Abstract
Introduction
Recent reports suggest that early salvage radiation (esRT) is non-inferior to adjuvant radiation (aRT) for adverse pathological features at radical prostatectomy. However, aRT was accepted as a standard treatment primarily based on effects on biochemical progression-free survival (bPFS). In order to understand the merits of esRT, the objective was to reassess if aRT vs. observation is associated with improved overall survival (OS).
Methods
A systematic review and meta-analysis of published randomized trials evaluating aRT was performed. The primary outcome was OS. Secondary outcomes were metastasis-free survival (MFS), loco-regional recurrence-free survival (RFS), bPFS, and adverse events. We performed a random-effects meta-analysis.
Results
Four randomized trials including 2068 patients with a median followup of 8.7–12.6 years were identified. While all trials reported a bPFS benefit, only one reported an OS benefit. Upon meta-analysis, no significant OS benefit was detected with aRT vs. observation (hazard ratio [HR] 0.90, 95% confidence interval [CI] 0.61–1.33), although consistent bPFS (HR 0.47, 95% CI 0.41–0.54) and local-RFS (HR 0.54, 95% CI 0.39–0.73) benefits were noted. There is an uncertain MFS benefit with aRT (HR 0.79, 95% CI 0.62–1.01), and the effect is largely driven by one trial with a notable risk of bias. There was also a risk of overtreatment, with 35–60% of patients being biochemical recurrence-free with observation alone. Adverse events risk was greater with aRT vs. observation.
Conclusions
Although aRT vs. observation provides a bPFS benefit related to local control, there is no clear OS or MFS benefit, a greater risk of adverse events, and a risk of overtreatment. By extension, these data have implications for patient selection and counselling for esRT.
Introduction
Approximately a third of patients undergoing radical prostatectomy (RP) for clinically localized prostate cancer (PCa) will have either a positive surgical margin (PSM), extraprostatic extension (EPE), or seminal vesicle invasion (SVI).1
Guidelines2,3 recommend offering adjuvant radiotherapy (aRT) to patients with one or more of these risk factors based on randomized trial data,4–9 with another trial recently published.10 However, aRT remains underused,11,12 and there has been interest in considering early salvage radiation (esRT) instead in the subset of men who experience biochemical progression. Recent conference presentations with early followup from the RAVES and RADICALS trials, which compared aRT vs. observation with esRT, found no difference in freedom from biochemical failure or local/distant failure, but did find greater odds of grade 2+ genitourinary toxicity with aRT.13,14
While there has since been enthusiasm to adopt esRT as standard of care for all, in order to understand the merits of esRT, it is important to also understand the merits of aRT. As such, we sought to resynthesize trials evaluating the oncological benefits and harms of aRT among patients who have adverse pathological features at RP.
Methods
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,15 we searched PubMed/Medline, clinicaltrials.gov, and the BioMed Central International Standard Randomized Controlled Trials Number (ISRCTN) Registry for published randomized clinical trials in humans from database inception to December 31, 2019 comparing participants who underwent either aRT or observation, without or without subsequent sRT, after RP demonstrating a PSM, EPE (pT3a), and/or SVI (pT3b). The search strategy was as follows: “(((adjuvant or postoperative) AND (radiotherapy OR radiation)) AND prostate cancer) AND (“randomized controlled trial” or “RCT” or “randomized clinical trial” or “randomised controlled trial” or “randomised clinical trial”)”. Multiple reports from the same clinical trial were analyzed as a single study, with priority given to more up-to-date results.
The primary outcome was overall survival (OS) and secondary outcomes were metastasis-free survival (MFS), biochemical progression-free survival (bPFS), loco-regional recurrence-free survival (RFS), and adverse events. The probability of bPFS was examined in the observation arms to assess risk of overtreatment of patients cured by RP alone.
Title and abstract screening, full-text review of selected papers, final study selection, and data abstraction was performed independently by two authors (BB and SL), with independent verification by coauthors. Risk of bias assessment was performed using the Cochrane Collaborative Risk-of-Bias tool for randomized trials.16
Heterogeneity was assessed using the Q-test and was quantified using I2 values.17 Publication bias could not be assessed using funnel plots due to the limited number of studies.
