Abstract
The cytoprotective transcriptor factor nuclear factor erythroid 2– related factor 2 (NRF2) is part of a complex regulatory network that responds to environmental cues. To better understand its role in a cluster of inflammatory and pro-oxidative burden of lifestyle diseases that accumulate with age, lessons can be learned from evolution, the animal kingdom and progeroid syndromes. When levels of oxygen increased in the atmosphere, mammals required ways to protect themselves from the metabolic toxicity that arose from the production of reactive oxygen species. The evolutionary origin of the NRF2–Kelch-like ECH-associated protein 1 (KEAP1) signalling pathway from primitive origins has been a prerequisite for a successful life on earth, with checkpoints in antioxidant gene expression, inflammation, detoxification and protein homoeostasis. Examples from the animal kingdom suggest that superior antioxidant defense mechanisms with enhanced NRF2 expression have been developed during evolution to protect animals during extreme environmental conditions, such as deep sea diving, hibernation and habitual hypoxia. The NRF2–KEAP1 signalling pathway is repressed in progeroid (accelerated ageing) syndromes and a cluster of burden of lifestyle disorders that accumulate with age. Compelling links exist between tissue hypoxia, senescence and a repressed NRF2 system. Effects of interventions that activate NRF2, including nutrients, and more potent (semi)synthetic NRF2 agonists on clinical outcomes are of major interest. Given the broad-ranging actions of NRF2, we need to better understand the mechanisms of activation, biological function and regulation of NRF2 and its inhibitor, KEAP1, in different clinical conditions to ensure that modulation of this thiol-based system will not result in major adverse effects. Lessons from evolution, the animal kingdom and conditions of accelerated ageing clarify a major role of a controlled NRF2–KEAP1 system in healthy ageing and well-being.
Keywords: chronic kidney disease, inflammation, NRF2–KEAP1, oxidative stress, progeria
NRF2 EMERGED IN EVOLUTION IN RESPONSE TO OXYGEN
Oxygen is critical for survival and cellular metabolism in all mammals. However, oxygen is also a toxic gas and during evolution mammals needed to develop efficient antioxidant systems to defend themselves against a constant bombardment from chemicals and reactive oxygen species (ROS), such as the by-products of oxidative metabolism, nutrients, ultraviolet radiation and the toxic metabolites of xenobiotics. Among several defense systems that evolved, such as autophagy and heat shock proteins, the nuclear factor erythroid 2–related factor 2 (NRF2) system was a dominant one. The network of hundreds of anti-oxidative, anti-inflammatory and bioenergetic genes regulated by NRF2 [1] is estimated to account for >1% of the total genome [2]. The subsequent evolution of cysteine-rich Kelch-like ECH-associated protein 1 (KEAP1) provided mammals with a more cultivated way to regulate NRF2 activity (like a molecular dimmer switch) in higher organisms [3]. Exposure to oxidants disrupts the interaction between NRF2 and KEAP1, which leads to translocation of NRF2 to the nucleus, which in turn increases the transcription of a battery of hundreds of cytoprotective genes that affect the proteasome [4]. In consideration of NRF2 and nuclear factor (NF)-κB being engaged in crosstalk, NRF2 also plays an important anti-inflammatory role [5]. Phylogenetic analyses of NRF2 sequences have shown that the major divergence in NRF2 occurred when the oceans released free oxygen to the atmosphere [6]. Thus, since rising levels of atmospheric oxygen in the Palezoic period (about 540 to 250 million years ago) appear to have driven the divergence of NRF2, the evolutionary origin of NRF2 is allied to the timing of the global transition from anaerobic to aerobic conditions. It can be assumed that the effective handling of powerful environmental electrophilic challenges and detoxification were prerequisites for the successful evolution from primitive short-lived organisms to long-lived mammals.
ENHANCED NRF2 EXPRESSION—A SURVIVAL MECHANISM IN THE ANIMAL KINGDOM
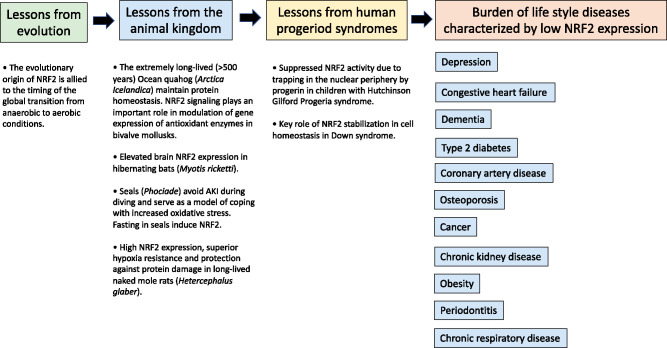
The conservation of NRF2, both in function and structure across species, implies that NRF2 plays a major role in protecting against the cytotoxic stress that occurs with the accumulation of damaged proteins, lipids and genetic material that promotes organ damage (Figure 1). The evolutionary development of the NRF2–KEAP1 pathway and its actions on protein turnover and glucose metabolism seems central for the cytoprotective signalling pathway [7]. Studies in Caenorhabditis elegans show that the NRF2 functional ortholog skinhead-1 (SKN-1) is involved in many homoeostatic functions and exerts considerable influence on the lifespan and healthspan of mammals [8]. A more elaborate system, evident in lower organisms and invertebrates (including model organisms Drosophila and zebrafish), has been conserved throughout the animal kingdom [9]. Compelling evidence for the importance of NRF2 for cytotoxic protection is provided by studies showing that mice lacking the NRF2 gene display enhanced sensitivity to toxins, carcinogens and organ damage [4]. Although the NRF2–KEAP1 system has not yet been extensively studied in the animal kingdom, there are cases exemplifying its importance for survival in extreme conditions (Figure 1).
FIGURE 1.
Since oxygen homoeostasis is of crucial importance to maintain survival, vertebrates developed a way to coordinate the oxygen levels in the intracellular compartments in order to maintain homoeostasis. In the living organism, hypoxia and inflammation commonly occur together and there is significant crosstalk between the transcription factors that are classically understood to respond to either hypoxia or inflammation. The evolutionary origin of NRF2 is allied to the timing of the global transition from anaerobic to aerobic condition. Superior anti-oxidant defense mechanisms with enhanced NRF2 expression may protect animals during extreme environmental conditions, such as deep sea diving, hibernation and habitual hypoxia. Studies of rare progeric diseases, such as HGPS, have increased our understanding of mechanisms that drive ageing and imply that therapeutic strategies that stimulate NRF2–KEAP1 could open up new avenues to slow down senescence and prevent diseases of ageing. Compelling evidence for a role of NRF2 in the susceptibility of numerous chronic burden of lifestyle diseases associated with inflammation and oxidative stress has been provided by studies that have shown associations between disease risk and genetic variations of the NRF2 gene. Thus, targeting the cytoprotective transcription factor NRF2 may have a positive effect on a cluster of burden of lifestyle diseases.
