2) | 15/483 (3.1) | – |
| Possible source of COVID-19, n/N (%) | ||||
| Family–home environment | 245/621 (39.5) | 41/85 (48.2) | 204/536 (38.1) | 0.349 |
| Workplace/nursing home, etc. | 15/621 (2.4) | 1/85 (1.2) | 14/536 (2.6) | – |
| Social life | 189/621 (30.4) | 26/85 (30.6) | 163/536 (30.4) | – |
| Travel | 17/621 (2.7) | 2/85 (2.4) | 15/536 (2.8) | – |
| Healthcare centre | 155/621 (25) | 15/85 (17.6) | 140/536 (26.1) | – |
| Medications, n/N (%) | ||||
| ACE inhibitors | 187/1043 (17.9) | 35/143 (24.5) | 152/900 (16.9) | 0.028 |
| ARBs | 151/1033 (14.6) | 27/141 (19.1) | 124/892 (13.9) | 0.101 |
| Calcium channel blockers | 388/1062 (36.5) | 64/148 (43.2) | 324/914 (35.4) | 0.068 |
| Beta-blockers | 343/1054 (32.5) | 69/150 (46) | 274/904 (30.3) | <0.001 |
| Other antihypertensives | 130/1006 (12.9) | 25/134 (18.7) | 105/872 (12) | 0.034 |
| Insulin | 219/1052 (20.8) | 46/144 (31.9) | 173/908 (19.1) | <0.001 |
| Oral antidiabetics | 126/1043 (12.1) | 23/144 (16) | 103/899 (11.5) | 0.123 |
| Statins | 143/1023 (14) | 29/138 (21) | 114/885 (12.9) | 0.010 |
| Antiaggregant or anticoagulants | 425/1056 (40.2) | 82/150 (54.7) | 343/906 (37.9) | <0.001 |
| Dialysis-related drugs, n/N (%) | ||||
| ESA | 62/337 (18.4) | 9/58 (15.5) | 53/279 (19) | 0.534 |
| Vitamin D and analogues | 52/337 (15.4) | 9/58 (15.5) | 43/279 (15.4) | 0.984 |
| Phosphate binders | 55/337 (16.3) | 11/58 (19) | 44/279 (15.8) | 0.549 |
| Tx immunosuppressive drugs, n (%) | ||||
| Corticosteroids | 76/78 (97.4) | 9/9 (100) | 67/69 (97.1) | 1.000 |
| Tacrolimus | 63/78 (80.8) | 7/9 (77.8) | 56/69 (81.2) | 1.000 |
| Cyclosporine-A | 7/78 (9) | 0/9 (0) | 7/69 (10.1) | 1.000 |
| MMF/MFA | 65/78 (83.3) | 9/9 (100) | 56/69 (81.2) | 0.342 |
| mTOR inhibitors | 8/78 (10.3) | 1/9 (11.1) | 7/69 (10.1) | 1.000 |
| Azathioprine | 6/78 (7.7) | 0/9 (0) | 6/69 (8.7) | 1.000 |
| Vascular access, n/N (%) | ||||
| Catheter | 104/363 (28.7) | 18/60 (30) | 86/303 (28.4) | 0.897 |
| A-V fistula | 258/363 (71.1) | 42/60 (70) | 216/303 (71.3) | – |
| A-V graft | 1/363 (0.3) | 0/60 (0) | 1/303 (0.3) | – |
| CRP, n/N (%) | ||||
| Normal | 189/1160 (16.3) | 3/172 (1.7) | 186/988 (18.8) | <0.001 |
| (x of upper normal value) | ||||
| 1–5 | 337/1160 (29.1) | 16/172 (9.3) | 321/988 (32.5) | – |
| 5–10 | 186/1160 (16) | 25/172 (14.5) | 161/988 (16.3) | – |
| 10–20 | 216/1160 (18.6) | 46/172 (26.7) | 170/988 (17.2) | – |
| >20 | 232/1160 (20) | 82/172 (47.7) | 150/988 (15.2) | – |
| CRP, n/N (%) (x of upper normal value) | ||||
| <10 | 712/1160 (61.4) | 44/172 (25.6) | 668/988 (67.6) | <0.001 |
| ≥10 | 448/1160 (38.6) | 128/172 (74.4) | 320/988 (32.4) | |