Study characteristics and outcomes were tabulated. Random-effects meta-analysis was performed using the inverse variance technique for pooling of hazard ratios. Forest plots were created using Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, Cochrane Collaboration, 2014).
Results
Literature search results
A total of 225 unique records were identified through our literature search. Seven reports on four multicenter, randomized trials were retained for the final analysis (Fig. 1).4–10
Fig. 1.
PRISMA diagram.
Study characteristics and limitations (Table 1)
Table 1.
Study characteristics
| Characteristic | ARO 96-02 | EORTC 22911 | SWOG 8794 | FP-FINROG-0301 |
|---|---|---|---|---|
| Sample size (n) | 307 | 1005 | 425 | 250 |
| Median followup (years) | aRT: 9.3 Obs: 9.4 |
10.6 | 12.6 | aRT: 9.3 Obs: 8.6 |
| Key inclusion and exclusion criteria | Inclusion:
|
Inclusion:
|
Inclusion:
|
Inclusion:
|
| Detectable postoperative PSA | 0% | 29.9% | 29.9% | aRT: 70% PSA <0.2–0.5 Obs: 65% PSA <0.2–0.5 |
| Radiation type and dose | 3- or 4-field technique; 60 Gy in 30 fractions | 2D radiation; 60 Gy in 30 fractions | 2D radiation; 60–64 Gy in 30–32 fractions | 3D-conformal RT; no nodal radiation; 66.6 Gy in 37 fractions |
| Primary outcome | bPFS | bPFS | MFS | bPFS |
| Secondary outcomes | MFS, OS | Local control, salvage XRT, MFS | PFS, PSA relapse-free interval | OS, CSS, adverse events |
| Rate of salvage XRT among patients with BCR in the control arm | NR | 43.4% | 33.2% | 86% |
| Median PSA at time of salvage XRT (μg/l) | NR | 1.7 | 0.75–1.0 | 0.7 |
| Biochemical PFS | 10-year estimate: aRT: 56.0% Obs: 35.0% |
10-year: aRT: 61.6% Obs: 41.1% |
Median followup: aRT: 60.7% Obs: 47.4% |
10-year estimate: aRT: 82% Obs: 61% |
| Local RFS | NR | 10-year: aRT: 83.4% Obs: 92.7% |
NR | NR |
| MFS | Median followup: aRT: 84.3% Obs: 85.1% |
10-year: aRT: 89.9% Obs: 89.0% |
Median followup: aRT: 56.5% Obs: 46% |
10-year estimate: aRT: 98% Obs: 96% |
| CSS | NR | 10-year: aRT: 96.1% Obs: 94.6% |
NR | 10-year estimate: aRT: 99% Obs: 99% |
| OS | Median followup: aRT: 86.5% Obs: 85.5% |
10-year:<:br>aRT: 76.9% Obs: 80.7% |
Median followup: aRT: 74.0% Obs: 66.0% |
10-year estimate:<:br>aRT: 92% Obs: 87% |
| Overall adverse event rates | Median followup: aRT: 21.9% Obs: 3.7% |
10-year: aRT: 70.8% Obs: 59.7% |
Median followup*: aRT: 23.8% Obs: 11.9% |
NR** |
| Grade 3+ adverse event rates | Median followup: aRT: 1% Obs: 0% |
10-year: aRT: 5.3% Obs: 2.5% |
NR | Median followup: aRT: 57% Obs: 40% |
10.6 years;
not reported in a cumulative fashion.
aRT: adjuvant radiation therapy; BCR: biochemical recurrence; bPFS: biochemical progression-free survival; CSS: cancer-specific survival; LRFS: local recurrence-free survival; MFS: metastasis-free survival; NR: not reported; Obs: observation; OS: overall survival; PCa: prostate cancer; PSM: positive surgical margins; RP: radical prostatectomy; RT: radiation therapy; SVI: seminal vesicle invasion; WHO: World Health Organization; XRT: radiation therapy.
