Abstract
Background
Protein-energy wasting, muscle mass (MM) loss and sarcopenia are highly prevalent and associated with poor outcome in haemodialysis (HD) patients. Monitoring of MM and/or muscle metabolism in HD patients is of paramount importance for timely detection of muscle loss and to intervene adequately. In this study we assessed the reliability and reproducibility of a simplified creatinine index (SCI) as a surrogate marker of MM and explored its predictive value on outcome.
Method
We included all in-centre HD patients from 16 European countries with at least one SCI. The baseline period was defined as 30 days before and after the first multifrequency bioimpedance spectroscopy measurement; the subsequent 7 years constituted the follow-up. SCI was calculated by the Canaud equation. Multivariate Cox proportional hazards models were applied to assess the association of SCI with all-cause mortality. Using backward analysis, we explored the trends of SCI before death. Bland–Altman analysis was performed to analyse the agreement between estimated and measured MM.
Results
We included 23 495 HD patients; 3662 were incident. Females and older patients have lower baseline SCI. Higher SCI was associated with a lower risk of mortality [hazard ratio 0.81 (95% confidence interval 0.79–0.82)]. SCI decline accelerated ∼5–7 months before death. Lean tissue index (LTI) estimated by SCI was correlated with measured LTI in both sexes (males: R2 = 0.94; females: R2 = 0.92; both P < 0.001). Bland–Altman analysis showed that measured LTI was 4.71 kg/m2 (±2 SD: −12.54–3.12) lower than estimated LTI.
Conclusion
SCI is a simple, easily obtainable and clinically relevant surrogate marker of MM in HD patients.
Keywords: creatinine, haemodialysis, lean body mass, muscle mass metabolism, simplified creatinine index
KEY LEARNING POINTS
What is already known about this subject?
Serum creatinine (SCr) is a routinely measured byproduct of skeletal muscle metabolism. SCr is a reliable indicator of an individual’s muscle mass (MM), nutritional status and physical activities in several clinical settings, including chronic kidney disease patients. It is well known that the levels of MM, nutritional status and physical activities are associated with clinical outcomes in dialysis patients.
What this study adds?
Creatinine kinetic models (CKMs) have been developed to calculate the creatinine appearance rate. Due to the complexity of CKMs, a simplified creatinine index (SCI) based on readily available parameters was developed and shown to be associated with outcomes in dialysis patients. In the current study, we further confirmed that a higher SCI value is associated with better survival in dialysis patients.
What impact this may have on practice or policy?
SCI can be used as a simple, easily obtainable and clinically relevant surrogate marker of MM in clinical settings.
INTRODUCTION
Protein-energy wasting (PEW) and muscle mass (MM) loss are highly prevalent in chronic haemodialysis (HD) patients and both are associated with poor patient outcomes [1, 2]. It is of paramount importance for clinicians to monitor body composition, including lean body mass (LBM) and MM alterations, in these patients for timely implementation of interventions to delay, halt or even reverse the deleterious process [3, 4]. Anthropometric methods such as subjective global assessment, skinfold thickness and mid-arm muscle circumference are commonly used to detect PEW, but their applicability and sensitivity in routine clinical practice are not convincing [5–7]. Serum biomarkers such as albumin and transthyretin are valid and clinically useful indicators of PEW with high predictive value of mortality [8–10]. However, these visceral proteins are poorly representative of nutritional status when referring to LBM or MM; both are strongly negatively influenced by inflammation [11–14]. Instrumental methods, including bioimpedance (BIA), dual-emission X-ray absorptiometry (DEXA), whole-body nitrogen content determined by neutron activation analysis and nuclear magnetic resonance imaging (MRI) or computer tomography [15–17] are of great value to measure LBM, yet they are still limited to clinical research and not ubiquitously used in clinical practice [18]. However, no one method, including DEXA, fulfils all requirements to be considered as a reference today [19].
Serum creatinine (SCr) is a routinely measured byproduct of skeletal muscle metabolism (MMet). SCr is a reliable indicator of an individual’s MM, nutritional status and physical activities in several clinical settings, including chronic kidney disease (CKD) patients [20]. However, in HD patients, the situation is more complex because urinary creatinine excretion has vanished in most patients. SCr levels fluctuate according to the intermittent nature of HD treatment and are inversely related to HD efficacy [21]. Regardless of these limitations, higher pre-HD SCr levels have been shown to associate with a reduced risk of mortality, clearly indicating its predominant role as a nutritional marker in dialysis patients. Several creatinine kinetic models (CKMs) have been developed to calculate the creatinine appearance rate from which the creatinine index (expressed in units of mg/kg/day) was deduced [22] or which is used to estimate the LBM (in kg) [23–26]. However, CKM requires measurements of post-HD SCr and rather complex mathematical algorithms. Therefore a simplified creatinine index (SCI) based on readily available parameters [i.e. patients’ demographics, single-pool Kt/V and pre-HD SCr] was developed [27, 28]. Several recent retrospective and prospective cohort studies demonstrated a high predictive value of SCI on HD patient outcomes [29, 30].
To further elucidate the predictive value of SCI as a marker of sarcopenia on patient outcomes, we examined the association between SCI and all-cause mortality in the database of the international MONitoring Dialysis Outcome (MONDO) Initiative [31]. Along with this we tested the reliability of SCI as a surrogate marker of MM in HD patients by comparing it to the instrumental one determined by multiple frequency bioimpedance spectroscopy (MF-BIS).
MATERIALS AND METHODS
For study enrolment, we considered all European adult HD patients documented in the MONDO Initiative. The MONDO Initiative is an international retrospective cohort study that comprises chronic HD patients from 41 countries. Prior to data transfer to the MONDO Initiative, the respective consortia remove all identifiable parameters as per Health Insurance Portability and Accountability Act, General Data Protection Regulation 2016/679 and locally applicable rules and regulations. Additionally, multiple levels of internal data validation control are applied before the database can be used for research. All the research activities conducted by the MONDO Initiative comply with national and international ethical, compliance and legal standards. This study was exempted by the Western Institutional Review Board (ES-16-005).