Hibernation is a survival strategy that has evolved in certain species to conserve energy during periods of nutrient shortfall. To survive long periods of hypoxia, cold weather and fasting (and increased oxidative stress that occurs during arousal in spring when organs are reperfused), hibernating animals are forced to maintain a careful balance between metabolic demand and supply. Food deprivation increases oxidative stress by increasing mitochondrial oxidant generation and depletion of antioxidants with decreased antioxidative capacity [10]. How hibernating species cope with ensuing increased oxidative stress is not well understood. While hibernating ground squirrels (Ictidomys tridecemlineatus) undergo repeated ischaemia–reperfusion cycles, which lead to high basal levels of reactive oxygen and nitrogen species, this does not seem to harm them [11]. As NRF2 exhibits elevated transcript levels in hibernating squirrels and NRF2 axis-associated micro ribonucleic acid (miRNA), miR-200a displays altered levels during hibernation [11], an interplay between non-coding RNAs and targets associated with oxidative stress may be operative. Thus, since NRF2 and Forkhead box O transcription factors, which play a role in ruling the expression of genes involved in proliferation, cell growth and longevity, have been shown to play major roles in the regulation of antioxidant defenses in the hibernating bat brain [12], an upregulation of the NRF2–KEAP1 system may be required to avoid organ damage during hibernation.
Seals (Phociade) have developed a remarkable capacity to protect their organs during prolonged periods of hypoxaemia. It has been reported that elephant seals can descend as far as 2000 m during diving for periods that can extend to up to 100 min per dive [13]. In order to secure sufficient perfusion of the brain and heart during apnoea-induced hypoxaemia in diving, there is an intense arterial constriction of peripheral vascular beds and splanchnic organs, including the kidneys [14]. The sustained vasoconstriction activated during diving leads to almost total discontinuation of kidney function—inulin clearance decreases >90% [15]—followed by reperfusion after diving [16]. Compared with the dog kidney, the seal kidney is extremely tolerant to repetitive ischaemia and acute kidney injury (AKI) [17] due to their highly developed antioxidant defense mechanisms [18]. As prolonged natural periods of fasting activate NRF2–KEAP1 in elephant seals [19], upregulation of the NRF2–KEAP1 pathway during fasting may contribute to seals’ capacity to protect their kidneys. Mammalian models that help us to study hypoxia–anoxia tolerance have relevance since we lack effective treatments to thwart ischaemia and hypoxia.
Another species that has developed a remarkable resistance to hypoxia is the naked mole rat (Heterocephalus glaber). During evolution they adapted to severe constant hypoxia in underground burrows and can survive prolonged periods of anoxia due to fructose-driven glycolysis [20]. The naked mole rat has emerged as a model organism for studies of ageing-related diseases and negligible senescence [21]. In contrast to other mammals, naked mole rats do not conform to the Gompertzian law of age-related mortality risk [22]. Naked mole rats live 7–10 times longer than similarly sized rodents of other species and, along with superior anoxia tolerance, they seem resistant to age-related diseases such as cancer, cardiovascular disease (CVD) and neurodegeneration [13]. In addition, naked mole rats experience no changes in body composition or decline in genomic and proteomic integrity due to elevated proteasome quality control mechanisms [23]. Whereas several mechanisms may contribute to the mole rats’ exceptional resilience and efficient breakdown and clearance of damaged proteins [24], increased expression of NRF2–KEAP1 was reported to be a major factor responsible for maintaining their damage control mechanisms [21].
Studies of other long-lived animals have provided valuable insights into mechanisms that characterize ageing [14]. In the long-lived (>500 years) ocean quahog (Arctica islandica), increased resistance to oxidative stress and maintenance of protein homoeostasis preserves cardiac function [25], a prerequisite for extreme longevity [26]. In accordance, a trans-criptomics-based screening recently identified that inhibition of Hsp90 defers ageing by improved protein homoeostasis in C. elegans [27]. Since inhibition of Hsp90 protects against atherosclerosis via cytoprotective mechanisms that are NRF2 dependent [28] and SKN-1/NRF2 is essential for the healthspan benefits of metformin in C. elegans [29], a role for NRF2 as a pro-longevity signalling pathway across phyla is supported.
STUDIES OF RARE PROGEROID DISORDERS IDENTIFY NRF2 AS A TARGET OF PREMATURE AGEING
ROS-induced premature cellular senescence is believed to contribute to ageing and age-related diseases. NRF2 activity decreases with ageing in mice [30] and when the NRF2 gene is inhibited, this promotes stress-induced premature senescence [31]. The senescence-associated decline of NRF2 in endothelial cells is mediated by miRNAs [32]. As both NRF2 deficiency [33] and hypoxia [34] exacerbate senescence, and endothelial senescence is protected via Klotho and NRF2 activation [35], the relationships between senescence, hypoxia and repressed NRF2 activity during ageing deserve attention. Studies in rare progeroid syndromes help us to better understand the role of NRF2 in the protection against oxidative stress–driven premature ageing (Figure 1). Recently Zamponi et al. [36] reported a key role for NRF2–KEAP1 stabilization in cellular homoeostasis in Down syndrome. Hutchinson–Gilford progeria syndrome (HGPS) is a rare premature ageing disorder due to an accumulation of progerin (mutant lamin A) in which carriers die of CVD as teenagers. Kubben et al. [37] identified the NRF2 antioxidant pathway as a driving mechanism in HGPS and showed that trapping of NRF2 at the nuclear periphery by progerin recapitulated the ageing defects and that reactivation of NRF2 reversed the progerin-associated nuclear ageing defects [37]. Taken together, studies of rare progeric diseases have helped to explain mechanisms that drive ageing and imply that therapeutic strategies that stimulate NRF2–KEAP1 may attenuate senescence and abrogate or ameliorate diseases of ageing.