The SWOG-87945,6 and ARO-96-028,9 trials studied patients with pT3 PCa with or without PSMs. EORTC-229114,7 and FinnProstate10 also included patients with pT2 disease and a PSM. Median followup ranged from 8.7–12.6 years.
Only the ARO-96-02 trial8,9 required patients to have a postoperative prostate-specific antigen (PSA) <0.1 μg/L. Contemporary definitions of aRT require this undetectable PSA level. The other trials included patients with a postoperative detectable PSA (29.9% of patients in EORTC-22911 had a PSA ≥0.2 μg/L; at least 29.9% of patients in SWOG-8794 had a postoperative PSA ≥0.2 μg/L; the FinnProstate trial required a PSA<0.5 μg/L and only 49.6% of patients had a confirmed PSA <0.2 μg/L). Thus, a considerable proportion of patients in the aRT arm of these three trials received sRT while some patients in the observation arm with detectable postoperative PSA did not receive appropriate sRT according to contemporary standards.
In patients randomized to observation or “watch-and-wait,” sRT was not uniformly administered upon biochemical recurrence (BCR) and, for those who did receive sRT, it was often administered late. In EORTC-22911,4,7 218 (82.3%) of 265 patients with BCR in the observation arm received active treatment, of whom 115 (43.4%) received sRT. Salvage treatment was initiated at a median PSA of 1.7 μg/L. In SWOG-8794,5,6 an estimated 64.0% experienced BCR after initially attaining an undetectable postoperative PSA (<0.2 μg/L), while 70 of 211 (33.2%) patients in the observation arm received sRT at a median PSA of 1.0 μg/L (interquartile range [IQR] 0.3, 1.5). In the FinnProstate trial,10 37 of 43 (86%) patients with BCR in the observation arm received sRT at a median PSA of 0.7 μg/L. The ARO-96-028,9 trial did not comment on use of sRT, although 49 of the 100 (49%) patients with BCR received salvage hormone therapy.
Risk of bias assessment
Two studies were assessed as having a low risk of bias,8–10 one was assessed as having a moderate risk of bias,4,7 and one was assessed as having a high risk of bias5,6 (Supplementary Tables 1, 2). Consideration as having a moderate/high risk of bias was driven by undertreatment in the observation arms due to the combination of including patients with postoperative detectable PSA who would warrant sRT according to contemporary standards, the low rates of sRT upon BCR in the control arms, and the late use of sRT when administered (Supplementary Table 2). Blinding of patients may not have been practically feasible. These other factors would all have biased results away from the null hypothesis.
Primary and secondary outcomes
The meta-analyses for primary and secondary outcomes are summarized in Fig. 2. We demonstrated no significant effect of aRT on OS (four trials: 95% confidence interval [CI] 0.90; 95% CI 0.61–1.33, p=0.59, I2=69%) (Fig. 2A). The FinnProstate trial was the only trial to provide an effect estimate for prostate cancer–specific mortality (PCSM), which demonstrated no effect (hazard ratio [HR] 1.00, 95% CI 0.06–15.91). Only two of 24 deaths were related to PCa. The effect on MFS for aRT vs. observation, despite being strongly driven by SWOG-8794 (weight=62.7%), did not reach statistical significance (three trials: HR 0.79, 95% CI 0.62–1.01, p=0.06, I2=7%) (Fig. 2B). There was a strong and consistent effect of aRT vs. observation on bPFS (four trials: pooled HR 0.47, 95% CI 0.41–0.54, p<0.001, I2=0%) (Fig. 2C). This effect was similar for loco-regional RFS (two trials: HR 0.54, 95% CI 0.39–0.73, p<0.001, I2=0%) (Fig. 2D).
Fig. 2.