| COVID-19 related clinic presentation at the time of diagnosis, n/N (%) | ||||
| Mild–moderate disease | 778/1155 (67.4) | 22/171 (12.9) | 756/984 (76.8) | <0.001 |
| Severe–critical disease | 377/1155 (32.6) | 149/171 (87.1) | 228/984 (23.2) | |
| Positive nasopharyngeal swab RT-PCR, n/N (%) | 655/1160 (56.5) | 106/172 (61.6) | 549/988 (55.6) | 0.139 |
| COVID-19 drug treatments, n/N (%) | ||||
| Oseltamivir | 749/1075 (69.7) | 129/161 (80.1) | 620/914 (67.8) | 0.002 |
| Macrolides | 917/1109 (82.7) | 144/160 (90) | 773/949 (81.5) | 0.008 |
| Chloroquine/hydroxychloroquine | 1127/1150 (98) | 165/171 (96.5) | 962/979 (98.3) | 0.137 |
| Lopinavir–ritonavir | 49/891 (5.5) | 21/127 (16.5) | 28/764 (3.7) | <0.001 |
| Favipiravir | 334/971 (34.4) | 109/145 (75.2) | 225/826 (27.2) | <0.001 |
| Glucocorticoids | 89/892 (10) | 37/121 (30.6) | 52/771 (6.7) | <0.001 |
| Tocilizumab | 25/886 (2.8) | 12/117 (10.3) | 13/769 (1.7) | <0.001 |
| Convalescent plasma | 5/883 (0.6) | 3/117 (2.6) | 2/766 (0.3) | 0.019 |
| Canakinumab/anakinra | 6/885 (0.7) | 2/118 (1.7) | 4/767 (0.5) | 0.185 |
| Any side effects related to these drugs, n/N (%) | 47/1050 (4.5) | 10/135 (7.4) | 37/915 (4) | 0.078 |
| Laboratory findings, median (Q1–Q3) | ||||
| Creatinine (mg/dL), median (Q1–Q3) | 1.58 (0.89–5.21) | 2.6 (1.41–5.90) | 1.4 (0.84–5.16) | <0.001 |
| Albumin (g/dL), median (Q1–Q3) | 3.67 (3.2–4) | 3.2 (2.8–3.6) | 3.7 (3.3–4) | <0.001 |
| Ferritin (ng/mL), median (Q1–Q3) | 394 (142–878) | 598 (309–1420) | 355 (126–815) | <0.001 |
| Haemoglobin (g/dL), mean ± SD | 11.8 (10.2–13.3) | 11.3 (9.6–12.8) | 11.9 (10.4–13.4) | 0.001 |
| Lymphocyte count (/mm3), median (Q1–Q3) | 1055 (619–1590) | 740 (400–1140) | 1100 (670–1650) | <0.001 |
| Platelet count (×1000/mm3), median Q1–Q3) | 209 (165–270) | 184.5 (139.5–262) | 214 (169–270) | 0.001 |
| Leukopaenia (<4000/mm3) | 212/1142 (18.6) | 39/170 (22.9) | 173/972 (17.8) | 0.112 |
| Lymphopaenia (<1500/mm3) | 629/1138 (55.3) | 146/168 (86.9) | 483/970 (49.8) | <0.001 |
| Anaemia (<10 g/dL) | 377/1140 (33.1) | 108/170 (63.5) | 269/970 (27.7) | <0.001 |
| Thrombocytopaenia (<150 × 103/mm3) | 198/1141 (17.4) | 72/171 (42.1) | 126/970 (13) | <0.001 |
| LDH (>2×upper limit of normal) | 277/1100 (25.2) | 108/161 (67.1) | 169/939 (18) | <0.001 |
| AST (>2×upper limit of normal) | 187/1123 (16.7) | 89/168 (53) | 98/955 (10.3) | <0.001 |
ADPCKD, autosomal dominant polycystic kidney disease; ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blocker; ESA, erythropoiesis stimulating agents; mTOR, mammalian target of rapamycin; MMF/MFA, mycophenolate mofetil/mycophenolic acid.
