Abstract
Introduction
The Canadian Kidney Cancer information system (CKCis) has prospectively collected data on patients with renal tumors since January 1, 2011 from 16 sites within 14 academic centers in six provinces. Canadian kidney cancer experts have used CKCis data to address several research questions. The goal of this study was to determine if the CKCis cohort is representative of the entire Canadian kidney cancer population, specifically regarding demographic and geographic distributions.
Methods
The CKCis prospective cohort was analyzed up to December 31, 2018. Baseline demographics and tumor characteristics were analyzed, including location of patients’ residence at the time of CKCis entry. Geographic data is presented by province, rural vs. urban via postal code information (2nd digit=0) and by Canadian urban boundary files. To determine the proportion of renal cell carcinoma (RCC) patients that CKCis captures, CKCis accruals were compared to projected Canadian Cancer Society RCC incidence in 2016–2017 and the incidence from the 2016 Canadian Cancer Registry. To determine if the CKCis baseline data is representative, it was compared to registry data and other published data when registry data was not available.
Results
This CKCis cohort includes 10 298 eligible patients: 66.6% male, median age 62.6 years; 14.6% had metastatic disease at the time of diagnosis and 70.4% had clear-cell carcinomas. The CKCis cohort captures about 1250 patients per year, which represents approximately 20% of the total kidney cancer incidence. The proportion of patients captured per province did vary from 13–43%. Rural patients make up 17% of patients, with some baseline differences between rural and urban patients. There appears to be no major differences between CKCis patient demographics and disease characteristics compared to national data sources. Canadian heat maps detailing patient location are presented.
Conclusions
CKCis contains prospective data on >10 000 Canadian kidney cancer patients, making it a valuable resource for kidney cancer research. The baseline demographic and geographic data do appear to include a broad cross-section of patients and seem to be highly representative of the Canadian kidney cancer population. Moving forward, future projects will include determining if CKCis cancer outcomes are also representative of the entire Canadian kidney cancer population and studying variations across provinces and within rural vs. urban areas.
Introduction
The Canadian Kidney Cancer information system (CKCis) is a prospective, national kidney cancer database that was initiated by Canadian kidney cancer clinicians and researchers in 2009. It was rolled out nationally and the system continues to prospectively collect de-identifiable data on patients with findings consistent with kidney cancer from January 1, 2011 onwards. CKCis is a source of data supporting many kidney cancer research initiatives and is used by many Canadian kidney cancer experts. Currently, data from patients with kidney tumors are collected from 16 sites within 14 academic centers (Supplementary Table 1) from six provinces.
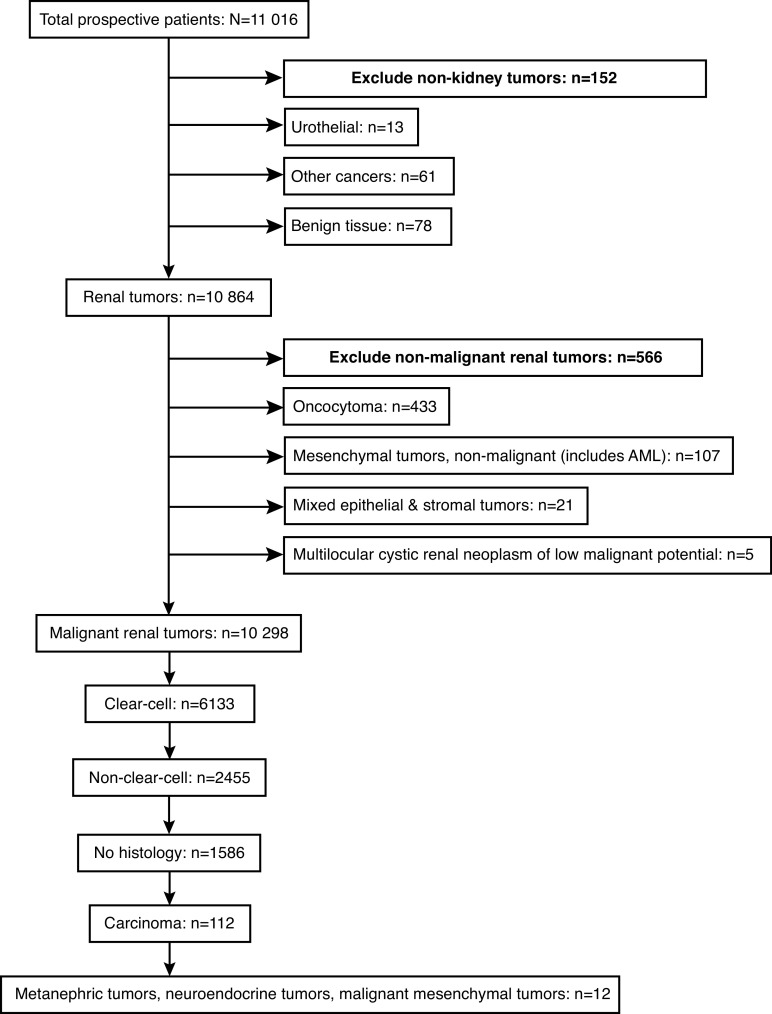
A potential concern with CKCis is whether the patients included, and data obtained from these sources, are generalizable to the entire Canadian population. Therefore, the objective of this study was to evaluate and analyze the CKCis kidney cancer patient population to determine if it is generalizable to the Canadian kidney cancer population. We report basic demographic data from CKCis, including age at diagnosis, sex, ethnicity, histology, and proportion who present with de novo metastatic disease. We compare those statistics to available Canadian data from the 2016 Canadian Cancer Registry (CCR) and the 2015–2017 Canadian Cancer Society (CCS), as well as non-Canadian sources where no Canadian data exists. Additionally, we present the geographic distribution of CKCis patients at the time of CKCis entry across provinces and by rural vs. urban location. This study includes CKCis patients with a malignant renal cancer or radiological/clinical features of malignancy but no pathological diagnosis, as shown in the consort diagram in Fig. 1.