CHRONIC BURDEN OF LIFESTYLE DISEASES IS RELATED TO LOW NRF2 EXPRESSION
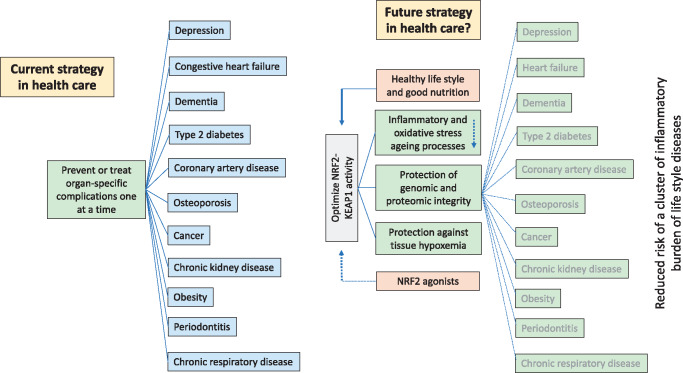
While the primary causes of ageing are not fully understood, an implicit component of many hypotheses to explain ageing is that ROS-mediated tissue and organ damage is likely to play a critical role [38]. Diseases of ageing are influenced by mechanisms that show strong evolutionary conservation and contribute to cancer, CVD and neurodegenerative diseases [39]. While worldwide life expectancy has nearly doubled in the last century due to improved hygiene and nutrition and better treatment for infectious diseases and CVD, further gains in life expectancy have stalled because of lifestyle diseases, including chronic kidney disease (CKD), obesity and type 2 diabetes mellitus—conditions in which inflammation and oxidative stress are prominent (Figure 2). It has been estimated that the proportion of the world population >60 years old will increase from 20% to 40% by 2050; the prevalence of burden of lifestyle diseases will increase with advanced age. Current approaches (i.e. addressing single problems within individual disease systems) will need to change in order to handle a growing burden of ageing-related diseases (Figure 2). This approach may lead to the alleviation of symptoms and organ damage, and limited prolongation of life, but since burden of lifestyle diseases occur in clusters and accumulate with age [39], the current strategy may not dramatically reduce the gap between lifespan and healthspan. The majority of chronic diseases that accumulate with age share common molecular mechanisms, such as inflammation, oxidative stress, mitochondrial dysfunction, metabolic imbalances and senescence [40] (Figure 3). An alternative strategy to improve health would be to target the whole ‘diseaseome’ simultaneously through a fundamental approach targeting underpinning mechanisms common to all burden of lifestyle diseases deemed related to ‘inflammaging’ (Figure 2). In our opinion, a strategy that targets the whole cluster of burden of lifestyle diseases simultaneously is more likely to significantly decrease the gap between ‘healthspan’ and ‘lifespan’. Since burden of lifestyle diseases usually manifest a repressed NRF2–KEAP1 signalling pathway [41], cytotoxic stress may play a role in predisposition and progression of the NRF2 diseasome. The introduction of modern Western diets with a high inflammatory index [42] together with the loss of ingredients in ancient foods that activate NRF2 [43] may in part contribute to burden of lifestyle disorders. As it has been hypothesized that centenarians in ‘Blue Zones’ have a constitutively upregulated NRF2 system [44], nutritional targeting of NRF2 may decrease the overall burden of lifestyle disorders.
FIGURE 2.
Burden of lifestyle diseases accumulate with age. The current approach in health care to target and treat individual burden of lifestyle diseases in isolation may be effective in the short term, but since burden of lifestyle diseases tend to cluster, this approach may not increase the number of healthy years of living much, with little impact on the gap between ‘lifespan’ and ‘healthspan’. An alternative approach would be to investigate if age-related diseases associated with inflammation and oxidative stress could be treated more effectively by modulating some fundamental mechanisms of ageing per se and decrease the risk of a cluster of burden of lifestyle diseases simultaneously. Since decreased expression of NRF2–KEAP1 characterizes burden of lifestyle diseases, studies should be conducted to find out if long-term NRF2 stimulation could maintain redox, protein and metabolic homoeostasis and prevent the ‘NRF2 diseasome’ cluster and increase the number of years of healthy living by reducing the risk of multiple diseases simultaneously.
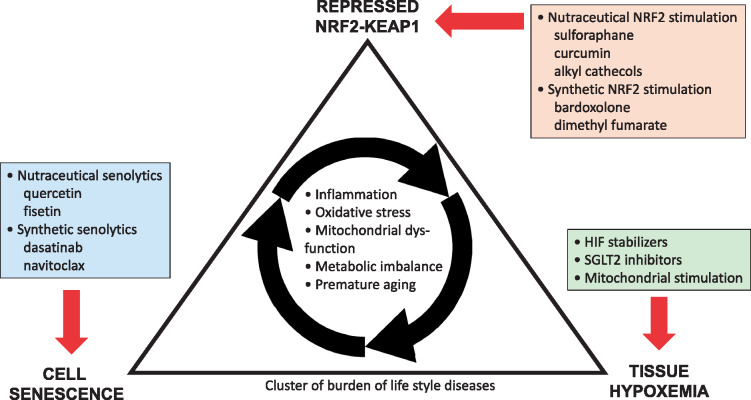
FIGURE 3.
Physiological clustering of cell senescence, tissue hypoxemia and repressed NRF2–KEAP1 expression seem to be drivers of a state of inflammation, increased oxidative stress, mitochondrial dysfunction and metabolic imbalance that drives a cluster of burden of lifestyle diseases that associate with premature ageing. Novel approaches to intervene in this scenario include nutraceutical and synthetic senolytic and NRF2-stimulating therapies, as well novel interventions targeting tissue hypoxia, such as sodium glucose cotransporter 2 inhibitors, stimulators of mitochondrial biogenesis and hypoxia-inducible factor (HIF) stabilizers.
CHRONIC BURDEN OF LIFESTYLE DISEASES IS RELATED TO TISSUE HYPOXIA
Considering that the NRF2 system evolved as a protective mechanism against oxidative stress when the oceans released free oxygen [6], it is evident that tissue hypoxaemia is a feature of age-associated burden of lifestyle diseases such as CKD [45], diabetes [46], obesity [47], cancer [48] and depression [49]. It is likely that mitochondria are a major target in hypoxic/ischaemic injury, as diseases that accumulate with age lead to dysregulation of mitochondrial molecular pathways [50]. Considering that NRF2 activation supports cell survival during hypoxia [51, 52], it could be hypothesized that adequate regulation of the NRF2–KEAP1 system may reduce the risk of tissue hypoxia in burden of lifestyle diseases. In fact, activation of NRF2 has been promoted as a prophylactic therapeutic strategy for acute mountain sickness [53]. It is pertinent to note that compared with other organs, the kidneys are particularly susceptible to hypoxia.