In the Cox regression survival analysis age, the severity of the disease, CRP and patient group (being in HD or CKD groups) were significantly related to mortality. Age, haemoglobin level, the severity of the disease, CRP and patient group (being in HD or CKD groups) were significantly related to composite outcome (dead or ICU admission) (Table 4). Kaplan–Meier plots of patient survival were presented in Figure 2. Of note, the in-hospital mortality rate and the composite outcome was not significantly different between the RT and control groups. No mortality difference was observed between the PSM-matched RT and control groups, but the mortality in the HD and CKD groups was significantly higher than in the control group. Additionally, we assessed the mortality rates between HD- and CKD-matched groups with the inclusion of only the patients with RT-PCR test confirmed COVID-19, and this analysis also revealed no difference between HD and CKD groups in terms of mortality (log-rank P = 0.72). When CKD group was subdivided according to eGFR, there was no mortality difference in three eGFR groups [26/92 (28.6%) patients in the <10 mL/min/1.73 m2 group; 31/110 (28.2%) patients in the 10–30 mL/min/1.73 m2 group; and 25/82 (30.5%) patients in the 30–60 mL/min/1.73 m2 group; P = 0.88].
Table 4.
Cox regression model created for the analysis of independent variables associated with in-hospital death or combined outcome (death or ICU admission)
| Characteristic | Dead |
Dead or ICU admission |
||
|---|---|---|---|---|
| P-value | HR (95% CI for HR) | P-value | HR (95% CI for HR) | |
| Age (years) | 0.017 | 1.019 (1.003–1.034) | 0.016 | 1.017 (1.003–1.032) |
| Gender (male) | 0.659 | 1.086 (0.753–1.566) | 0.476 | 1.131 (0.806–1.586) |
| Diabetes mellitus | 0.885 | 1.027 (0.715–1.477) | 0.622 | 1.089 (0.776–1.527) |
| Hypertension | 0.189 | 0.721 (0.443–1.174) | 0.487 | 0.850 (0.536–1.346) |
| Cardiovascular disease | 0.262 | 1.242 (0.850–1.815) | 0.814 | 1.043 (0.736–1.478) |
| COPD | 0.310 | 0.806 (0.531–1.223) | 0.283 | 0.809 (0.550–1.190) |
| Albumin (g/dL) | 0.731 | 0.948 (0.697–1.288) | 0.294 | 0.859 (0.647–1.141) |
| Haemoglobin (g/dL) | 0.059 | 1.091 (0.997–1.194) | 0.040 | 1.093 (1.004–1.189) |
| Lymphocyte count (/mm3) | 0.650 | 1.000 (1.000–1.000) | 0.459 | 1.000 (1.000–1.000) |
| Platelet count (×1000/mm3) | 0.073 | 0.998 (0.996–1.000) | 0.591 | 1.000 (0.998–1.001) |
| CRP increase ≥10× of upper limit | 0.004 | 1.811 (1.213–2.704) | 0.005 | 1.693 (1.169–2.451) |
| Clinic presentationa | <0.001 | 5.819 (3.460–9.789) | <0.001 | 4.805 (3.037–7.602) |
| COVID-19 diagnosis by RT-PCR | 0.535 | 1.121 (0.781–1.608) | 0.906 | 0.980 (0.703–1.366) |
| Patient group, control group (reference) | 0.013 | 0.028 | ||
| HD group | 0.011 | 2.325 (1.210–4.467) | 0.008 | 2.257 (1.236–4.121) |
| RT group | 0.169 | 1.897 (0.761–4.727) | 0.138 | 1.872 (0.818–4.286) |
| CKD group | 0.001 | 2.880 (1.524–5.442) | 0.003 | 2.440 (1.351–4.405) |
Severe–critical disease/mild–moderate disease.
DISCUSSION
This study, comparing hospitalized COVID-19 patients with various kidney disease groups (Stages 3–5 CKD, HD and RT), has shown that mortality was significantly higher in kidney patients than in patients without kidney disease. The highest mortality rate was observed in the CKD group rather than the HD or RT patients. Similar results persisted in the combined outcome (mortality or ICU admission). The mortality rate in RT patients (11.1%) was lower than previously reported studies. Adjusted mortality and combined outcomes in the RT group did not differ from the control group, whereas the mortality of HD and CKD groups were similar in terms of both outcomes.