In the current study we limited the inclusion to patients with at least one baseline measurement of SCr and spKt/V. The baseline period was defined as 30 days before and 30 days after the first MF-BIS measurement on HD. The subsequent 7 years on HD was defined as the follow-up period, in which all-cause mortality was recorded. In 2006, MF-BIS (Body Composition Monitor; Fresenius Medical Care, Bad Homburg, Germany) was introduced as part of routine clinical care in most of the participating clinics. All the BIA measurements were measured by multifrequency (50 frequencies) MF-BIS, which is a three-compartment model, differentiating between lean tissue index (LTI), fat tissue index (FTI) and an ‘overhydration’ compartment [32]. All the values were performed before the dialysis at the midweek session with the patient in a supine position. Laboratory and clinical parameters were obtained from patients’ electronic medical records. In case multiple measurements of laboratory or clinical variables were available during baseline, arithmetic averages were calculated. Patients were followed until transferred to non-participating clinics, treatment modality change, transplantation, recovery of renal function, death or the study end (31 December 2012).
Calculation of SCI
SCI was calculated using the Canaud formula [27]:
| (1) |
LTI was estimated using the formula by Canaud et al. [29]:
| (2) |
The estimated LTI was then compared with LTI values obtained from MI-BIS measurements.
The following analyses were performed:
descriptive of baseline characteristics for all patients, patients who died and patients who survived during the follow-up;
comparison of baseline SCI values in both sexes across different age strata (18–29, 30–39, 40–49, 50–59, 60–69, 70–79 and >80 years);
association of SCI with all-cause mortality using Cox proportional hazards models for unadjusted models and the fully adjusted model with adjustment for age, gender, diabetic and cardiovascular disease (CVD) as comorbidities, body mass index (BMI), dialysis vintage, serum albumin and C-reactive protein (CRP);
description of the SCI dynamics before death in both sexes;
relationships and agreements between LTI computed by Equation (2) and LTI measured by MF-BIS in both sexes;
sensitivity analyses for (3) and (4) in patients whose baseline vintages were <12 and >18 months were also conducted to account for the potential impact of residual renal function on the outcomes;
to correct for the effect of fluid status on SCr levels, subset analyses for (3) and (4) in patients who with normohydrated and overhydrated pre-dialytic fluid status in the baseline were performed. Normohydrated was defined as relative fluid status <15% for males and <13% for females. Fluid status was calculated as (overhydration/extracellar water) × 100.
To explore non-linear relationships between SCr, measured LTI, estimated LTI and outcomes, we constructed cubic spline functions with four degrees of freedom in the unadjusted and fully adjusted models. Subset analyses were also performed in patients with baseline vintages <12 and >18 months and normohydrated and overhydrated fluid status.
Statistical analyses
We report mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. The continuous variables were compared via one-way analysis of varaiance or Kruskal–Wallis tests based on their distribution, and categorical variables were compared via two-sample t-tests between patients who survived and those who died. P-values <0.05 or a 95% CI not including null were considered statistically significant.
We built Cox proportional hazards regression models in the unadjusted and adjusted models to assess the association between baseline SCI values and all-cause mortality. Point estimates and 95% CIs of hazard ratios (HRs) were reported. For the analysis of SCI trends before death, a backward process was applied that started at the time of death and went backward in time for up to 24 months. A cubic B-spline function with 95% CI was fitted to investigate SCI trajectories in both sexes. The first derivative of these splines was approximated using finite differences. Simple linear regression was used to assess the relation between equation-derived and measured LTI. Bland–Altman analysis [33] was performed to analyse the agreement between equation-derived and measured LTI at different levels of measured LTI. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.4.4 (R Foundation, Vienna, Austria) [34].
RESULTS
In total, 23 495 incident and prevalent HD patients from 16 European countries were included in the study; 2194 patients (9.4%) died during follow-up. The average follow-up time for all patients was 330 days, 265 days for patients who died and 337 days for patients who survived. The average age was 61.0 ± 15.0 years, 57% of patients were male, ~24% of them with diabetics as a comorbidity and the average dialysis vintage as 4.4 ± 4.7 years. Patients who died tended to have lower SCI, LTI, post-HD weight, SCr, albumin and phosphate and higher CRP and neutrophil:lymphocyte ratio (NLR) and a higher percentage patients with overhydrated status (52% versus 32%; Table 1).