Fig. 1.
Consort diagram: Canadian Kidney Cancer information system cohort January 1, 2011 to December 31, 2018. AML: angiomyolipoma.
Methods
All CKCis participating centers have obtained the appropriate local ethics board approval to include de-identified patient information. For this project, the cohort was restricted to all kidney cancer patients, both localized and metastatic, diagnosed from January 1, 2011 to December 31, 2018. Baseline demographics obtained include sex, age, ethnicity (as self-identified by patient), province at diagnosis, postal code at time of entry into CKCis, pathology, and stage (localized or metastatic). De novo metastatic disease was defined as meta-static disease found before or within three months of a kidney cancer diagnosis. Geographic data will be presented by province, rural vs. urban via postal code information (2nd digit=0 represents rural) and by the Forward Sortation Area boundary (FSA), which are based on Canadian postal codes. Geographic prevalence maps were created using ESRI ArcPro software.
To compare CKCis baseline demographic data with Canadian data, we used: 1) 2016 CCR data for sex, median age, rural residence (by postal code), and pathology; and 2) 2016 Canadian census data for race. In CKCis, patients self-identify as one race, which includes Caucasian. Caucasian is not an option in the Canadian census; however, we can discern whether a participant identifies as a visible minority through the census. Visible minority is defined by the Employment Equity Act as “persons, other than Aboriginal peoples, who are non-Caucasian in race or non-white in color.” Thus, for the purpose of this project, anyone who did not identify as a visible minority was considered Caucasian.1
To determine the number of kidney cancer patients entered into CKCis (the numerator) as a proportion of all patients diagnosed with kidney cancer in Canada (the denominator), Canadian data was obtained from :1) the CCR for the most recent year available (2016); and 2) the most recent CCS estimated yearly incidence data (2015–2017).2–4 These data will be presented for all of Canada and by province. It is recognized that these administrative databases have limitations, however, they are the only source of national data.
The CCR is a census of people diagnosed with cancer in Canada. It contains administrative information on cancer incidences, and individual and tumor characteristics from provincial and territorial cancer registries.5 The data were available from all provinces except for Quebec. Analyses using the CCR focused on individuals diagnosed with renal cell carcinoma (RCC) (tumor topography coded as C64 in the ICD-O-3). Clear-cell adenocarcinoma was determined by tumor histology (coded as 8310 in the ICD-O-3). To protect respondents’ confidentiality, statistics are only released if based on five or more individuals. Provinces were aggregated when a cell of the table did not meet this minimum.
All eligible patients in the CKCis cohort were analyzed. For the comparison with 2016 CCR data, we used the 2016 CKCis cohort. Some CKCis sites do manage and enter data for patients who do not reside in their province (e.g., patients from Saskatchewan managed in Alberta). Therefore, for province-specific information, only patients who resided in the six participating provinces at the time of their diagnosis were analyzed.
Statistical analysis
Patients who enrolled in CKCis with a primary or metastatic diagnosis between January 1, 2011 and December 31, 2018 were included. Percentages are used to summarize categorical variables. Age is summarized using the median and range. Chi-squared tests were used when comparing the percentage of males and rural residency from the CKCis data with the CCR data. All the statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, U.S.).