So far, the impact of COVID-19 on CKD, HD and RT patients has not been well defined. In this analysis, the risk of unadjusted and adjusted mortality rates and combined mortality or ICU admission in the CKD group was remarkably higher than in the control group. Moreover, outcomes did not differ when the CKD group was divided into three subgroups according to eGFR levels. CKD patients appear to be at higher risk for severe COVID-19 [6]. In a study from Wuhan, 101 patients with elevated baseline serum creatinine had in-hospital mortality of 33.7% [7]. In a preliminary data of a meta-analysis, a significant association between CKD and severe COVID-19 was observed [odds ratio = 3.03, 95% confidence interval (CI) 1.09–8.47] [8, 9].
Another remarkable observation in our study is that CKD patients had higher mortality than HD and RT patients. This had already been noted in a smaller sample size study from Cremona, Italy, in which the mortality rate was 35.3% in the patients with normal renal function, 88.2% in the CKD 3–5 group, 30.0% in the HD group and 36.4 in the RT group [10]. Of note, in both reports, age was higher for the CKD group, and this might be the reason, especially considering that in our cohort the difference disappeared after PSM.
The frequency of CKD Stage 3 and above is very high around the world, as in Turkey, and cardiovascular complications are major causes of mortality in these patients [11–13]. The highest mortality of the CKD group may be attributed to advanced age and a high burden of comorbidities (especially cardiovascular) in this study. Similar to our study, a cross-sectional, multicentre, nationwide study from Italy also showed that age and presence of CKD were independently associated with mortality in hospitalized patients with COVID-19 [14].
The mortality difference between the CKD and HD groups, however, became insignificant after PSM matching. This finding suggests that mortality in CKD patients is not different from that in HD patients when baseline characteristics, comorbidities and severity of the COVID-19 are similar. Uraemia is a state of chronic immune activation and chronic immune suppression, and uraemic patients have an increased risk of infection due to disordered natural and adaptive immunity [15]. Increased production and decreased clearance of pro-inflammatory cytokines can lead to inflammation and derangements in extracellular volume and play a role in high mortality in these patients [16, 17]. In addition, these patients may be vulnerable to any kind of medical treatment and invasive intervention [18, 19], and therefore such approaches may be less applicable during the COVID-19 pandaemic. All these data show that Stages 3–5 CKD patients may be the group with the highest mortality during the pandaemic. More detailed studies are certainly needed to clarify a causal link, but it is reasonable to warn health authorities on this issue, and efforts should be made to take more precautionary measures for kidney patients.
The mortality rate in our HD group (16.2%) is more favourable compared with previously published reports from Italy (23%), Spain (28%) and New York (31%), which involved fewer patients than the present series [20–22]. We have found that the adjusted in-hospital mortality rate was two times higher in HD patients than in the control group. Although our HD patients had high rates of comorbidities and antihypertensive use, these patients also had more severe clinical presentation, worse initial and in-hospital laboratory values, and increased inflammatory parameters. Antiviral drug use was higher in the HD group as compared with other groups. All these parameters were predictors of worse outcomes during COVID-19 [9]. Hence, health centres should be aware of these data and pay more attention to HD patients’ burden during the pandaemic. On the other hand, the HD centres were found as a major source of infection in (47.8%) our HD patients, which was much higher than other groups. HD centres have become high-risk places for HD patients during this pandaemic [23, 24] and the risk of an HD patient getting COVID-19 at a healthcare facility cannot be totally eliminated, even if maximum precautions are taken [25]. A study from the UK suggests that patients undergoing HD at home are relatively protected compared with patients receiving HD in-centre, so there may be disease transmission in the centre [26]. Hence, home-based dialytic therapies may be a safer option compared with centre-based HD treatment.