ACUTE KIDNEY DISEASE—A MODEL OF A CLINICAL CONDITION OF TISSUE HYPOXIA
Most major causes of AKI produce conditions of hypoxia within the kidney. It was recently proposed that an intermittent decline in kidney function due to episodes of transient renal ischaemia, toxins, drug toxicity volume depletion (in other words, episodes of AKI), with improved management of hypertension and diabetes, are responsible for driving CKD progression more so than a decline in kidney function related to the primary disease process [54]. As NRF2–KEAP1 determines the sensitivity of kidneys to various insults that can result in AKI [55] and T-lymphocyte activation of NRF2 protects against AKI [56], a patient with preserved NRF2–KEAP1-based cytoprotective pathways may better resist repetitive minor episodes of AKI. Recent data showing that a youthful systemic milieu diminished renal ischaemia injury in elderly mice reperfusion [57] and that NRF2 activation conferred protection from ischaemic AKI in mice [58] support such a hypothesis.
MODULATION OF THE NRF2 TRANSCRIPTION FACTOR—IS IT SAFE?
As NRF2 is dysregulated in numerous human conditions that often occur in clusters, it represents an attractive target for modulation. Since arresting the ageing process per se limits the overall burden of chronic diseases, it would be of interest to target fundamental mechanisms of ageing (Figure 2). It is still unexplored whether activation of NRF2 increases lifespan, delays ageing or prevents burden of lifestyle diseases. It has been suggested that targeting NRF2 with diverse natural phytochemicals and/or synthetic compounds may be an option to prevent a cluster of age-related chronic inflammatory diseases. Although there are at least 30 patents for NRF2 modulators [41], their clinical benefit has not yet been realized. Some important considerations need to be taken into account when targeting NRF2. Since there is compelling evidence that polymorphisms [59] and epigenetic mechanisms [60] modulate the transcriptional activity of the NRF2 promoter, these factors need to be taken into account. Animal studies on deactivation of KEAP1 show that activation of NRF2 signalling modulates pathways beyond detoxification and cytoprotection, with the major cluster of genes associated with lipid metabolism [61]. Moreover, in keeping with the theory of reductive stress [i.e. a harmful disturbance in the redox state and overproduction of reduced glutathione and nicotinamide adenine dinucleotide phosphate (NADPH)] and the observation that NRF2 activity varies significantly depending on the physiological and pathological context, properly timed manipulation of the NRF2 pathway is critical [62]. Studies are needed to evaluate if the ‘hit and run’ approach, shown to be effective for senolytics [63], should also be used when NRF2–KEAP1 is targeted. The seasonal changes in NRF2–KEAP1 expression that occur in the animal kingdom may support such an approach [19]. The correct dosage of activators for stress sensors and their putative interactions with disease‐related pathways remains a concern [64]. NRF2 hyperactivation could promote oncogenesis [4]; therefore safety signals for increased risk of cancer should be taken seriously and the ‘sweet spot’ for NRF2 activation needs to be identified. Moreover, different NRF2 agonists have different potentials for carcinogenic effects [65]. As constitutive over-activation of NRF2 may be oncogenic, promote hyperplasia and increase susceptibility to atherosclerosis [3], NRF2 may have evolved in response to different selective pressures during evolution. Thus, as the global characterization of NRF2 activation is biphasic [3], both increased and depressed NRF2 expression should be avoided.
Nutrients that stimulate NRF2
Since a recent systematic analysis from 195 countries showed that 22% of all global deaths are attributable to the individual’s diet [66], the impact of the “foodome” on age related health deserves more attention. Several naturally occurring chemicals extracted from plants with anti-inflammatory and antioxidative properties have NRF2-inducing effects [67], and humans have been safely ingesting NRF2 activators as part of their diet for millennia. Not surprisingly, Sardinian and Okinawan diets are both rich in pickled foods that contain alkyl catechols [68]. Alkyl catechols are known NRF2 agonists [43] and it can be hypothesized that NRF2 activation might be responsible, in part, for prolonged life expectancy in these populations. Additionally, alkyl catechols such as quercetin [43] are proven senolytic agents, which might be expected to enhance healthspan via nutritional intake. Compared with synthetic chemicals that stimulate NRF2, the stimulatory effect of phytochemicals, such as flavonoids and diterpenoids, has been regarded as relatively weak. However, in a comparative study of 54 natural compounds, 7 were more potent than the classic synthetic NRF2 activator tert-butylhydroquinone [69] (Table 1). As a diet low in starch may result in impaired NRF2 activity in mice [70], and as the bioavailability of polyphenols and dietary fibres can be affected by molecular interactions between bioactive compounds and the food matrix [71], the impact of the gut microbiota on these factors relative to NRF2 activation deserves attention. Recent studies show that natural NRF2 activators have significant clinical effects. Urolithin, a microbial metabolite derived from polyphenolics of pomegranate fruits and berries, upregulated epithelial tight junctions and attenuated colitis via stimulation of NRF2-dependent pathways [72]. Sulphoraphane (provided as concentrated broccoli sprout extract), an electrophilic activator of NRF2, reduced fasting blood glucose and glycated hemoglobin in obese patients with dysregulated type 2 diabetes [73]. Moreover, >100 clinical trials report that curcumin from the turmeric plant is safe and effective in a number of burden of lifestyle diseases [74], and its potential as a potent NRF2 activator has been discussed [75]. Several other natural agents, including carnosol, andrographolide and trans-chalone, stimulate NRF2. For nephrologists, it is of interest that cocoa flavanol mitigated endothelial dysfunction in haemodialysis patients [76].
Table 1.
Ranking NRF2 activating efficacy (based on an increase in the luminal signal in the AREc32 cell line) of 2 synthetic and 54 natural products
| Potency | Family | Substance | Source |
|---|---|---|---|
| 1 | CDDO-lm | Synthetic | |
| 2 | Diterpenoid | Andrographolide | Herbaceous plant |
| 3 | Diterpenoid | trans-chalcone | Tomato skin |
| 4 | Isothiocyanates | Sulforaphane | Broccoli |
| 5 | Zingiberaceae | Curcumin | Tumeric |
| 6 | Flavonoid | Flavone | Spices and red-purple plant foods |
| 7 | Diterpenoid | Kahweol | Coffee beans |
| 8 | Diterpenoid | Carnosol | Rosemary (herb) |
| 9 | tBHQ | Synthetic |
Natural compounds that did not activate NRF2: resveratrol and oleanolic acid. CDDO-lm, 2-cyano-3,12-dioxooleana-1,9(11)-diene-28-oic acid-limidazole; tBHW, tert-butylhydroquinone.