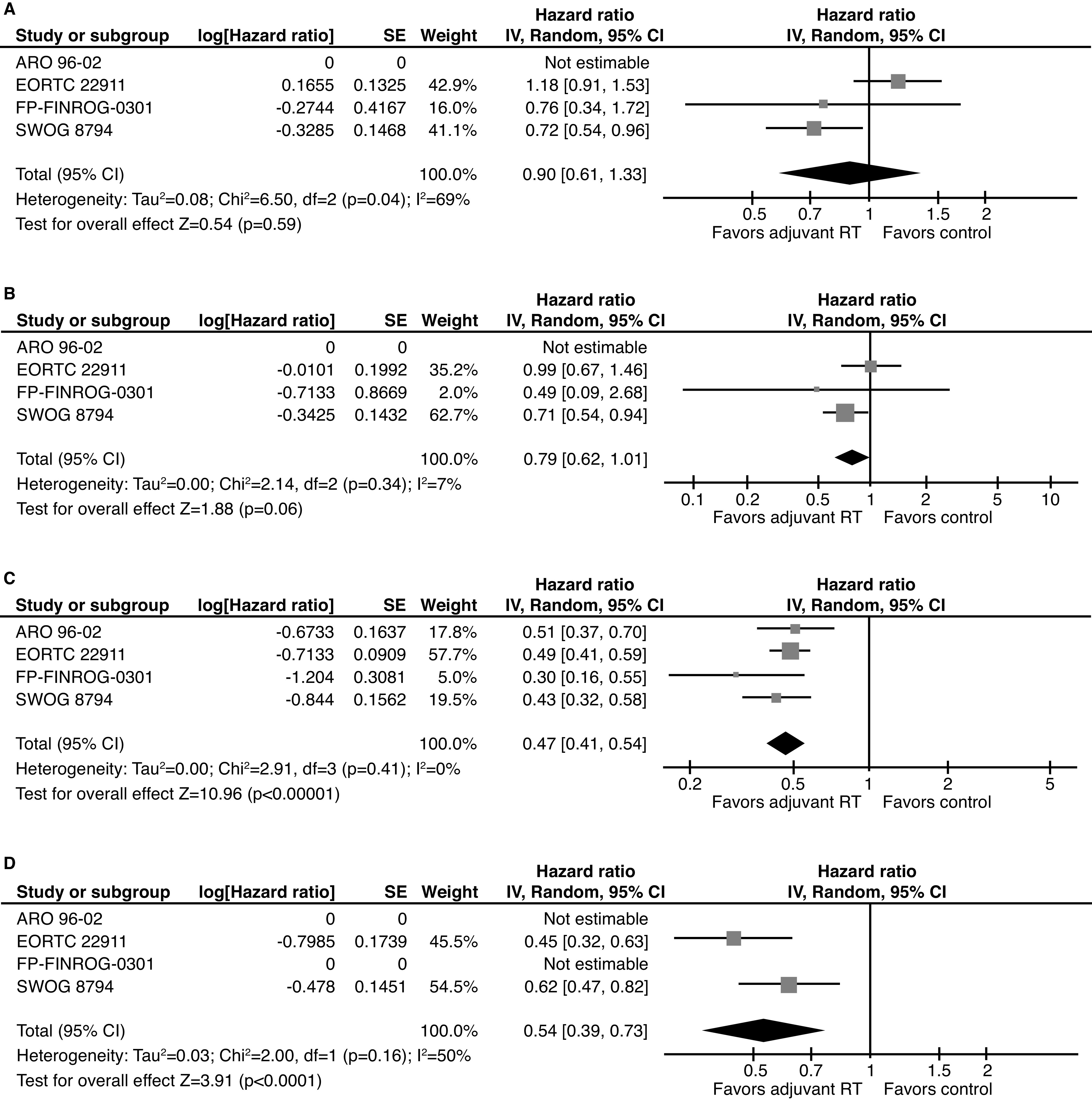
Summary of studies evaluating the association between adjuvant radiotherapy and oncologic and survival outcomes in patients with adverse features after prostatectomy. (A) Overall survival. (B) Metastasis-free survival. (C) Biochemical progression-free survival. (D) Loco-regional recurrence-free survival. Note that ARO-96-02 reported on metastasis-free and overall survival but a hazard ratio was not reported and, therefore, this trial could not contribute to the meta-analysis of these outcomes. CI: confidence interval; IV: inverse variance; RT: radiation therapy.