Mortality in our transplant patient group was much lower than in many series [27, 28]. There are important differences in age, transplant duration, race, donor source and treatments, which might affect the results. In a single-centre Italian study including 20 patients, mortality was 25% and ICU admission was 20% [27]. The transplant duration of patients in that series was significantly higher than in our series, and 75% of patients were treated with lopinavir/ritonavir, which was not superior to standard care in a randomized study [29]. In a report from New York, 10 of the 36 (28%) kidney transplant recipients died [28]. The characteristics of the patients were very different from our study, i.e. 75% of those patients had received a deceased donor kidney. Although they concluded that RT patients had high mortality, recent data from New York showed an almost similar mortality rate (21%) among hospitalized patients [30]. The crude mortality rate of our transplant patients was much higher than the control group (11.1% versus 4.0%). However, the difference disappeared after PSM analyses. In the Cox regression model, the hazard ratio (HR) was 1.897 and the CI was large (95% CI 0.761–4.727). Hence, this may be due to the small sample size of the RT patients. Therefore, we were unable to assess whether RT patients were or were not at increased risk by this study. The low mortality of our RT group may be, in part, explained by the relatively young age of the group. Hence, RT mortality may not be so high when compared with other patients in their own population. There might be a possibility of the anti‐inflammatory and immune balancing effects of immunosuppressives that could diminish the clinical expression of COVID-19 [31–34]. The significantly higher mortality rate in HD patients compared with RT patients with COVID-19 is a very important concern, and HD patients have a high risk of dialysis centre-related viral transmission. This point should be carefully considered for HD patients with suitable donors in the pandaemic era, for which there has been an ongoing dramatic decrease in the deceased donor pool and in overall solid-organ transplantation procedures during the pandaemic period [35, 36].
The overall mortality of the patients in this study was lower than in previous reports. This finding may be related to the demographic, geographic and racial characteristics, and treatment approaches of the patients. There may also be a role of health systems and health infrastructure. National COVID-19 guidelines are regularly updated and followed by all healthcare facilities and government institutions dynamically. Moreover, all COVID-19 treatments and hospitalizations in Turkey are covered by the government, even in private hospitals. CT is easily available and used commonly for diagnosis, which was found to be used in 90% of patients in all kidney patient groups. National online coordination of hospital and ICU bed facilities and prompt hospitalization may have played a role in these favourable outcomes.
This study has several limitations. This was a retrospective study and the groups were not randomized. Urinalysis and kidney images were not routinely analysed because they were not regularly available in the pandaemic. Several data, such as blood pressure, blood oxygen saturation, changes in kidney functions and ICU care details, were not included in the dataset. Another concern is that patients without a positive RT-PCR were also included in the study. Only 56.4% of patients were diagnosed based on RT-PCR test. RT-PCR-negative patients were only included if their clinical findings and CT findings were strongly suggestive of COVID-19. However, RT-PCR test parameter as a variable in Cox analyses did not show a significant effect on the results. On the other hand, we planned to include one control group patient per patient from the CKD groups (HD, RT and Stages 3–5 CKD) in the study, based on the order of arrival of the patients. However, it was not possible to match one control group patient to each CKD patient. The researchers and their centres that participated in our study were all nephrology centres. During the pandaemic, they also hospitalized COVID-19 patients who did not have any nephrological problem. Hence, sometimes patients came consecutively from CKD groups, and sometimes a candidate patient had exclusion criteria (such as acute kidney injury). Thus, the number of control group patients was not equal to the sum of the number of CKD groups. As we used PSM analyses, it should be considered that we can reduce the bias due to confounding variables, but this only accounts for observed covariates. Hence, factors that cannot or are not observed are not accounted for with this method.
In conclusion, hospitalized COVID-19 patients with CKDs, including Stages 3–5 CKD, HD and RT, have significantly higher mortality than patients without kidney disease. Stages 3–5 CKD patients may have an in-hospital mortality rate as high as HD patients, which may be in part because of similar age and comorbidity burden. We were unable to assess if RT patients were or were not at increased risk for in-hospital mortality by this study, because of relatively small sample size of the RT patients. Further research is warranted to find measures and treatments to decrease the strikingly high mortality in CKD patients with COVID-19 and to assess the risk of RT patients.
ACKNOWLEDGEMENTS
We thank OMEGA Contract Research Organization in Turkey for data processing and statistical analysis. We also thank Prof. Mehmet Sukru Sever for his critical reading of the manuscript.