Table 1.
Baseline characteristics of the study population
| Characteristics | All patients (n = 23 495) | Survivors (n = 21 301) | Deceased (n = 2194) | Δ (survivors − deceased) | P-value |
|---|---|---|---|---|---|
| Patients | |||||
| Age (years) | 61.0 ± 15.0 | 61.2 ± 15.0 | 69.5 ± 12.8 | −8.3 ± 14.8 | <0.001 |
| Males (%) | 56.8 | 56.7 | 58.0 | − 1.3 | >0.05 |
| Urea reduction ratio (%) | 74.9 ± 7.7 | 75.0 ± 7.6 | 74.3 ± 8.3 | 0.6 ± 7.7 | <0.001 |
| Diabetics (%) | 24.2 | 23.4 | 32.0 | − 8.6 | <0.001 |
| Body height (cm) | 164.3 ± 9.9 | 164.4 ± 9.9 | 163.9 ± 9.7 | 0.5 ± 9.9 | 0.01 |
| Dialysis vintage (years) | 4.4 ± 4.7 | 4.5 ± 4.7 | 4.0 ± 4.2 | 0.5 ± 4.7 | <0.001 |
| Biomarkers | |||||
| SCI (mg/kg/day) | 19.3 ± 2.6 | 19.3 ± 2.6 | 18.0 ± 2.4 | 1.3 ± 2.6 | <0.001 |
| LTM (kg) | 34.3 ± 10.4 | 34.6 ± 10.4 | 30.9 ± 9.4 | 3.7 ± 10.3 | <0.001 |
| Measured LTI (kg/m2) | 12.5 ± 3.1 | 12.6 ± 3.1 | 11.4 ± 3.0 | 1.2 ± 3.1 | <0.001 |
| Estimated LTI (kg/m2) | 17.2 ± 3.5 | 17.4 ± 3.5 | 16.0 ± 3.5 | 1.4 ± 3.5 | <0.001 |
| ECW (L) | 16.8 ± 3.5 | 16.8 ± 3.5 | 16.6 ± 3.6 | 0.2 ± 4.7 | <0.001 |
| Overhydrated status (%) | 39 | 32 | 52 | −20 | <0.001 |
| BMI (kg/m2) | 26.0 ± 5.3 | 26.1 ± 5.3 | 25.2 ± 5.5 | 0.9 ± 5.3 | <0.001 |
| Post-HD body weight (kg) | 70.3 ± 15.6 | 70.6 ± 15.6 | 67.8 ± 16.2 | 2.8 ± 15.6 | <0.001 |
| SCr (mg/dL) | 7.8 ± 2.4 | 7.9 ± 2.4 | 6.8 ± 2.2 | 1.1 ± 2.4 | <0.001 |
| Serum albumin (g/dL) | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.6 ± 0.5 | 0.2 ± 0.4 | <0.001 |
| nPCR (g/kg/day) | 1.05 ± 0.2 | 1.05 ± 0.2 | 1.04 ± 0.2 | 0.01 ± 0.2 | >0.05 |
| Serum phosphate (mg/dL) | 4.8 ± 1.4 | 4.8 ± 1.4 | 4.5 ± 1.5 | 0.3 ± 1.4 | <0.001 |
| Total cholesterol (mg/dL) | 173.1 ± 43.9 | 173.8 ± 43.7 | 167.8 ± 45.3 | 6.4 ± 43.9 | <0.001 |
| Triglycerides (mg/dL) | 163.8 ± 99.5 | 165.1 ± 99.8 | 152.0 ± 96.0 | 0.9 ± 4.4 | <0.001 |
| Low-density protein (mg/dL) | 100.6 ± 37.1 | 101.0 ± 43.7 | 97.4 ± 37.2 | 3.6 ± 37.1 | 0.007 |
| CRP (mg/dL) | 13.2 ± 19.5 | 12.1 ± 17.9 | 23.5 ± 28.7 | −11.4 ± 19.2 | <0.001 |
| Neutrophil:lymphocyte ratio | 3.2 ± 2.7 | 3.1 ± 2.5 | 4.2 ± 4.0 | −1.1 ± 2.7 | <0.001 |
| spKt/V | 1.70 ± 0.3 | 1.70 ± 0.3 | 1.65 ± 0.4 | 0.05 ± 0.3 | 0.003 |
Values presented as mean ± SD unless staed otherwise. TM: lean tissue mass; LTI = LTM/(body height in metres)2; estimated LTI = (SCI * Post-HD weight * 0.029 + 7.38)/[body height (m)]2; ECW: extracellular water; nPCR: normalized protein catabolic rate.
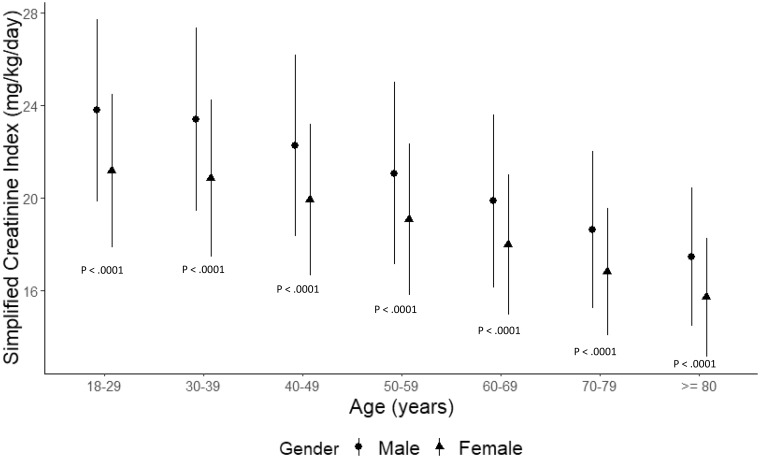
The total population SCI stratified by age is presented in Figure 1. Across all age strata, SCI values were statistically higher in males than females. The highest SCI was observed in males 18–29 years of age [SCI 23.8 (95% CI 19.8–27.7) mg/kg/day]. In both sexes, SCI levels decreased with age, with the lowest SCI in patients >80 years of age [males: 17.4 (95% CI 14.4–20.4); females: 15.7 (95% CI 13.1–18.3) mg/kg/day].
FIGURE 1.
Simplified creatinine index stratified by age and gender. Data present mean (95% CI).
The predictive value of SCI, unadjusted and adjusted, on all-cause mortality is presented in Table 2. In general, higher SCI [unadjusted HR 0.81 (95% CI 0.79–0.82); adjusted HR 0.90 (95% CI 0.86–0.93)], BMI [unadjusted HR 0.97 (95% CI 0.96–0.98); adjusted HR 0.98 (95% CI 0.97–0.99)] and serum albumin [unadjusted HR 0.30 (95% CI 0.28–0.33); adjusted HR 0.49 (95% CI 0.44–0.55)] are associated with lower risk of all-cause mortality. In contrast, patients with diabetes and CVD as comorbidities, who were older and with higher CRP were at a higher risk of a poor outcome. Sensitivity analyses for patients with vintages <12 and >18 months showed similar findings (Table 3). A subset analysis conducted in patients with normohydrated and overhydrated status also yielded similar observations (Table 4).
Table 2.
Predictive value of SCI for all-cause mortality by unadjusted and adjusted Cox proportional hazards model
| Hazard ratio |
||
|---|---|---|
| Parameter | Univariate | Adjusted |
| Age (per year) | 1.04 (1.04–1.04)* | 1.02 (1.02–1.03 * |
| Gender (male:female) | 1.06 (0.98–1.16) | 1.31 (1.14–1.49)* |
| Diabetic (yes:no) | 1.44 (1.32–1.58 * | 1.20 (1.06–1.35)* |
| Comorbid CVD (yes:no) | 1.25 (1.11–1.34)* | 1.13 (1.05–1.26)* |
| BMI (per kg/m2) | 0.97 (0.96–0.98)* | 0.98 (0.97–0.99)* |
| Vintage (per year) | 0.98 (0.97–0.98)* | 1.01 (1.00–1.02)* |
| Serum albumin (per mg/dL) | 0.30 (0.28–0.33)* | 0.49 (0.44–0.55)* |
| CRP (μg/mL) | 1.02 (1.02–1.02)* | 1.01 (1.01 -1.02)* |
| SCI (per mg/kg/day) | 0.81 (0.79–0.82)* | 0.90 (0.86–0.93)* |
Indicates statistical significance.
Table 3.