Subset meta-analyses were performed for the bPFS endpoint for three of the trials.4,7–10 The benefit of aRT over observation was generally consistent across all analyzable subsets (Table 2). Stronger point estimates for the effect of aRT were noted among patients with any positive margins (EORTC 22911 and ARO 96-02: HR 0.43, 95% CI 0.35–0.52, p<0.001, I2=0%), T2-margin-positive disease (ARO 96-02 and FinnProstate: HR 0.21, 95% CI 0.04–1.01, p=0.05, I2=77%), extracapsular extension (EORTC 22911, ARO 96-02, and FinnProstate: HR 0.43, 95% CI 0.35–0.53, p<0.001, I2=0%), and Gleason 6 PCa (ARO 96-02 and FinnProstate: HR 0.29; 95% CI 0.08–1.04, p=0.06, I2=6%). However, effect estimates of aRT on PFS remained significant for patients with negative margins (EORTC 22911 and ARO 96-02: HR 0.65, 95% CI 0.49–0.85, p=0.002, I2=0%), SVI (ARO 96-02 and EORTC 22911: HR 0.62, 95% CI 0.48–0.82, p<0.001, I2=0%), and Gleason 7 PCa (ARO 96-02 and FinnProstate: HR 0.48;, 95% CI 0.34–0.67, p<0.001, I2=0%). The SWOG 8794 trial5,6 focused subset analyses on MFS and noted a greater benefit in patients with Gleason 7–10 PCa rather than Gleason 2–6 disease, although 100 patients had missing data The bPFS in the observation arms ranged from 35–60.6% (Table 1), which is approximately indicative of the number of patients in both arms cured by surgery alone.
Table 2.
Subgroup analyses using biochemical progression-free survival endpoint
| Subgroup | Trials included | Pooled effect (HR, 95% CI, p, I2) |
|---|---|---|
| Margin negative (R0) | ARO-96-02 EORTC-22911 |
HR 0.65, 95% CI 0.49–0.85 p=0.002, I2=0% |
| Margin positive (R1) | ARO-96-02 EORTC-22911 |
HR 0.43, 95% CI 0.35–0.52 p<0.001, I2=0% |
| T2R1 | ARO-96-02 FinnProstate |
HR 0.21, 95% CI 0.04–1.01 p=0.05, I2=77% |
| Extracapsular extension (regardless of margin) | ARO-96-02 EORTC-22911 FinnProstate |
HR 0.43, 95% CI 0.35–0.53 p<0.001, I2=0% |
| Seminal vesicle invasion | ARO-96-02 EORTC-22911 |
HR 0.62, 95% CI 0.48–0.82 p<0.001, I2=0% |
| Gleason score 6 | ARO-96-02 FinnProstate |
HR 0.29, 95% CI 0.08–1.04 p=0.06, I2=64% |
| Gleason score 7 | ARO-96-02 FinnProstate |
HR 0.48, 95% CI 0.34–0.67,M p<0.001, I2=0% |
Note for subgroup analysis: Thompson excluded as subgroup results only provided for metastasis-free survival. CI: confidence interval; HR: hazard ratio.