It is conceivable that some nutrients downregulate the NRF2–KEAP1 pathway (Figure 4). Indeed, radical changes in food preservation of our Western diet have resulted in the absence of alkyl catechols found in traditionally fermented foods and may have resulted in serious negative consequences for NRF2-mediated cell protection [43]. Fermented cabbage (kimchi) stimulates NRF2, reduces lipid peroxidation and alleviates hepatic steatosis in mice [80]. Increased intake of red and processed meat is associated with premature ageing [81] and burden of lifestyle diseases such as CKD [82]. NRF2-dependent antioxidant responses appear to be involved in the resistance of pre-neoplastic colon cells to cytotoxic and genotoxic stressors in beef-fed rats [83]. Since red meat stimulates the production of uraemic toxins by the gut microbiota and indoxyl sulphate downregulates the expression of NRF2 [84], the link between red meat and NRF2 may be indirect. The increase in the consumption of sugar-added foods and sweetened beverages with a high fructose content correlates with an increased risk of burden of lifestyle diseases. Notably, plant-based flavone prevented high fructose–induced metabolic syndrome by inhibiting the binding of KEAP1 to NRF2 [85]. While a good deal of evidence has been accumulated to date, more studies are required to fully understand the effects of various dietary components and specific foods on the NRF2–KEAP1 pathway and links to human disease.
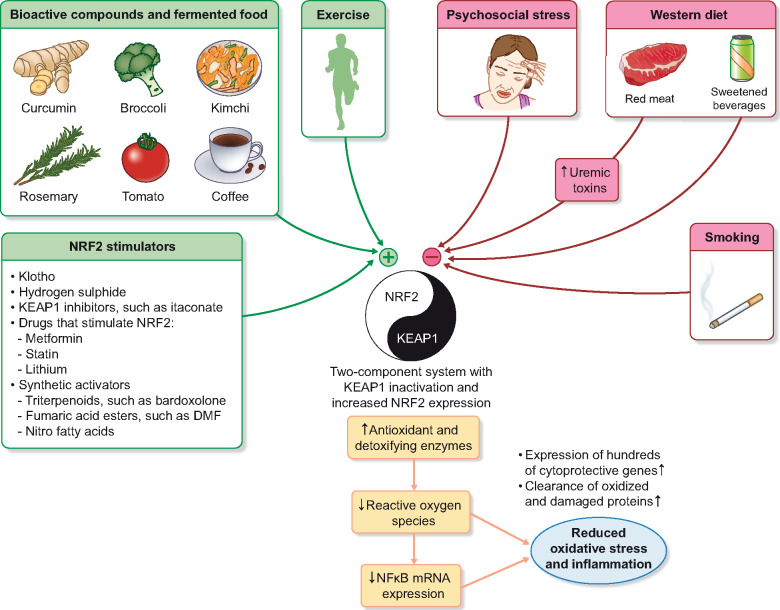
FIGURE 4.
An unhealthy lifestyle may, via continuous depression of NRF2, affect redox, protein and metabolic homoeostasis and increase the risk of burden of lifestyle diseases. Whereas the typical Western diet seems to inhibit NRF2 cell defense pathways, diets based on bioactive compounds and fermented products have been shown to activate this cytoprotective system. While physical activity stimulates NRF2–KEAP1 [77] and contributes to stress resilience, cigarette smoking [78] and psychological stress [79] blocks the protective expression of the NRF2–KEAP1 pathway. NRF2 and KEAP1 can be seen as having a ‘yin and yang’ relationship in which graded inhibition of KEAP1 increased the expression of NRF2, which in turn increases the expression of hundreds of cytoprotective genes. A comprehensive overview of the molecular mechanisms governing the functions of KEAP1 and NRF2 was published by Yamamoto et al. [5]. Studies need to test if the various synthetic NRF2 stimulators (such as BARD) and KEAP1 inhibitors (such as itaconate) can protect against the plethora of inflammatory diseases that accumulate with age.
Klotho and hydrogen sulphide—emerging NRF2 activators
Klotho protects cells from inflammation and oxidative stress and in mice klotho promotes longevity and protects against CVD and CKD [86]. Klotho exerts its anti-ageing mechanism via multiple mechanisms. Although the premature ageing phenotype of Klotho−/− mice has been attributed to the absence of Klotho-mediated suppression of the insulin–insulin-like growth factor-1 pathway [86], it was also reported that Klotho exerts its protective effects by augmenting NRF2 expression and inactivating NF-κB activation, both in vitro and in vivo [87]. Since restored NRF2 activity reversed Klotho deficiency and attenuated inflammation and oxidative stress in rats with CKD [88], the relation between NRF2 and Klotho may be bidirectional. Thus, as soluble Klotho protected against angiotensin II–mediated apoptosis and senescence in human vascular smooth muscle cells (VSMCs) via NRF2 [89], targeting NRF2 represents a novel therapeutic strategy against VSMC dysfunction. Activation of the NRF2 signalling pathway prevented hyperphosphataemia-induced vascular calcification by inducing autophagy in VSMCs [90].
Hydrogen sulphide (H2S), previously considered a toxic air pollutant, is now recognized as an important signalling molecule that modulates thiol-based redox switches that promote anti-inflammatory and antioxidative effects [91]. The beneficial effects of protein restriction on ageing in the animal kingdom [92] are believed to be mediated by the sulphur-containing amino acid methionine and activation of the trans-sulphuration pathway. During cellular stress, H2S-mediated trans-sulphuration of KEAP1 promotes nuclear translocation of NRF2 and stimulates the NRF2–KEAP1 pathway. Since H2S attenuates ageing-related kidney dysfunction by enhancing NRF2 nuclear translocation [93] and attenuates VSMC calcification via activation of NRF2–KEAP1 [94], this gas should be of definite interest to nephrologists.