Predictive value of SCI for all-cause mortality categorized by dialysis vintage
| Hazard ratio (unadjusted) |
Hazard ratio (adjusted) |
|||
|---|---|---|---|---|
| Parameter | Vintage <12 months | Vintage >18 months | Vintage <12 months | Vintage >18 months |
| Age (per year) | 1.04 (1.03–1.05)* | 1.04 (1.04–1.05)* | 1.03 (1.02–1.04)* | 1.02 (1.01–1.03)* |
| Gender (male:female) | 0.96 (0.82–1.12)* | 1.13 (1.02–1.26)* | 1.19 (0.95–1.48) | 1.52 (1.28–1.80)* |
| Diabetic (yes:no) | 1.04 (0.88–1.23) | 1.70 (1.52–1.90)* | 0.92 (0.75–1.13) | 1.43 (1.22–1.67)* |
| Comorbid CVD (yes:no) | 1.05 (1.02–1.35)* | 1.08 (1.02–1.45)* | 1.13 (1.05–1.26)* | 1.25 (1.18–1.53)* |
| BMI (per kg/m2) | 0.95 (0.93–0.96)* | 0.98 (0.97–0.99)* | 0.97 (0.95–0.99)* | 1.00 (0.98–1.01) |
| Vintage (per year) | 0.72 (0.55–0.95)* | 0.98 (0.97–0.99)* | 1.30 (0.92–1.84) | 1.01 (0.99–1.03) |
| Serum albumin (per mg/dL) | 0.30 (0.26–0.35)* | 0.29 (0.26–0.33)* | 0.44 (0.36–0.52)* | 0.51 (0.43–0.60)* |
| CRP (per μg/mL) | 1.02 (1.02–1.02)* | 1.02 (1.01–1.02)* | 1.01 (1.01–1.02)* | 1.01 (1.01–1.01)* |
| SCI (per mg/kg/day) | 0.82 (0.79–0.85)* | 0.80 (0.78–0.82)* | 0.91 (0.85–0.97)* | 0.86 (0.82–0.90)* |
Indicates statistical significance.
Table 4.
Predictive value of SCI for all-cause mortality categorized by baseline hydration status
| Hazard ratio (unadjusted) |
Hazard ratio (adjusted) |
|||
|---|---|---|---|---|
| Parameter | Normohydrated patients | Overhydrated patients | Normohydrated patients | Overhydrated patients |
| Age (per year) | 1.05 (1.05–1.06)* | 1.03 (1.03–1.04)* | 1.03 (1.02–1.04)* | 1.02 (1.02–1.03)* |
| Gender (male:female) | 0.98 (0.86–1.10) | 0.91 (0.80–1.02) | 1.34 (1.12–1.60)* | 1.09 (0.91– 1.31) |
| Diabetic (yes:no) | 1.59 (1.39–1.81)* | 1.17 (1.04–1.33)* | 1.14 (0.96–1.36) | 1.16 (1.22–1.67)* |
| Comorbid CVD (yes:no) | 1.45 (1.32–1.53)* | 1.23 (1.08–1.33)* | 1.18 (1.08–1.28)* | 1.25 (0.98–1.36)* |
| BMI (per kg/m2) | 0.99 (0.98–1.00)* | 0.97 (0.95–0.98)* | 0.99 (0.98, 1.01) | 0.98 (0.96–0.99) |
| Vintage (per year) | 0.98 (0.96–0.99)* | 0.97 (0.96–0.98)* | 1.01 (0.99–1.03) | 1.00 (0.99–1.02) |
| Serum albumin (per mg/dL) | 0.23 (0.24–0.32)* | 0.36 (0.32–0.41)* | 0.48 (0.41–0.57)* | 0.52 (0.45–0.61)* |
| CRP (per μg/mL) | 1.02 (1.01–1.02)* | 1.02 (1.02–1.02)* | 1.01 (1.01–1.02)* | 1.01 (1.01–1.02)* |
| SCI (per mg/kg/day) | 0.79 (0.77–0.81)* | 0.83 (0.81–0.85)* | 0.90 (0.86–0.95)* | 0.92 (0.87–0.97)* |
Indicates statistical significance.
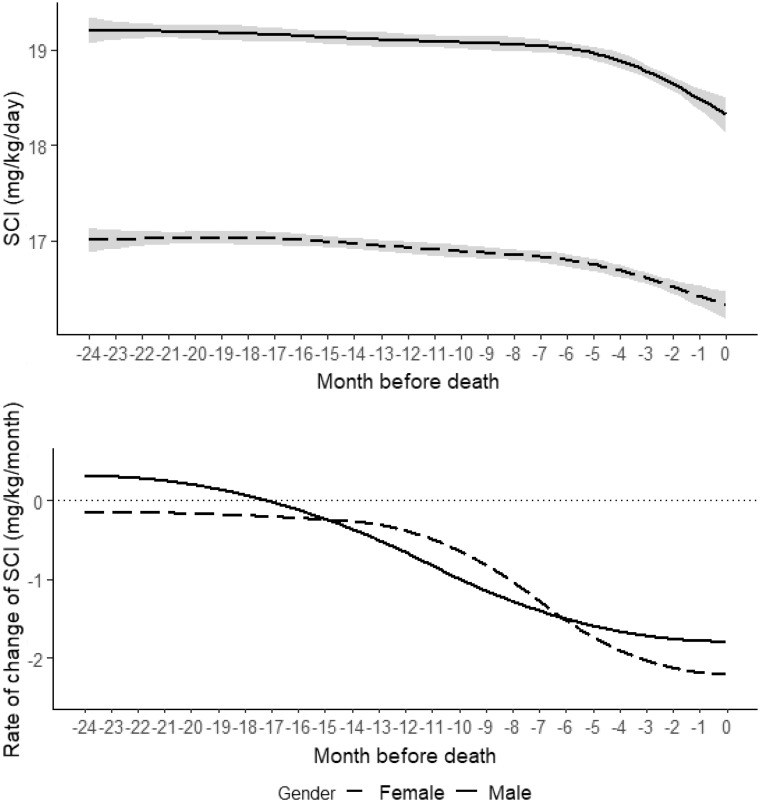
SCI dynamics in the 2 years before death are depicted in Figure 2 for both sexes. In general, SCI values were higher in males. An accelerated SCI decline began 5–7 months before death and was more pronounced in males (Figure 2). Similar observations were seen in patients with vintages <12 and >18 months, with normohydrated and overhydrated status (Supplementary data, Figures S1 and S2).