Toxicities
Variability between trials in the assessment and reporting of adverse events precluded meta-analysis. There were no grade 5 adverse events and grade 4 events were rare. In the FinnProstate trial, the probability of any adverse events using Common Terminology Criteria for Adverse Events (CTCAE) v4.03 criteria was higher in the adjuvant vs. observation arm (96% vs. 84.7%), including gastrointestinal (77.0% vs. 12.9%), urinary (88.1% vs. 62.1%), and erectile (56.3% vs. 41.9%) disorders. There was also a greater number of grade 3–4 toxicities (55.6% vs. 40.3%). The median number of all adverse events (6 [range 0–17] vs. 1.5 [range 0–11]) and grade 3–4 adverse (1 [range 0–6] vs. 0 [range 0–3]) was higher in the aRT vs. observation arm. The ARO-96-02 trial only provided toxicity data in its initial report, using the Radiation Therapy Oncology Group (RTOG)/EORTC classification, which did not capture urinary incontinence.9 At a median followup of 53.7 months, the cumulative incidence of any adverse event was 21.9% vs. 3.7% with aRT vs. observation, and grade 3 adverse events were confined to the aRT arm. In the EORTC trial, the 10-year cumulative incidence of any (70.8% vs. 59.7%) and grade 3 (5.3% vs. 2.5%) RTOG/EORTC late toxicity was higher with aRT vs. observation. In the SWOG trial, the probability of any complication was higher with aRT vs. observation (23.8% vs. 11.9%), including rectal complications (3.3% vs. 0%), urethral stricture (17.8% vs. 9.5%), and total urinary incontinence (6.5% vs. 2.8%).
Discussion
In an updated meta-analysis, we found a consistent benefit to aRT vs. observation (with possible late salvage therapy in a subset of eligible patients), with respect to bPFS and local RFS. However, for more clinically relevant endpoints, such as MFS and OS, the benefit ranges from uncertain to non-existent, respectively. Further, the apparent (non-significant) MFS effect is largely driven by one trial (SWOG-8794), which has a notable risk of bias. While we could not perform quantitative meta-analysis, qualitative synthesis demonstrated an increased risk of toxicity with the aRT strategy. In addition to uncertain benefit with some toxicity, there was also a moderate risk of overtreatment, with 35–60% of patients being BCR-free with observation alone.
If aRT were a drug being subjected to modern standards and scrutiny, it would be unlikely to receive regulatory approval on the basis of these combined results. Given that the bPFS benefit with aRT likely reflects local control rather than a true OS benefit, we must also expect that the benefits of esRT is similar, at best. In men with early BCR, a nuanced discussion may be warranted contrasting the expected benefits and potential toxicities of esRT, particularly in those who are still recovering continence or men with shorter life expectancy.
The robustness of the primary endpoints needs to be considered when interpreting these trials. While the validity of MFS as a surrogate endpoint for OS has been established,18 similar validation for bPFS is not yet available. Furthermore, in these trials, most patients with BCR die from causes other than PCa.4–10 As such, evidence for a benefit of MFS and/or OS are required to justify support for the routine use of aRT rather than the bPFS benefits noted in this meta-analysis. Furthermore, given that not all BCRs translate into PCa mortality, not all patients may warrant esRT upon BCR. BCR may be an indicator for potential risk for metastasis, risk of needing secondary outcomes, and may have quality of life implications.19 However, it may be better for future trials to quantify these endpoints directly and leave BCR as a secondary endpoint rather than relying on BCR alone.
An editorial that accompanied the recent publication of the FinnProstate trial highlighted many of the notable features of this study and the resulting extant literature base to guide decisions regarding postoperative radiotherapy.20 Notably, FinnProstate included men with pT2 disease and PSMs and provides the most relevant data on this subgroup of men. Additionally, the cohort was accrued and treated in a more contemporary era and the radiotherapy dose used was closer to contemporary practices. However, many men enrolled had elevated PSA levels at trial entry and, thus, this (like the EORTC and SWOG trials) is not a true adjuvant trial. Unlike the remainder of the literature, salvage radiotherapy was quite reliably used in this trial (86%), though it was at a median PSA of 0.7 ng/mL, more accurately described as late salvage radiotherapy.
We were unable to identify a particular subset of patients who derived greater or lesser benefit, although analyses were limited to the bPFS outcome. A secondary analysis of EORTC-22911 suggested patients with PSMs derive a bPFS benefit, while those with negative margins did not.21 However, one observational study with 20 years’ median followup in patients with pT2N0R1 PCa found that this did not translate into a PCSM or OS benefit, despite replicating the magnitude of bPFS benefit seen in the trials.22 Most patients in this study died of non-PCa causes, which is similar to the FinnProstate trial,10 the only one of the four trials to report on cause of death.