Results
Baseline patient characteristics
Fig. 1 depicts the prospective CKCis cohort. Patients with non-kidney tumors were excluded (n=152), as well as those with benign renal pathology, which include oncocytomas, benign mesenchymal tumors (like angiomyolipomas), mixed epithelial and stromal tumors, and multilocular cystic renal neoplasms of low malignant potential (n=566). The eligible CKCis cohort for this project includes 10 298 patients. Sixty-five (0.6%) patients are from outside of the participating provinces.
Baseline demographics for the entire cohort, as well as by province, are shown in Table 1. In the entire cohort, the median age was 62.6 years and the range 61.4 years (AB) to 64.4 years (NS). The range of ages was 19 –96 years. Male patients made up 66.6% of the cohort and Caucasians made up 85.6%.
Table 1.
Baseline demographics of patients, 2011–2018
| Canada* | BC | AB | MB | ON | QC | NS | |
|---|---|---|---|---|---|---|---|
| n | 10 298 | 698 | 1392 | 444 | 4121 | 2600 | 978 |
| Age, median (yrs) | 62.6 | 62.4 | 61.4 | 61.6 | 62.6 | 62.8 | 64.4 |
| Male (%) | 66.6 | 70.2 | 70.4 | 66.2 | 65.3 | 66.0 | 66.6 |
| Caucasian (%) | 85.6 | 64.5 | 80.7 | 82.6 | 83.7 | 91.0 | 98.7 |
| Rural Residence (%) | 17.0 | 9.7 | 15.0 | 23.9 | 15.1 | 15.7 | 32.0 |
| De novo metastatic (%) | 14.6 | 19.9 | 17.2 | 16.7 | 14.7 | 9.4 | 19.1 |
| Metastatic any time (%) | 31.1 | 41.1 | 35.6 | 42.1 | 31.2 | 22.8 | 34.4 |
Includes 65 patients from Saskatchewan, Newfoundland, PEI, Yukon, Nunavut, and Northwest Territories.
Baseline disease characteristics
A wide variety of malignant histologies are captured in CKCis, as well as 15.4% of patients with no pathological diagnosis. The reasons for no pathology varies but include small renal masses on active surveillance or tumors in patients too ill for a biopsy or for whom a biopsy would not appear to change management. Among all patients, 59.6% have clear-cell carcinoma. Among those with pathology, clear-cell histology was seen in 70.4% of patients. Of the non-clear-cell cancers, papillary and chromophobe made up the majority at 13.5% and 6.6%, respectively. Nephrectomy or partial nephrectomy specimens were used to make the diagnosis in 86.0% of patients, biopsy or fine needle aspirate in 13.6%, and metastectomy in 0.5%.
De novo metastatic disease was seen in 14.6% of patients and metastatic disease at any time was seen in 31.1%.
Geographic distribution of patients
The number of CKCis patients per province is shown in Table 1. Geographic distribution of accrued prevalence rates for Canada by FSA are shown in Fig. 2 for the entire country and by province/region. The areas with the highest concentration correspond to CKCis sites, especially in Southern Ontario, the Ottawa region, Montreal, and mainland Nova Scotia. However, the figures also show that patients are accrued from areas throughout the entire participating provinces.
Fig. 2.