FIGURE 2.
Trends and rates of change of SCI in the 2 years preceding death for males and females. Solid line: males; dashed line: females.
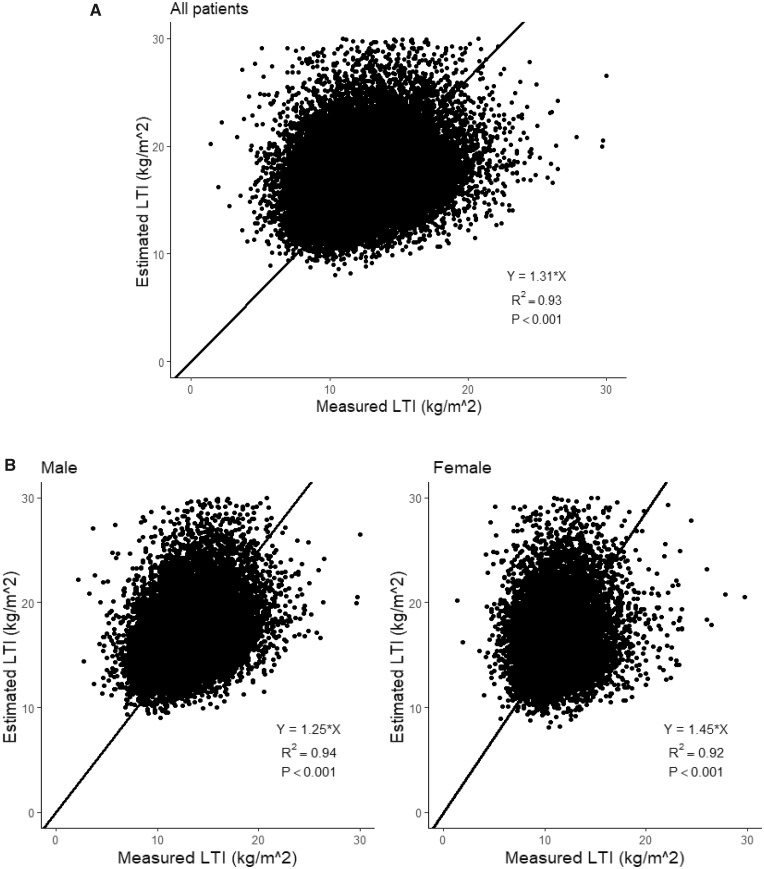
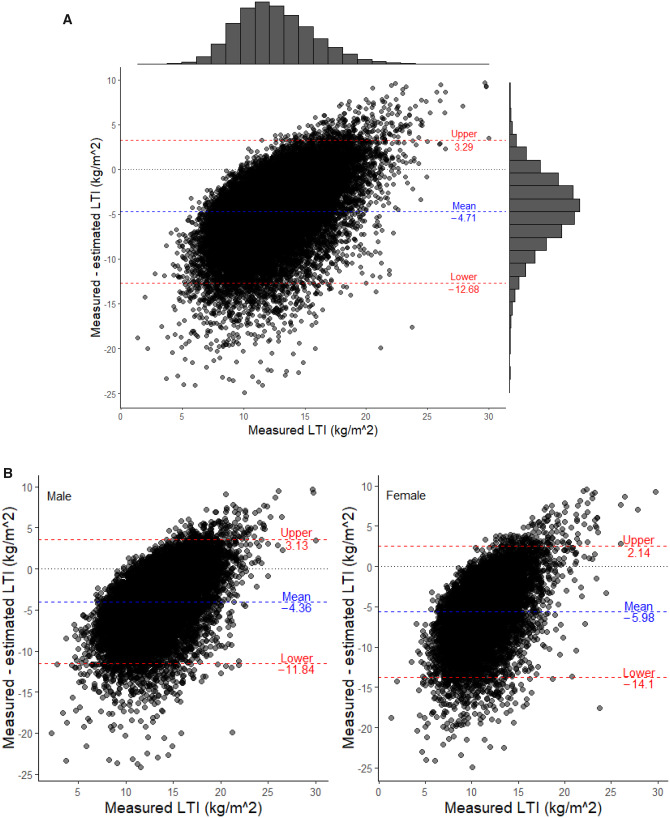
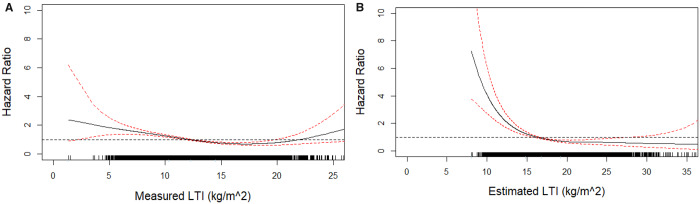
LTI estimated using Equation (2) was correlated with measured LTI in both sexes (all patients: R2 = 0.93; males: R2 = 0.94; females: R2 = 0.92; all P < 0.001; Figures 3A and B). Bland–Altman analysis (Figure 4A) showed that estimated LTI was 4.71 kg/m2 (±2 SD −12.68–3.29) higher than LTI measured by MF-BIS in all patients, 4.36 kg/m2 (±2 SD −11.84–3.13) higher than LTI measured in males (Figure 4B) and 5.98 kg/m2 (±2 SD −14.10–2.14) higher than LTI measured in females (Figure 4B). Cubic spline analyses revealed that higher SCI (>20.5 mg/kg/day), measured LTI (>12.8 kg/m2) and estimated LTI (>16.1 kg/m2) values are associated with lower risk of mortality in the unadjusted and adjusted models (Supplementary Figures S3; Figure 5A and B). This effect seemed more pronounced when using the estimated LTI.
FIGURE 3.
Best-fit linear regression analysis (setting constant term α = 0) for all patients, males and females. (A) All patients. (B) Stratified by males and females.
FIGURE 4.
Modified Bland–Altman analysis assessing the differences between LTI measured by MF-BIS versus estimated LTI. (A) All patients. The two red dashed lines indicate the upper (mean + 2 SD) and lower limits (mean − 2SD) of the ΔLTI (measured − estimated LTI). The histogram on top of the figure represents the distribution of the measured LIT, the histogram on the right side indicates the distribution of the difference between measured and estimated LIT. (B) Stratified by males and females. The two red dashed lines indicate the upper (mean + 2 SD) and lower limits (mean − 2 SD) of the ΔLTI (measured − estimated LTI).