Synthetic NRF2 agonists
Among synthetic NRF2 activators, bardoxolone methyl (BARD) has the highest potency towards NRF2 [95]. Dimethyl fumarate (DMF) is the only Federal Drug Administration (FDA)- and European Medical Agency (EMA)- approved drug currently registered as an NRF2 activator. It is used clinically for the treatment of psoriasis and multiple sclerosis [41], but since activation of NRF2 by DMF attenuated vitamin D3–induced vascular calcification in an in vivo mouse model [96], its role in the prevention of vascular stiffness deserves attention. Among a number of synthetic triterpenoids based on oleanolic acid, BARD has attracted interest in the renal community, since it increases estimated glomerular filtration rate (eGFR) due to a presumed increase in the glomerular surface area for filtration and suppression of fibrosis, glomerulosclerosis and interstitial fibrosis in rodent models of CKD. These renal protective effects were confirmed when BARD increased eGFR in a study (Trial to Determine the Effects of Bardoxolone Methyl on eGFR in Patients With Type 2 Diabetes and Chronic Kidney Disease) of type 2 diabetics with CKD Stage 3 [97]. However, when the Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes (BEACON) trial in 2185 type 2 diabetics with CKD 4 was terminated because of a significant increase in congestive heart failure (CHF) in patients treated with BARD [98], the interest was dampened. The BEACON trial shows that NRF2 stimulation can have harmful effects. Since the post hoc analysis shows that CKD 4 patients who developed CHF had elevated mean B-type natriuretic peptide concentrations before randomization (BARD modulation of the endothelin pathway promotes acute water and sodium retention), patients at increased risk could be identified [99]. The possibility to identify patients at risk for CHF together with the post hoc analysis of BEACON, which showed that BARD improved kidney function [100], has renewed the hope to arrest progression of kidney disease with BARD. As the NRF2-mediated oxidative stress pathway serves as the hub for clusters of inflammation- and metabolism-related transcriptional networks associated with impaired kidney function from multiple a etiologies [101], the NRF2–KEAP1 system seems to affect a final common pathway of CKD progression independent of aetiology. Clinical studies with BARD in patients with Alport syndrome, immunoglobulin A nephropathy, autosomal dominant polycystic kidney disease, type 1 diabetes and focal segmental glomerulosclerosis are ongoing.
SUMMARY
To better identify new therapeutic targets for burden of lifestyle diseases and decrease the gap between ‘lifespan’ and ‘healthspan’, we need a mechanistic-based approach linking premature ageing to a whole cluster of chronic inflammatory diseases (geroscience) rather than the organ-based approach to disease that we apply today. Accumulating evidence based on observations from survival mechanisms developed during evolution in the animal kingdom and rare progeroid syndromes suggests that low expression of the cytoprotective and DNA repair system NRF2–KEAP1 contributes to an age-related diseasome. As a master regulator of cellular homoeostasis, NRF2 represents an attractive target for a cluster of chronic diseases characterized by inflammation, oxidative stress and tissue hypoxia. The NRF2 ‘drugome’ includes both nutraceuticals (such as sulphoraphane) and synthetic NRF2 agonists (such as BARD). Better understanding of the biological function, activation and regulation of NRF2–KEAP1 should help us identify the ‘sweet spot’ of optimal activity and identify those patients in whom NRF2 should be targeted with caution. Considering the physiological clustering of tissue hypoxia, senescence and a repressed NRF2–KEAP1 system, it would be of interest to study whether a combination of NRF2 agonists, senolytics [63] and drugs that reduce cortical renal hypoxemia, such as sodium glucose cotransporter 2 inhibitors [102], would have salubrious effects in CKD and other burden of lifestyle diseases than each therapy in isolation (Figure 3).
ACKNOWLEDGEMENTS
The Heart and Lung Foundation, Karolinska Institutet Diabetic Theme Centre, INTRICARE, CaReSyAn and ‘Njurfonden’ supported Peter Stenvinkel’s research.
CONFLICT OF INTEREST STATEMENT
Colin Meyer and Geoff Block are employed by REATA. Peter Stenvinkel and Glenn Chertow are members of the REATA scientific advisory board.
REFERENCES
- 1. Malhotra D, Portales-Casamar E, Singh A. et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 2010; 38: 5718–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holmstrom KM, Baird L, Zhang Y. et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2013; 2: 761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maher J, Yamamoto M.. The rise of antioxidant signaling—the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol 2010; 244: 4–15 [DOI] [PubMed] [Google Scholar]
- 4. Cuadrado A, Rojo AI, Wells G. et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 2019; 18: 295–317 [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto M, Kensler TW, Motohashi H.. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 2018; 98: 1169–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gacesa R, Dunlap WC, Barlow DJ. et al. Rising levels of atmospheric oxygen and evolution of Nrf2. Sci Rep 2016; 6: 27740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copple IM, Dinkova-Kostova AT, Kensler TW. et al. NRF2 as an emerging therapeutic target. Oxid Med Cell Longev 2017; 2017: 8165458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackwell TK, Steinbaugh MJ, Hourihan JM. et al. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med 2015; 88: 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuse Y, Kobayashi M.. Conservation of the Keap1-Nrf2 system: an evolutionary journey through stressful space and time. Molecules 2017; 22: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stankovic M, Mladenovic D, Ninkovic M. et al. Effects of caloric restriction on oxidative stress parameters. Gen Physiol Biophys 2013; 32: 277–283 [DOI] [PubMed] [Google Scholar]
- 11. Frigault JJ, Gaudet JD, Morin PJ.. Investigating Nrf2-associated non-coding RNAs in the hibernating ground squirrel, Ictidomys tridecemlineatus. J Therm Biol 2018; 75: 38–44 [DOI] [PubMed] [Google Scholar]
- 12. Yin Q, Ge H, Liao CC. et al. Antioxidant defenses in the brains of bats during hibernation. PLoS One 2016; 11: e0152135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stenvinkel P, Painer J, Kuro-O M. et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol 2018; 14: 265–284 [DOI] [PubMed] [Google Scholar]
- 14. Bron KM, Murdaugh HV Jr, Millen JE. et al. Arterial constrictor response in a diving mammal. Science 1966; 152: 540–543 [DOI] [PubMed] [Google Scholar]
- 15. Davis RW, Castellini MA, Kooyman GL. et al. Renal glomerular filtration rate and hepatic blood flow during voluntary diving in Weddell seals. Am J Physiol 1983; 245: R743–R748 [DOI] [PubMed] [Google Scholar]
- 16. Zapol WM, Liggins GC, Schneider RC. et al. Regional blood flow during simulated diving in the conscious Weddell seal. J Appl Physiol Respir Environ Exerc Physiol 1979; 47: 968–973 [DOI] [PubMed] [Google Scholar]
- 17. Halasz NA, Elsner R, Garvie RS. et al. Renal recovery from ischemia: a comparative study of harbor seal and dog kidneys. Am J Physiol 1974; 227: 1331–1335 [DOI] [PubMed] [Google Scholar]
- 18. Zenteno-Savin T, Clayton-Hernandez E, Elsner R.. Diving seals: are they a model for coping with oxidative stress? Comp Biochem Physiol C Toxicol Pharmacol 2002; 133: 527–536 [DOI] [PubMed] [Google Scholar]