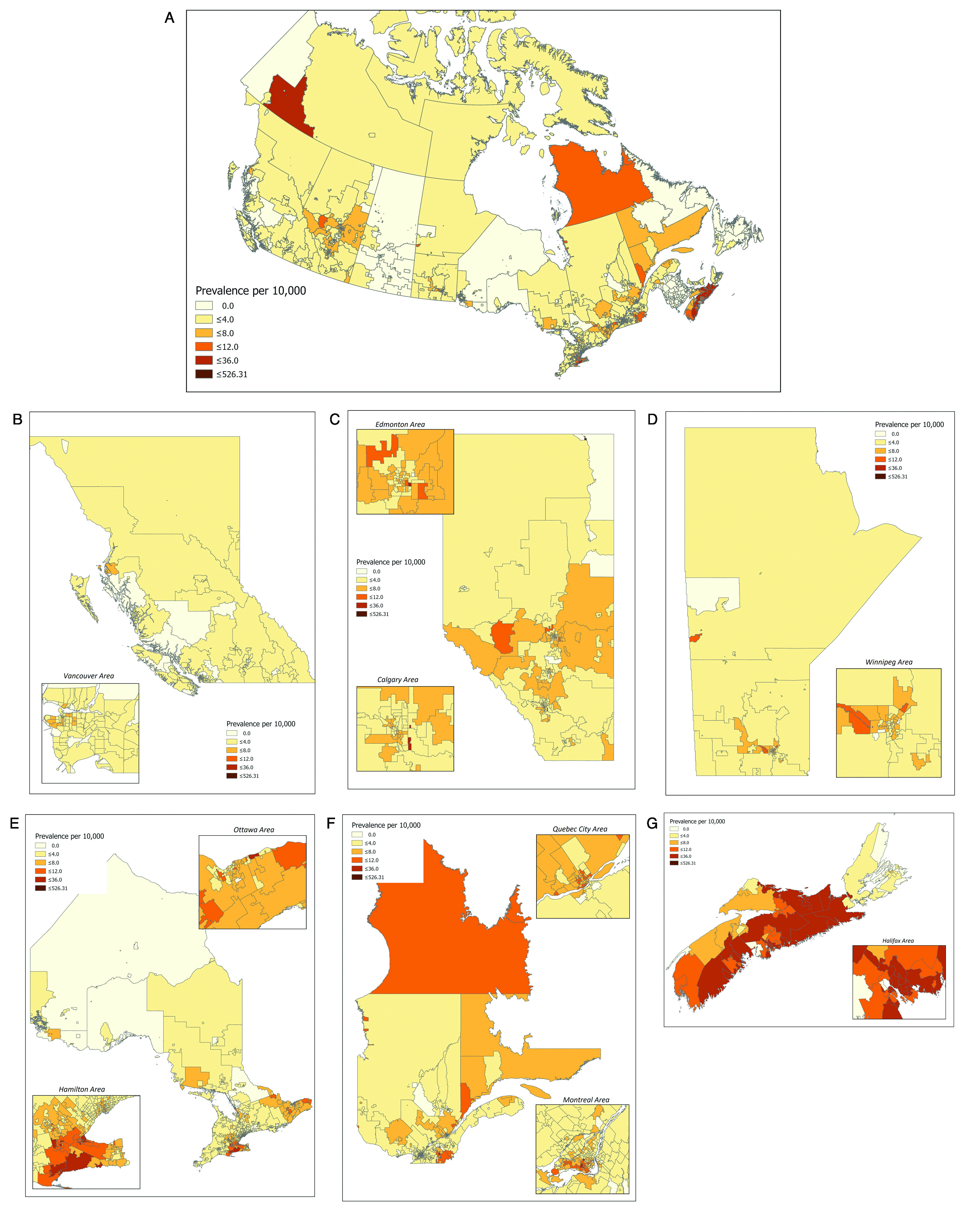
Canadian Kidney Cancer information system national and provincial accrual via forward sortation area geography (FSA) 2011–2018. (A) Canada; (B) British Columbia; (C) Alberta; (D) Manitoba; (E) Ontario; (F) Quebec; (G) Nova Scotia.
The proportion of patients who live in a rural location is 17.0%, with variation across the provinces ranging from 9.7% (BC) to 32.0% (NS). To note, rural and urban populations do show some differences in terms of baseline demographics. The mean age at presentation, percentage of males, and proportion of patients presenting with de novo metastatic disease are not statistically different. However, more patients with rural postal codes are Caucasian (90.9% vs. 84.6%, p<0.0001), have metastatic disease at any time (33.1% vs. 30.7%, p=0.04), and have clear-cell pathology (63.6% vs. 58.7%, p<0.0001).
CKCis baseline data compared to Canadian kidney cancer population
Table 2 compares the baseline 2016 CKCis data with that of the entire Canadian kidney cancer population from the 2016 CCR for age, sex, and rural residence, along with the proportion who present with de novo metastatic disease and clear-cell pathology. There was no statistically significant difference between the CKCis data and the CCR concerning male population, rural residence, and clear-cell pathology.
Table 2.
2016 CKCis demographics compared to national RCC data
| CKCis cohort | National RCC data | p | Data source | |
|---|---|---|---|---|
| Age, median (years) | 63 | 64 | NA^ | CCR, 2016 |
| Male | 65.5% | 64.8% | 0.60 | CCR, 2016 |
| Rural residence | 17.0% | 18.7% | 0.12 | CCR, 2016 |
| Race, Caucasian | 86.4% | 77.7% | NA^ | Canadian census, 2016 |
| De novo metastatic | 14.7% | 16% | NA^ | SEER data, 2009–2015 |
| Clear-cell histology (in those with a cancer diagnosis) | 58.6% (all patients) 71.4% (in only those with pathology) |
59.6% | 0.46 | CCR, 2016 |
No formal statistical comparison was performed due to lack of data.