FIGURE 5.
(A) Association of measured LTI values with all-cause mortality. Model adjusted for age, gender, BMI, dialysis vintage, diabetes and CVD as comorbidities, serum albumin and CRP. (B) Association of estimated LTI values with all-cause mortality. Model adjusted for age, gender, BMI, dialysis vintage, diabetes and CVD as comorbidities, serum albumin and CRP.
DISCUSSION
Our study in >20 000 HD patients from a large international population showed that SCI is associated with clinically relevant outcomes. While our results corroborate earlier findings and support SCI’s generalizability and use in different dialysis populations [20, 35–37], we also add new insights to the existing literature [38]. In our study, we showed that SCI tends to be higher in male and younger HD dialysis patients. In patients who died, SCI declined months before death in both male and female patients. We also observed that a higher SCI was associated with a survival benefit in both males and females. From the current study, we observed that LTI derived from SCI and MF-BIS were strongly related, the former tended to generally overestimate LTI as compared with the measured method.
The assessment of MM or muscle turnover is of critical importance in the nutritional management of CKD patients to quantify sarcopenia and predict outcome. The various methods (e.g. clinical and anthropometrics, instrumental, biomarkers or imaging) and threshold values used to measure LBM are hampered by the tools' intrinsic limitations. No one method, including DEXA, fulfils all requirements to be considered as a reference today [19]. Our study indicated that SCI was lower in older patients of both sexes. Considering SCI differences noted in patients between 18 and 29 years and >80 years of age, one can estimate an active MM decrease of ~27% in males and ~26% in females. This value exceeds the 20% decrease in active MM reported in a healthy sex- and age-matched population [2, 3]. The lower SCI in older patients in our study cohort supports the notion that MM is much lower in HD patients compared with a healthy, age-matched population. The decrease in SCI most likely shows a loss of active MM and a slowdown of MMet, indicating poor physical performance, low appetite and high prevalence of inflammation, particularly in older HD patients [2].
The predictive value of SCI and SCI changes on patient outcomes are significant in both unadjusted and adjusted Cox proportional hazards regression models. Higher SCI by 1 mg/kg/day is associated with a 19% lower HR for all-cause mortality in unadjusted analysis and 10% in the adjusted analysis. These findings were consistent in patients with normohydrated and overhydrated fluid status [32]. The finding supports the hypothesis that SCI can be viewed as a skeletal muscle nutritional surrogate in dialysis patients. In the backward analysis, SCI showed a clear declining trend 5–7 months before death in both sexes. The validity of SCI as a predictor of mortality corroborated some preliminary results from the Dialysis Outcomes and Practice Patterns Study (Bernard Canaud, unpublished data), where the HR of death was reduced by 16.5% per 1 SCI unit higher (mg/kg/day). By nature, this cross-sectional study could not address the effects of advanced CKD and its metabolic derangements (e.g. uraemic toxins accumulation, metabolic acidosis and hormonal disturbances) on MM alteration before initiation of renal replacement therapy. This point deserves further and dedicated study specifically addressing this question.
Albumin is the most used nutritional marker in CKD patients and is strongly associated with patient outcomes [39, 40]. However, albumin is also affected by fluid status and inflammation, rendering it a composite marker [41]. Compared with albumin, SCr is a more specific and stable marker of somatic proteins [20, 42]. In a study of 179 prevalent HD patients, Vernaglione et al. [21] concluded that creatinine metabolism is not influenced by the inflammatory acute phase response. Another interesting finding of our study is that SCI declines approximately at the same time before death as serum albumin, creatinine, phosphate and normalized protein catabolic rate [43, 44]. The rapid decline of SCI ~6 months before death suggests that muscle wasting is triggered by catabolic factors and accelerated ageing processes noted in uraemia and, possibly, precipitated by reduced physical activity, protein intake, muscle synthesis and deconditioning. Therefore, based on our current and previous analysis [27], we can support the hypothesis that SCI is a clinically relevant indicator of malnutrition.
Substantial losses in LBM and sarcopenia are associated with malnutrition and PEW, phenomena common in HD patients. The association between PEW and mortality in CKD and end-stage renal disease patients is well established [1]. Prior to death, certain characteristic changes were observed in chronic HD patients, such as accelerated weight loss (interdialytic weight gain, post-HD weight), increased inflammation (CRP, albumin and NLR), worsened nutritional status (decline in SCr, phosphate, potassium and normalized protein catabolic rate) and cardiovascular status [44–47]. Albumin is a marker of visceral protein considered to be a late indicator of malnutrition, taking several weeks before being reduced [5]. Therefore we suggest that timely monitoring of surrogate markers of MM could facilitate early detection of sarcopenia trends, offering opportunities to implement interventions that might delay, halt or even reverse such deleterious dynamics. In this context, SCI change over time appears to be an interesting and easy access marker of active MM and a surrogate of nutritional status that deserves monitoring. Rapid and/or significant decline of SCI over a brief period may indicate MM wasting and thus trigger further diagnostic and therapeutic steps.
In our study, the comparison of LTI as computed by SCI versus LTI as measured by MF-BIS shows, despite a very strong relation, a general underestimation of the measured method (MF-BIS) by the estimated method (SCI), except at the upper ranges. Interestingly, this observation is in some disagreement with a recent study analysing D3-creatine dilution used as a more accurate measurement of skeletal MM [48]. In this study, instrumental measurement (e.g. DEXA) of MM overestimates the value obtained by the D3-creatine dilution method in all age and gender groups by almost 25%. These observations do not call into question the validity of one or the other of the methods of the muscular mass measurement but underline the fact that each of these methods does not measure the same component of the skeletal muscle. LTI derived from SCI measures skeletal MM and function at the molecular level, while LTI derived from MF-BIS tends to measure MM at the tissue organ level that includes water, i.e. lipid and fibrotic tissue, both of which increase with advancing age and reduce the accuracy of the measurement of anatomical MM [48]. This apparent discrepancy between MM measurements underlines the importance of selecting one tool with a validated method for monitoring dialysis patients and must not be attached to compare the methods to each other. In that case, time trends analysis of skeletal MM in dialysis patients is of crucial importance to detect unusual changes for working up and intervening earlier. Further studies on outcome predictive value of each one or in combination are warranted to support the validity of this concept.