- 19. Vázquez-Medina JP, Soñanez-Organis JG, Rodriguez R. et al. Prolonged fasting activates Nrf2 in post-weaned elephant seals. J Exp Biol 2013; 216: 2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park TJ, Reznick J, Peterson BL. et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 2017; 356: 307–311 [DOI] [PubMed] [Google Scholar]
- 21. Lewis KN, Wason E, Edrey YH. et al. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci USA 2015; 112: 3722–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruby JG, Smith M, Buffenstein R.. Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age. Elife 2018; 7: e31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis KN, Andziak B, Yang T. et al. The naked mole-rat response to oxidative stress: just deal with it. Antioxid Redox Signal 2013; 19: 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pérez VI, Buffenstein R, Masamsetti V. et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA 2009; 106: 3059–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sosnowska D, Richardson C, Sonntag WE. et al. A heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living noncolonial animal. J Gerontol A Biol Sci Med Sci 2014; 69: 1448–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gruber H, Wessels W, Boynton P. et al. Age-related cellular changes in the long-lived bivalve A. islandica. Age (Dordr) 2015; 37: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janssens GE, Lin XX, Millan-Ariño L. et al. Transcriptomics-based screening identifies pharmacological inhibition of Hsp90 as a means to defer aging. Cell Rep 2019; 27: 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lazaro I, Oguiza A, Recio C. et al. Interplay between HSP90 and Nrf2 pathways in diabetes-associated atherosclerosis. Clin Investig Arterioscler 2017; 29: 51–59 [DOI] [PubMed] [Google Scholar]
- 29. Onken B, Driscoll M.. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One 2010; 5: e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duan W, Zhang R, Guo Y. et al. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. In Vitro Cell Dev Biol Anim 2009; 45: 388–397 [DOI] [PubMed] [Google Scholar]
- 31. Volonte D, Liu Z, Musille PM. et al. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell 2013; 24: 1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuosmanen SM, Sihvola V, Kansanen E. et al. MicroRNAs mediate the senescence-associated decline of NRF2 in endothelial cells. Redox Biol 2018; 18: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fulop GA, Kiss T, Tarantini S. et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 2018; 40: 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Regina C, Panatta E, Candi E. et al. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech Ageing Dev 2016; 159: 14–21 [DOI] [PubMed] [Google Scholar]
- 35. Romero A, San Hipólito-Luengo Á, Villalobos LA. et al. The angiotensin-(1-7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell 2019; 18: e12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zamponi E, Zamponi N, Coskun P. et al. Nrf2 stabilization prevents critical oxidative damage in Down syndrome cells. Aging Cell 2018; 17: e12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubben N, Zhang W, Wang L. et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell 2016; 165: 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11: 298–300 [DOI] [PubMed] [Google Scholar]
- 39. Niccoli T, Partridge L.. Ageing as a risk factor for disease. Curr Biol 2012; 22: R741–52 [DOI] [PubMed] [Google Scholar]
- 40. Fougère B, Boulanger E, Nourhashémi F. et al. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci 2017; 72: 1218–1225 [DOI] [PubMed] [Google Scholar]
- 41. Cuadrado A, Manda G, Hassan A. et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev 2018; 70: 348–383 [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Arellano A, Martínez-González MA, Ramallal R. et al. Dietary inflammatory index and all-cause mortality in large cohorts: the SUN and PREDIMED studies. Clin Nutr 2019; 38: 1221–1231 [DOI] [PubMed] [Google Scholar]
- 43. Senger DR, Li D, Jaminet S-C. et al. Activation of the Nrf2 cell defense pathway by ancient foods: disease prevention by important molecules and microbes lost from the modern Western diet. PLoS One 2016; 11: e0148042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davinelli S, Willcox DC, Scapagnini G.. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun Ageing 2012; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palm F, Koeners MP.. Editorial: hypoxia in kidney disease. Front Physiol 2018; 9: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gerber PA, Rutter GA.. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 2017; 26: 501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hosogai N, Fukuhara A, Oshima K. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56: 901–911 [DOI] [PubMed] [Google Scholar]
- 48. Zelenka J, Koncošová M, Ruml T.. Targeting of stress response pathways in the prevention and treatment of cancer. Biotechnol Adv 2018; 36: 583–602 [DOI] [PubMed] [Google Scholar]
- 49. Kious BM, Kondo DG, Renshaw PF.. Living high and feeling low: altitude, suicide, and depression. Harv Rev Psychiatry 2018; 26: 43–56 [DOI] [PubMed] [Google Scholar]
- 50. Ham PB, Raju R.. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol 2017; 157: 92–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kolamunne RT, Dias IH, Vernallis AB. et al. Nrf2 activation supports cell survival during hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of reactive oxygen and nitrogen species. Redox Biol 2013; 1: 418–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao S, Chao D, Zhou H. et al. A novel mechanism for cytoprotection against hypoxic injury: δ-opioid receptor-mediated increase in Nrf2 translocation. Br J Pharmacol 2015; 172: 1869–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lisk C, McCord J, Bose S. et al. Nrf2 activation: a potential strategy for the prevention of acute mountain sickness. Free Radic Biol Med 2013; 63: 264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johnson RJ, Rodriguez-Iturbe B.. Rethinking progression of CKD as a process of punctuated equilibrium. Nat Rev Nephrol 2018; 14: 411–412 [DOI] [PubMed] [Google Scholar]
- 55. Shelton LM, Park BK, Copple IM.. Role of Nrf2 in protection against acute kidney injury. Kidney Int 2013; 84: 1090–1095 [DOI] [PubMed] [Google Scholar]
- 56. Noel S, Martina MN, Bandapalle S. et al. T lymphocyte-specific activation of Nrf2 protects from AKI. J Am Soc Nephrol 2015; 26: 2989–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu D, Lun L, Huang Q. et al. Youthful systemic milieu alleviates renal ischemia-reperfusion injury in elderly mice. Kidney Int 2018; 94: 268–279 [DOI] [PubMed] [Google Scholar]
- 58. Liu M, Reddy NM, Higbee EM. et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int 2014; 85: 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arisawa T, Tahara T, Shibata T. et al. Nrf2 gene promoter polymorphism and gastric carcinogenesis. Hepatogastroenterology 2008; 82–83: 750–754 [PubMed] [Google Scholar]
- 60. Yu S, Khor TO, Cheung KL. et al. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One 2010; 5: e8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yates MS, Tran QT, Dolan PM. et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 2009; 30: 1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dodson M, de la Vega MR, Cholanians AB. et al. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol 2019; 59: 555–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tchkonia T, Kirkland JL.. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA 2018; 320: 1319–1320 [DOI] [PubMed] [Google Scholar]