FUNDING
The study was funded by Turkish Society of Nephrology.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Naicker S, Yang CW, Hwang SJ. et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; 97: 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basile C, Combe C, Pizzarelli F. et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 2020; 35: 737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang W, Xu Y, Gao R. et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323: 1843–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidance to COVID-19 (SARS Cov2 Infection) (Scientific Board Study) Republic of Turkey Ministry of Health (published on April 14). https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf (18 April 2020, date last accessed)
- 5. Levey AS, Stevens LA, Schmid CH. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Luo Y, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry BM, Lippi G.. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020; 52: 1193–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malberti F, Pecchini P, Marchi G, Foramitti M.. When a nephrology ward becomes a COVID-19 ward: the Cremona experience. J Nephrol 2020; 33: 625–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahmoodi BK, Matsushita K, Woodward M. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet 2012; 380: 1649–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foley RN, Murray AM, Li S. et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16: 489–495 [DOI] [PubMed] [Google Scholar]
- 13. Suleymanlar G, Utas C, Arinsoy T. et al. A population-based survey of Chronic REnal Disease In Turkey–the CREDIT study. Nephrol Dial Transplant 2011; 26: 1862–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iaccarino G, Grassi G, Borghi C. et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian society of hypertension. Hypertension 2020; 76: 366–372 [DOI] [PubMed] [Google Scholar]
- 15. Syed-Ahmed M, Narayanan M.. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis 2019; 26: 8–15 [DOI] [PubMed] [Google Scholar]
- 16. Arici M, Walls J.. End-stage renal disease, atherosclerosis, and cardiovascular mortality: Is C-reactive protein the missing link? Kidney Int 2001; 59: 407–414 [DOI] [PubMed] [Google Scholar]
- 17. Meyer TW, Hostetter TH.. Uremia. N Engl J Med 2007; 357: 1316–1325 [DOI] [PubMed] [Google Scholar]
- 18. Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 2009; 4: 1275–1283 [DOI] [PubMed] [Google Scholar]
- 19. Schlieper G, Hess K, Floege J. et al. The vulnerable patient with chronic kidney disease. Nephrol Dial Transplant 2016; 31: 382–390 [DOI] [PubMed] [Google Scholar]
- 20. Alberici F, Delbarba E, Manenti C. et al. Management of patients on dialysis and with kidney transplant during SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep 2020; 5: 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sánchez-Álvarez JE, Fontán MP, Martín CJ. et al. Situación de la infección por SARS-CoV-2 en pacientes en tratamiento renal sustitutivo. Informe del Registro COVID-19 de la sociedad española de nefrología (SEN). Nefrología 2020; 40: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valeri AM, Robbins-Juarez SY, Stevens JS. et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 2020; 31: 1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiong F, Tang H, Liu L. et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan. J Am Soc Nephrol 2020; 31: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Y, Diao B, Lv X. et al. 2019 Novel Coronavirus Disease in Hemodialysis (HD) Patients: Report from One HD Center Wuhan, China: medRxiv, 2020 10.1101/2020.02.24.20027201 (18 April 2020, date last accessed); preprint: not peer reviewed [DOI]
- 25. Scarpioni R, Manini A, Valsania T. et al. Covid-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol 2020; 9: 1–5 [PubMed] [Google Scholar]
- 26. Corbett RW, Blakey S, Nitsch D. et al. ; for the West London Renal and Transplant Centre. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol 2020; 31: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alberici F, Delbarba E, Manenti C. et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int 2020; 97: 1083–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akalin E, Azzi Y, Bartash R. et al. Covid-19 and kidney transplantation. N Engl J Med 2020; 382: 2475–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao B, Wang Y, Wen D. et al. A Trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med 2020; 382: 1787–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richardson S, Hirsch JS, Narasimhan M. et al. ; and the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernández-Ruiz M, Andrés A, Loinaz C. et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 2020; 20: 1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romanelli A, Mascolo S.. Immunosuppression drug-related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant 2020; 20: 1947–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abadja F, Atemkeng S, Alamartine E. et al. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation 2011; 92: 396–403 [DOI] [PubMed] [Google Scholar]
- 34. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Domínguez-Gil B, Coll E, Fernández-Ruiz M. et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant 2020; 20: 2593–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loupy A, Aubert O, Reese P. et al. Organ procurement and transplantation during the COVID-19 pandemic. Lancet 2020; 395: e95–e96 [DOI] [PMC free article] [PubMed] [Google Scholar]