One of the limitations of our study is observational, it draws on a convenience sample and is limited to measurements gathered as part of standard care. Prospective studies with specifically designed investigations are warranted to further explore the relationship between SCI and MM/MMet. Second, our patients were mostly European Caucasian, possibly limiting the generalizability of the SCI to other populations. Of note, the SCI has been used in European and Asian dialysis patients with a comparable clinical predictive value. Further studies should address its utility in other races and ethnicities. Third, residual renal function is not considered in the current SCI computation, limiting its use to anuric patients. However, we attempted to partly address this issue by performing sensitivity analyses for patients with dialysis vintages <12 months and >18 months, as in the latter group residual renal function is likely (nearly) absent.
Ideally, a marker should reflect MM status and its temporal trajectory with a clinically actionable sensitivity and specificity. In addition, it should be inexpensive and operationally simple to obtain. To that end, several technical, functional and biochemical methods are used, such as BIA to determine LBM, anthropometry and DEXA [49], quantitation of muscle strength [50], the creatinine index from creatinine kinetics [28] and the SCI by Canaud [27]. We consider the SCI advantageous since it uses routinely collected variables. The SCI can thus be used to monitor a patient’s MM in a routine clinical setting and as a tool for population-level investigations. However, whether SCI could potentially replace or complete technical and functional test warrants future studies. It is an important open question if SCI reflects the ‘active MM’ or the ‘static MM’ as currently measured by BIA and DEXA. Specifically designed studies comprising imaging (e.g. DEXA and MRI), functional and biochemical measurements are necessary to address that question.
CONCLUSION
Nutritional status is an important determinant of patient outcomes and reflected in MM/MMet. In this study, the SCI is a reliable and inexpensive marker of MMet. SCI is strongly associated with HD patient outcomes and declines 5–7 months prior to death. Its prognostic value compares with albumin, but with the advantage that its computation is typically collected monthly; in contrast, albumin is measured less frequently by some dialysis providers in Europe and abroad.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the Steering Committee of the MONDO Initiative for the opportunity to conduct this research.
AUTHORS’ CONTRIBUTIONS
B.C. proposed the research idea and took the lead in manuscript writing. L.U. and X.Y. developed the study plan and performed the statistical analysis.J.K. and P.K. provided critical inputs during the development of the manuscript. Y.W. provided valuable inputs regarding the statistical methods applied. All the other co-authors provided comments and inputs on the analysis plan and manuscript writing.
CONFLICT OF INTEREST STATEMENT
L.U. and B.C. are employees of Fresenius Medical Care (FMC) and may hold stock in the company. P.K., J.R. and X.Y. are employees of the Renal Research Institute, a wholly owned subsidiary of FMC. P.K. holds stock in FMC and receives author royalties from UpToDate. All other co-authors declare no conflict of interests.
(See related article by Delgado and Johansen. Revisiting serum creatinine as an indicator of muscle mass and a predictor of mortality among patients on hemodialysis. Nephrol Dial Transplant 2020; 35: 2033--2035)
REFERENCES
- 1. Ikizler TA, Wingard RL, Harvell J. et al. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int 1999; 55: 1945–1951 [DOI] [PubMed] [Google Scholar]
- 2. Cano NJ, Roth H, Aparicio M. et al. Malnutrition in hemodialysis diabetic patients: evaluation and prognostic influence. Kidney Int 2002; 62: 593–601 [DOI] [PubMed] [Google Scholar]
- 3. Tellado JM, Garcia-Sabrido JL, Hanley JA. et al. Predicting mortality based on body composition analysis. Ann Surg 1989; 209: 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beddhu S, Pappas LM, Ramkumar N. et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 2003; 14: 2366–2372 [DOI] [PubMed] [Google Scholar]
- 5. de Mutsert R, Grootendorst DC, Boeschoten EW. et al. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am J Clin Nutr 2009; 89: 787–793 [DOI] [PubMed] [Google Scholar]
- 6. Johansen KL, Lee C.. Body composition in chronic kidney disease. Curr Opin Nephrol Hypertens 2015; 24: 268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rymarz A, Szamotulska K, Niemczyk S.. Comparison of skinfold thicknesses and bioimpedance spectroscopy to dual-energy X-ray absorptiometry for the body fat measurement in patients with chronic kidney disease. Nutr Clin Pract 2017; 32: 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Combe C, Chauveau P, Laville M. et al. Influence of nutritional factors and hemodialysis adequacy on the survival of 1,610 French patients. Am J Kidney Dis 2001; 37(1 Suppl 2): S81–S88 [DOI] [PubMed] [Google Scholar]
- 9. Cano NJ. Metabolism and clinical interest of serum transthyretin (prealbumin) in dialysis patients. Clin Chem Lab Med 2002; 40: 1313–1319 [DOI] [PubMed] [Google Scholar]
- 10. Gama-Axelsson T, Heimburger O, Stenvinkel P. et al. Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol 2012; 7: 1446–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menon V, Greene T, Wang X. et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int 2005; 68: 766–772 [DOI] [PubMed] [Google Scholar]
- 12. Honda H, Qureshi AR, Heimburger O. et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 2006; 47: 139–148 [DOI] [PubMed] [Google Scholar]
- 13. de Mutsert R, Grootendorst DC, Indemans F. et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 2009; 19: 127–135 [DOI] [PubMed] [Google Scholar]
- 14. Marcelli D, Di Benedetto A, Ciotola A. et al. Subjective global assessment scores have poor correlation with serum albumin in obese hemodialysis patients by Eric D. Erb, Rosa K. Hand, and Alison L. Steiber. J Ren Nutr 2014; 24: 432–433 [DOI] [PubMed] [Google Scholar]
- 15. Furstenberg A, Davenport A.. Comparison of multifrequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry assessments in outpatient hemodialysis patients. Am J Kidney Dis 2011; 57: 123–129 [DOI] [PubMed] [Google Scholar]
- 16. Carrero JJ, Johansen KL, Lindholm B. et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 2016; 90: 53–66 [DOI] [PubMed] [Google Scholar]
- 17. Zhou Y, Hoglund P, Clyne N.. Comparison of DEXA and bioimpedance for body composition measurements in nondialysis patients with CKD. J Ren Nutr 2019; 29: 33–38 [DOI] [PubMed] [Google Scholar]