- 64. Vaziri ND, Liu S, Farzaneh SH. et al. Dose-dependent deleterious and salutary actions of the Nrf2 inducer dh404 in chronic kidney disease. Free Radic Biol Med 2015; 86: 374–381 [DOI] [PubMed] [Google Scholar]
- 65. To C, Ringelberg CS, Royce DB. et al. Dimethyl fumarate and the oleanane triterpenoids, CDDO-imidazolide and CDDO-methyl ester, both activate the Nrf2 pathway but have opposite effects in the A/J model of lung carcinogenesis. Carcinogenesis 2015; 36: 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019; 393: 1958–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Esgalhado M, Stenvinkel P, Mafra D.. Nonpharmacologic strategies to modulate nuclear factor erythroid 2-related factor 2 pathway in chronic kidney disease. J Ren Nutr 2017; 27: 282–291 [DOI] [PubMed] [Google Scholar]
- 68. Willcox DC, Scapagnini G, Willcox BJ.. Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev 2014; 136–137: 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu KC, McDonald PR, Liu J. et al. Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med 2014; 80: 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vaziri ND, Liu SM, Lau WL. et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 2014; 9: e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. González-Aguilar GA, Blancas-Benítez FJ, Sáyago AS.. Polyphenols associated with dietary fibers in plant foods: molecular interactions and bioaccessibility. Curr Opin Food Sci 2017; 13: 84–88 [Google Scholar]
- 72. Singh R, Chandrashekharappa S, Bodduluri SR. et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun 2019; 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Axelsson AS, Tubbs E, Mecham B. et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 2017; 9: eaah4477 [DOI] [PubMed] [Google Scholar]
- 74. Kunnumakkara AB, Bordoloi D, Padmavathi G. et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 2017; 174: 1325–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tu ZS, Wang Q, Sun DD. et al. Design, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative death. Eur J Med Chem 2017; 134: 72–85 [DOI] [PubMed] [Google Scholar]
- 76. Rassaf T, Rammos C, Hendgen-Cotta UB. et al. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double–blind, randomized, placebo–controlled trial. Clin J Am Soc Nephrol 2016; 11: 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fratta Pasini AM, Stranieri C, Rigoni AM. et al. Physical exercise reduces cytotoxicity and up-regulates nrf2 and upr expression in circulating cells of peripheral artery disease patients: an hypoxic adaptation? J Atheroscler Thromb 2018; 25: 808–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Garbin U, Fratta Pasini A, Stranieri C. et al. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favoring inflammation. PLoS One 2009; 4: e8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang J, Yao W, Dong C. et al. Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci 2018; 268: 865–870 [DOI] [PubMed] [Google Scholar]
- 80. Woo M, Kim M, Noh JS. et al. Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress. J Funct Foods 2017; 32: 218–225 [Google Scholar]
- 81. McClelland R, Christensen K, Mohammed S. et al. Accelerated ageing and renal dysfunction links lower socioeconomic status and dietary phosphate intake. Aging (Albany NY) 2017; 8: 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Haring B, Selvin E, Liang M. et al. Dietary protein sources and risk for incident chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Ren Nutr 2017; 27: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Surya R, Helies-Toussaint C, Martin OC. et al. Red meat and colorectal cancer: Nrf2-dependent antioxidant response contributes to the resistance of preneoplastic colon cells to fecal water of hemoglobin- and beef-fed rats. Carcinogenesis 2016; 37: 635–645 [DOI] [PubMed] [Google Scholar]
- 84. Bolati D, Shimizu H, Yisireyili M. et al. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-κB. BMC Nephrol 2013; 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang M, Jiang ZH, Li CG. et al. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed Pharmacother 2018; 105: 1283–1290 [DOI] [PubMed] [Google Scholar]
- 86. Kurosu H, Yamamoto M, Clark JD. et al. Suppression of aging in mice by the hormone Klotho. Science 2005; 309: 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo Y, Zhuang X, Huang Z. et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 238–251 [DOI] [PubMed] [Google Scholar]
- 88. Son YK, Liu S, Farzaneh SH. et al. Nrf2 activator, dh404, restores renal Klotho expression and attenuates oxidative stress and inflammation in rats with CKD. J Applied Health Sci Int 2015; 2: 22–34 [Google Scholar]
- 89. Maltese G, Psefteli PM, Rizzo B. et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med 2017; 21: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yao L, Wang J, Tian BY. et al. Activation of the Nrf2-ARE signaling pathway prevents hyperphosphatemia-induced vascular calcification by inducing autophagy in renal vascular smooth muscle cells. J Cell Biochem 2017; 118: 4708–4715 [DOI] [PubMed] [Google Scholar]
- 91. Longen S, Beck KF, Pfeilschifter J.. H2S-induced thiol-based redox switches: biochemistry and functional relevance for inflammatory diseases. Pharmacol Res 2016; 111: 642–651 [DOI] [PubMed] [Google Scholar]
- 92. Sanchez-Roman I, Barja G.. Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction. Exp Gerontol 2013; 48: 1030–1042 [DOI] [PubMed] [Google Scholar]
- 93. Hou CL, Wang MJ, Sun C. et al. Protective effects of hydrogen sulfide in the ageing kidney. Oxid Med Cell Longev 2016; 2016: 7570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aghagolzadeh P, Radpour R, Bachtler M. et al. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/NRF2/NQO1 activation. Atherosclerosis 2017; 265: 78–86 [DOI] [PubMed] [Google Scholar]
- 95. Copple IM, Shelton LM, Walsh J. et al. Chemical tuning enhances both potency toward nrf2 and in vitro therapeutic index of triterpenoids. Toxicol Sci 2014; 140: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ha CM, Park S, Choi YK. et al. Activation of Nrf2 by dimethyl fumarate improves vascular calcification. Vascul Pharmacol 2014; 63: 29–36 [DOI] [PubMed] [Google Scholar]
- 97. Pergola PE, Raskin P, Toto RD. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011; 365: 327–336 [DOI] [PubMed] [Google Scholar]
- 98. de Zeeuw D, Akizawa T, Audhya P. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013; 369: 2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chin MP, Reisman SA, Bakris GL. et al. Mechanisms contributing to adverse cardiovascular events in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. Am J Nephrol 2014; 39: 499–508 [DOI] [PubMed] [Google Scholar]
- 100. Chin MP, Bakris GL, Block GA. et al. Bardoxolone methyl improves kidney function in patients with chronic kidney disease stage 4 and type 2 diabetes: post-hoc analyses from bardoxolone methyl evaluation in patients with chronic kidney disease and type 2 diabetes study. Am J Nephrol 2018; 47: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Martini S, Nair V, Keller BJ. et al. Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol 2014; 25: 2559–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kamezaki M, Kusaba T, Komaki K. et al. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep 2018; 8: 4029. [DOI] [PMC free article] [PubMed] [Google Scholar]