CCR: Canadian Cancer Registry; CKCis: Canadian Kidney Cancer information system; RCC: renal cell carcinoma; SEER: Surveillance, Epidemiology, and End Results.
Proportion of patients entered into CKCis compared to entire Canadian RCC population
Table 3 reports CKCis Canadian and provincial accrual data, along with CCS estimated incidence data and CCR incidence data from 2016. In 2016, CKCis captured 20.7% of the Canadian kidney cancer population (excluding data from Quebec) when compared to the CCR, and 19.6% of the population (including Quebec) when compared to the CCS. The provinces varied from a low of 12.7% accrual (BC) to a high of 51.4% (NS). Table 3 also shows the differences between the CCS estimated incidence and the actual incidence data obtained from the CCR, with notable differences in some provinces. Overall, the CCS estimated incidences are higher than the incidence data obtained from CCR, with the exception of BC.
Table 3.
CKCis incidence compared to Canadian Cancer Registry and Canadian Cancer Society (2016) data
| CCR 2016 | CCS 2016 | Absolute difference between CCR and CCS | CKCis 2016 | % patients captured vs. CCR | % patient captured vs. CCS | |
|---|---|---|---|---|---|---|
| Canada | 4395 (excl QC) | 6400 (total) 4750 (excl QC) |
−355 | 1255 (total) 908 (excl QC) |
20.7 (excl QC) | 19.6 (total) 19.1 (excl QC) |
| BC | 690 | 630 | +60 | 80 | 11.6 | 12.7 |
| AB | 515 | 600 | −85 | 186 | 36.1 | 31.0 |
| MB | 205 | 275 | −70 | 59 | 28.8 | 21.5 |
| ON | 2265 | 2320 | −55 | 467 | 20.6 | 20.1 |
| QC | 1650 | 347 | 21.0 | |||
| NS | 210 | 250 | −40 | 108 | 51.4 | 43.2 |
CCR: Canadian Cancer Registry; CCS: Canadian Cancer Society; CKCis: Canadian Kidney Cancer information system; excl QC: excluding Quebec.
The CKCis accrual rate compared to CCS data in 2015 was 21.2%, and in 2017 was 18.7%. Provincial accrual rates again varied from 15.3–40.4%. This data is shown in Supplementary Table 2.
Discussion
The publication and use of real-world data and evidence has become an integral part of research in oncology. Realworld data may come from many different sources, including individual institutions, specific regions, collaborations between investigators/clinicians, or at national and international levels.
CKCis, as a multiprovincial source of real-world information, is an invaluable resource for clinicians, researchers, patients, and administrators to discover new information about the epidemiology, management, outcomes, and trends over time and across the country for the Canadian kidney cancer population. The data, however, are gathered from select Canadian academic centers, thus, it is important to understand if patients are representative of the entire Canadian kidney cancer population. Our hypothesis was that patients in CKCis represent the RCC population from many parts of the country, with baseline patient and tumor characteristics similar to the entire kidney cancer population. CKCis results were compared to estimates published by the CCS and actual data obtained from the CCR.
This study supports our hypothesis that the CKCis population is similar to the entire kidney cancer population of Canada in terms of baseline demographic and tumor characteristics. The median age at the time of diagnosis in CKCis (63 years) appears similar to the CCR (64 years). This is also in line with the U.S. Surveillance, Epidemiology, and End Results (SEER) database from 2012–2016, which reports a median age of 64 years at the time of diagnosis.6 Males make up 66.6% of the CKCis population (65.5% in 2016), which is similar to the CCR at 64.9%, and the CCS, which ranged between 62.9% and 65.3% in 2015 and 2019, respectively.7
In terms of race, CKCis contains 86% of patients who self-identify as Caucasian. In the 2016 Canadian census, 22% of the population identified as a visible minority and, thus, the assumption for this study is that 78% of the Canadian population was Caucasian in 2016.8 Thus, CKCis may have an over-representation of Caucasians. To note, the “visible minority” definition does not include Aboriginal peoples and depending on how these patients self-identify, they would be included in the non-Caucasian category of CKCis but not the Canadian census. In a previous publication from CKCis, 2.3% of patients self-identified as Indigenous.9
In CKCis, 70% of patients had clear-cell RCC when analyzing only patients who had a histological diagnosis. This is similar to the often-reported 75–85% in the international literature.10 In CKCis, 59% of patients had clear-cell RCC when including patients with and without pathology, which is almost identical to that seen in the CCR data. The reason for the lower proportion of clear-cell RCC in the CCR and CKCis data is likely due to the inclusion of kidney cancers that are included based only on radiological imaging. Patients can be entered into provincial and territorial cancer registries and coded as kidney cancer with no pathology if the radiological findings are in keeping with kidney cancer. If the diagnosis is made radiologically, the pathology is usually entered as “malignant neoplasm” or “renal cell carcinoma, NOS,” which would be considered non-clear-cell and, thus, give a higher proportion of non-clear-cell RCCs. Research conducted with Nova Scotia provincial registry data found 11% of patients were diagnosed radiologically (and therefore would all be considered non-clear-cell). In that study, there was also up to 15.4% discordance between the NS Registry data and the diagnosis from actual pathology reports.11 De novo metastatic disease was diagnosed in 15% of patients in CKCis. Comparable data is not available from the CCR or any other Canadian source. The U.S. SEER data from 2009–2015 reports 16% of patients are diagnosed with de novo metastatic disease.6 There was considerable variation in this variable across the country, from 9.4% (QC) to 19.9% (BC), which will need further exploration.