- 18. Marcelli D, Wabel P, Wieskotten S. et al. Physical methods for evaluating the nutrition status of hemodialysis patients. J Nephrol 2015; 28: 523–530 [DOI] [PubMed] [Google Scholar]
- 19. Buckinx F, Landi F, Cesari M. et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018; 9: 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel SS, Molnar MZ, Tayek JA. et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle 2013; 4: 19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vernaglione L, Marangi AL, Cristofano C. et al. Predictors of serum creatinine in haemodialysis patients: a cross-sectional analysis. Nephrol Dial Transplant 2003; 18: 1209–1213 [DOI] [PubMed] [Google Scholar]
- 22. Canaud B, Garred LJ, Argiles A. et al. Creatinine kinetic modelling: a simple and reliable tool for the assessment of protein nutritional status in haemodialysis patients. Nephrol Dial Transplant 1995; 10: 1405–1410 [PubMed] [Google Scholar]
- 23. Keshaviah PR, Nolph KD, Moore HL. et al. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol 1994; 4: 1475–1485 [DOI] [PubMed] [Google Scholar]
- 24. Lo WK, Prowant BF, Moore HL. et al. Comparison of different measurements of lean body mass in normal individuals and in chronic peritoneal dialysis patients. Am J Kidney Dis 1994; 23: 74–85 [DOI] [PubMed] [Google Scholar]
- 25. Bhatla B, Moore H, Emerson P. et al. Lean body mass estimation by creatinine kinetics, bioimpedance, and dual energy x-ray absorptiometry in patients on continuous ambulatory peritoneal dialysis. ASAIO J 1995; 41: M442–M446 [DOI] [PubMed] [Google Scholar]
- 26. Canaud B, Leblanc M, Garred LJ. et al. Protein catabolic rate over lean body mass ratio: a more rational approach to normalize the protein catabolic rate in dialysis patients. Am J Kidney Dis 1997; 30: 672–679 [DOI] [PubMed] [Google Scholar]
- 27. Canaud B, Granger Vallee A, Molinari N. et al. Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis patients. PLoS One 2014; 9: e93286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daugirdas JT, Depner TA.. Creatinine generation from kinetic modeling with or without postdialysis serum creatinine measurement: results from the HEMO study. Nephrol Dial Transplant 2017; 32: 1926–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desmeules S, Levesque R, Jaussent I. et al. Creatinine index and lean body mass are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant 2004; 19: 1182–1189 [DOI] [PubMed] [Google Scholar]
- 30. Terrier N, Jaussent I, Dupuy AM. et al. Creatinine index and transthyretin as additive predictors of mortality in haemodialysis patients. Nephrol Dial Transplant 2007; 23: 345–353 [DOI] [PubMed] [Google Scholar]
- 31. von Gersdorff GD, Usvyat L, Marcelli D. et al. Monitoring dialysis outcomes across the world – the MONDO Global Database Consortium. Blood Purif 2013; 36: 165–172 [DOI] [PubMed] [Google Scholar]
- 32. Wizemann V, Wabel P, Chamney P. et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 24: 1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giavarina D. Understanding Bland Altman analysis. Biochem Med 2015; 25: 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. . http//www.R-project.org/ (accessed January 2020). [Google Scholar]
- 35. Huang CY, Lee SY, Yang CW. et al. A simpler creatinine index can predict long-term survival in chinese hemodialysis patients. PLoS One 2016; 11: e0165164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamada S, Taniguchi M, Tokumoto M. et al. Modified creatinine index and the risk of bone fracture in patients undergoing hemodialysis: the Q-cohort study. Am J Kidney Dis 2017; 70: 270–280 [DOI] [PubMed] [Google Scholar]
- 37. Molina P, Vizcaino B, Molina MD. et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: the ProtEin Stores prEservaTion (PESET) study. Nephrol Dial Transplant 2018; 33: 1223–1235 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki Y, Matsuzawa R, Kamiya K. et al. Trajectory of lean body mass assessed using the modified creatinine index and mortality in hemodialysis patients. Am J Kidney Dis. 2020; 75: 195–203 [DOI] [PubMed] [Google Scholar]
- 39. Kaysen GA, Johansen KL, Cheng SC. et al. Trends and outcomes associated with serum albumin concentration among incident dialysis patients in the United States. J Ren Nutr 2008; 18: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dalrymple LS, Johansen KL, Chertow GM. et al. Longitudinal measures of serum albumin and prealbumin concentrations in incident dialysis patients: the comprehensive dialysis study. J Ren Nutr 2013; 23: 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alves FC, Sun J, Qureshi AR. et al. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One 2018; 13: e0190410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thongprayoon C, Cheungpasitporn W, Kashani K.. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis 2016; 8: E305–E311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Usvyat LA, Barth C, Bayh I. et al. Interdialytic weight gain, systolic blood pressure, serum albumin, and C-reactive protein levels change in chronic dialysis patients prior to death. Kidney Int 2013; 84: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ye X, Dekker MJE, Maddux FW. et al. Dynamics of nutritional competence in the last year before death in a large cohort of US hemodialysis patients. J Ren Nutr 2017; 27: 412–420 [DOI] [PubMed] [Google Scholar]
- 45. Kotanko P, Thijssen S, Usvyat L. et al. Temporal evolution of clinical parameters before death in dialysis patients: a new concept. Blood Purif 2009; 27: 38–47 [DOI] [PubMed] [Google Scholar]
- 46. Kotanko P, Kooman J, van der Sande F. et al. Accelerated or out of control: the final months on dialysis. J Ren Nutr 2014; 24: 357–363 [DOI] [PubMed] [Google Scholar]
- 47. Wong MMY, Thijssen S, Wang Y. et al. Prediction of mortality and hospitalization risk using nutritional indicators and their changes over time in a large prevalent hemodialysis cohort. J Ren Nutr 2020; 30: 69–78 [DOI] [PubMed] [Google Scholar]
- 48. Evans WJ, Hellerstein M, Orwoll E. et al. D3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle 2019; 10: 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marcelli D, Brand K, Ponce P. et al. Longitudinal changes in body composition in patients after initiation of hemodialysis therapy: results from an international cohort. J Ren Nutr 2016; 26: 72–80 [DOI] [PubMed] [Google Scholar]
- 50. Isoyama N, Qureshi AR, Avesani CM. et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9: 1720–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.