The geographic inclusion of the CKCis population is vast and from all parts of the participating six provinces, with a small number from surrounding provinces and territories. Given CKCis centers are in larger cities, we were concerned that rural patients may be under-represented; however, rural patients make up 17.0% of the CKCis population, which is not significantly different than the CCR at 18.7%. Statistics Canada indicates 18.9% of Canadians in 2011 lived in rural areas.12 A limitation to the geography data is the recording of the patients’ postal code at the time of their first contact with a CKCis site, which may not consider moves pre and post.
In terms of the proportion of kidney cancer patients that CKCis represents, CKCis captured about 20% of the Canadian kidney cancer population. It is difficult to know what proportion of patients are treated within the participating centers and simply not accrued into CKCis vs. the proportion of patients managed outside of CKCis participating centers. The accrual of patients within CKCis does vary by site and province for many reasons but a major reason is the variation in consent processes across the country and even within provinces. For example, some centers have a waiver of consent and it is likely most patients seen at that center are captured. Other centers have the option of telephone consents or waiver of consent for deceased patients. Some centers require all patients to sign an in-person consent form, which would result in a lower accrual rate. Now that we have seen the regional and provincial variations, it will allow CKCis administrators and individual sites to strategize on how to maximize accrual at their site. These data also allow CKCis to monitor their success so that with each additional year, the capture rate should improve. A very realistic goal is for CKCis to capture data on one in four, or 25% of kidney cancer patients.
Conclusions
Kidney cancer care is delivered in all regions and in multiple centers across Canada. Due to logistics and resource constraints, not every patient with kidney cancer can be entered in CKCis. However, the key with a national database that cannot collect data on every individual kidney cancer patient is that it should still represent the entire population under study. The conclusion from this study is that the CKCis population has similar baseline patient and tumor characteristics to the general Canadian kidney cancer population and does not appear to be biased despite coming from academic centers only. These data suggest that research published using CKCis data are representative and generalizable to the Canadian population. This study has provided a benchmark for patient accrual that we can use to monitor new strategies and improvements in CKCis infrastructure. It has also raised a number of future research questions, such as determining if CKCis cancer outcomes are also representative of the entire Canadian kidney cancer population and studying variations across provinces and within rural vs. urban areas.
Supplementary Information
Supplementary Table 1.
CKCis sites
| Centre(s) | Location | Uro-oncologist | Medical oncologist |
|---|---|---|---|
| University Health Network | Toronto | Antonio Finelli* | Aaron Hansen |
| Capital Health QEII Hospital | Halifax | Ricardo Rendon | Lori Wood* |
| McGill University Health Centre, Jewish General Hospital | Montréal | Simon Tanguay | François Patenaude |
| St. Joseph’s Healthcare Hamilton, Juravinski Cancer Centre | Hamilton | Anil Kapoor* | Aly-Khan Lalani |
| Tom Baker Cancer Centre | Calgary | Bimal Bhindi | Daniel Heng* |
| Cross Cancer Institute | Edmonton | Adrian Fairey | Naveen Basappa* |
| Vancouver General Hospital | Vancouver | Alan So* | Christian Kollmannsberger |
| St. Boniface Hospital | Winnipeg | Darrel Drachenberg* | Jeffrey Graham |
| Sunnybrook Hospital | Toronto | Laurence Klotz | Georg Bjarnason* |
| The Ottawa Hospital | Ottawa | Luke Lavallée*/ Rodney Breau | Neal Reaume/ Christina Canil |
| Centre hospitalier de l'Université de Montréal | Montréal | Denis Soulières* | Jean-Baptiste Lattouf |
| Centre hospitalier universitaire de Québec | Québec | Frédéric Pouliot* | Vincent Castonguay |
| London Health Sciences Centre | London | Nicholas Power* | Eric Winquist |
| Centre hospitalier universitaire de Sherbrooke | Sherbrooke | Patrick Richard* | Michel Pavic |
Principal investigator.
CKCis: Canadian Kidney Cancer information system.
Supplementary Table 2.
CKCis incidence compared to CCS (2015 and 2017)
| CCS 2015 | CKCis 2015 | % patients captured | CCS 2017 | CKCis 2017 | % patients captured | |
|---|---|---|---|---|---|---|
| Canada | 6200 | 1314 | 21.2 | 6600 | 1234 | 18.7 |
| BC | 550 | 84 | 15.3 | 700 | 107 | 15.3 |
| AB | 580 | 202 | 34.8 | 610 | 113 | 18.5 |
| MB | 240 | 53 | 22.1 | 235 | 38 | 16.2 |
| ON | 2450 | 520 | 21.2 | 2450 | 534 | 21.8 |
| QC | 1580 | 349 | 22.1 | 1710 | 329 | 19.2 |
| NS | 250 | 101 | 40.4 | 260 | 104 | 40.0 |
CCS: Canadian Cancer Society; CKCis: Canadian Kidney Cancer information system.
Acknowledgements
The CCR was accessed through a Statistics Canada Research Data Centre, thus we would like to acknowledge that “this research was supported by funds to the Canadian Research Data Centre Network (CRDCN) from the Social Sciences and Humanities Research Council (SSHRC), the Canadian Institute for Health Research (CIHR), the Canadian Foundation for Innovation (CFI), and Statistics Canada. Although the research and analysis are based on data from Statistics Canada, the opinions expressed do not represent the views of Statistics Canada.”
Footnotes
Competing interests: Dr. Tanguay has been an advisory board member for Pfizer; and has received travel grants from Sanofi. Dr. Basappa has been an advisory board member for Astellas, AstraZeneca, BI, BMS, Janssen, Novartis, and Pfizer; and has received honoraria from Astellas, BMS, Janssen, Novartis, and Pfizer. Dr. Kapoor has been an advisory board member for and participated in clinical trials supported by Amgen, Astellas, GSK, Janssen, Novartis, Pfizer, and Sanofi. Dr. Heng has been a consultant for BMS, Ipsen, Merck, Novartis, and Pfizer. Dr. Pouliot has been an advisory board member for Amgen, Astellas, Astra Zeneca, Bayer, Janssen, Sanofi, and Tersera; a speakers’ bureau member for Astellas and Janssen; and has received payment, honoraria and/or grants from Amgen, Astellas, Astra Zeneca, Bayer, Janssen, Sanofi, and Tersera. Dr. Finelli has been an advisory board member for AbbVie, Amgen, Astellas, Bayer, Janssen, Roche, and Sanofi. Dr. Lavallée has been an advisory board member for AbbVie, Bayer, Ferring, Sanofi and Tersera; and has received an unrestricted research grant from Sanofi. Dr. So has been an advisory board member for AbbVie, Amgen, Astellas, Bayer, Janssen, Ferring, and Tersera; and has participated in clinical trials supported by Astellas, Ferring, and Janssen. Dr. Soulières has been an advisory board member for Bayer, BMS, Merck, and Novartis; has received honoraria from BMS, Merck, and Pfizer; and has participated in clinical trials supported by BMS, Merck, Novartis, and Pfizer. Dr. Bjarnason has been an advisory board member for and received honoraria for CME talks from Ipsen, MBS, Merck, and Pfizer; hold stocks in Abbott and Pfizer; and has participated in clinical trials supported by Ipsen, MBS, Merck, and Pfizer. Dr. Richard has been an advisory board member for BMS and Sanofi; has been a speakers’ bureau member for AbbVie, Amgen, Astellas, Ferring, and Janssen; and has participated in clinical trials supported by Calithera and Lidds Pharma. Dr. Wood has received research funding from Aragon Pharmaceuticals, AstraZeneca, BMS, Exelixis, Merck, Novartis, Pfizer, and Roche. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed
References
- 1.Statistics Canada. Dictionary, Census of Population. 2016. [Accessed March 6, 2020]. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011101.
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics. 2015. [Accessed Jan 2, 2020]. Available at: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2015-EN.pdf.
- 3.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics. 2016. [Accessed Jan 2, 2020]. Available at: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2016-EN.pdf.
- 4.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics. 2017. [Accessed Jan 9, 2020]. Available at: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2017-EN.pdf?la=en.
- 5.Statistics Canada. Canadian Cancer Registry (CCR) [Accessed March 9, 2020]. [updated: 2020 Jan 28]. Available at: https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207.
- 6.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Bethesda, MD: SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer; [Accessed Jan 9, 2020]. Available at: https://seer.cancer.gov/statfacts/html/kidrp.html. [Google Scholar]
- 7.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics. 2019. [Accessed Jan 2, 2020]. Available at: https://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2019-EN.pdf.
- 8.Statistics Canada. Number and proportion of visible minority population in Canada 1981–2036. [Accessed March 6, 2020]. Available at: https://www.statcan.gc.ca/eng/dai/btd/othervisuals/other010.
- 9.Wong ECL, Breau RH, Mallick R, et al. Renal cell carcinoma in the Canadian Indigenous population. Curr Oncol. 2019;26:e367–71. doi: 10.3747/co.26.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins MB, Choueiri TK. Epidemiology, pathology, and pathogenesis of renal cell carcinoma. UpToDate [serial on the Internet] Jan, 2020. [Accessed Jan 9, 2020]. [updated Jan 21, 2020]. Available at: https://www.uptodate.com/contents/epidemiology-pathology-and-pathogenesis-of-renal-cell-carcinoma?search=kidney%20cancer%20pathology&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1%20-%20H17.
- 11.Himmelman JG, Merrimen J, Matheson K, et al. Accuracy of kidney cancer diagnosis and histological subtype within Canadian cancer registry data. Can Urol Assoc J. 2017;11:E326–9. doi: 10.5489/cuaj.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Canada. Canada’s rural population since 1851[February 2012] [Accessed Jan 9, 2020]. Available at: https://www12.statcan.gc.ca/census-recensement/2011/as-sa/98-310-x/98-310-x2011003_2-eng.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
CKCis sites
| Centre(s) | Location | Uro-oncologist | Medical oncologist |
|---|---|---|---|
| University Health Network | Toronto | Antonio Finelli* | Aaron Hansen |
| Capital Health QEII Hospital | Halifax | Ricardo Rendon | Lori Wood* |
| McGill University Health Centre, Jewish General Hospital | Montréal | Simon Tanguay | François Patenaude |
| St. Joseph’s Healthcare Hamilton, Juravinski Cancer Centre | Hamilton | Anil Kapoor* | Aly-Khan Lalani |
| Tom Baker Cancer Centre | Calgary | Bimal Bhindi | Daniel Heng* |
| Cross Cancer Institute | Edmonton | Adrian Fairey | Naveen Basappa* |
| Vancouver General Hospital | Vancouver | Alan So* | Christian Kollmannsberger |
| St. Boniface Hospital | Winnipeg | Darrel Drachenberg* | Jeffrey Graham |
| Sunnybrook Hospital | Toronto | Laurence Klotz | Georg Bjarnason* |
| The Ottawa Hospital | Ottawa | Luke Lavallée*/ Rodney Breau | Neal Reaume/ Christina Canil |
| Centre hospitalier de l'Université de Montréal | Montréal | Denis Soulières* | Jean-Baptiste Lattouf |
| Centre hospitalier universitaire de Québec | Québec | Frédéric Pouliot* | Vincent Castonguay |
| London Health Sciences Centre | London | Nicholas Power* | Eric Winquist |
| Centre hospitalier universitaire de Sherbrooke | Sherbrooke | Patrick Richard* | Michel Pavic |
Principal investigator.
CKCis: Canadian Kidney Cancer information system.
Supplementary Table 2.
CKCis incidence compared to CCS (2015 and 2017)
| CCS 2015 | CKCis 2015 | % patients captured | CCS 2017 | CKCis 2017 | % patients captured | |
|---|---|---|---|---|---|---|
| Canada | 6200 | 1314 | 21.2 | 6600 | 1234 | 18.7 |
| BC | 550 | 84 | 15.3 | 700 | 107 | 15.3 |
| AB | 580 | 202 | 34.8 | 610 | 113 | 18.5 |
| MB | 240 | 53 | 22.1 | 235 | 38 | 16.2 |
| ON | 2450 | 520 | 21.2 | 2450 | 534 | 21.8 |
| QC | 1580 | 349 | 22.1 | 1710 | 329 | 19.2 |
| NS | 250 | 101 | 40.4 | 260 | 104 | 40.0 |
CCS: Canadian Cancer Society; CKCis: Canadian Kidney Cancer information system.