It is plausible that, instead of a single factor identifying a subset of patients who benefit greatest from aRT, multiple adverse factors are required. Observational research suggested that aRT was only associated with survival benefit in patients with at least two of the following risk factors: Gleason score ≥8, pT3/pT4 disease, and positive lymph nodes.23 Meanwhile, a secondary analysis of EORTC 22911 argued against aRT for patients with a negative surgical margin.21
Limitations
There are relevant limitations to this study. The meta-analysis is limited to using data that has been reported; not all studies have reported all of the outcomes we wished to synthesize. There are subtle differences in study inclusion criteria and study design. This may explain some of the heterogeneity noted. It has previously been shown that early sRT provides improved outcomes compared to late sRT.24 In the studies included in this review, aRT was compared to late or nonexistent sRT. The early data with short followup from the RAVES and RADICALS trials, comparing aRT vs. observation with esRT, were recently presented at the ASTRO and ESMO annual meetings, respectively. They found no difference in freedom from biochemical failure or local/distant failure, but did find greater odds of grade 2+ genitourinary toxicity with aRT.13,14 These results are likely to impact treatment going forward. However, the role of esRT needs to be contextualized in the setting of an unclear OS and MFS benefit with even aRT.
Conclusions
The present study synthesizes and weighs the relative oncological benefit vs. toxicities for aRT vs. observation after RP demonstrating adverse pathological features. Given the absence of an OS benefit and an uncertain MFS benefit, aRT for all such patients likely represents over-treatment.
These data also have implications for the merits of esRT, which also may not provide OS benefit. Furthermore, when compared to aRT, esRT would reduce but not eliminate overtreatment, especially since many BCRs do not translate into PCa mortality. As such, in the context of the preliminary data from RADICALS and RAVES, esRT should be considered but should not be the mandatory in all men. Observation with esRT upon BCR may be appropriate in some men, but a more nuanced discussion weighing benefit and toxicity risk may be warranted for others. Further work is needed to identify patients who would benefit most from aRT and esRT.
Supplementary Information
Supplementary Table 1.
Risk of bias assessment for using Cochrane collaborative tool
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinded outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| ARO-96-02 | Low | Low | High | High | Low | Low | Low | Low |
| EORTC 22911 | Low | Low | High | High | Low | Low | Moderate | Moderate |
| SWOG 8794 | Low | Low | High | High | Low | Low | High | High |
| FP-FINROG-0301 | Low | Low | High | High | Low | Low | Low | Low |
Supplementary Table 2.
Specific sources of potential bias in randomized adjuvant radiation trials
| Feature predisposing to bias | Inclusion of patients with postoperatively detectable PSA | Low rates of salvage treatment in control arms | Very late use of salvage radiation (PSA >1.0 ng/ml) |
|---|---|---|---|
| SWOG-8794 | Yes | Yes | Yes |
| EORTC-22911 | Yes | Yes | Yes |
| ARO-96-02 | No | Unknown | Unknown |
| FinnProstate | Yes | No | No |
PSA: prostate-specific antigen.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed
References
- 1.Sooriakumaran P, Srivastava A, Shariat SF, et al. A multinational, multi-institutional study comparing positive surgical margin rates among 22393 open, laparoscopic, and robot-assisted radical prostatectomy patients. Eur Urol. 2014;66:450–6. doi: 10.1016/j.eururo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO guideline. J Urol. 2013;190:441–9. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: A randomized controlled trial (EORTC trial 22911) Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: A randomized clinical trial. JAMA. 2006;296:2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomized controlled trial (EORTC trial 22911) Lancet (London, England) 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 8.Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year followup of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–50. doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Wiegel T, Bottke D, Steiner U, et al. Phase 3 postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 10.Hackman G, Taari K, Tammela TL, et al. Randomized trial of adjuvant radiotherapy following radical prostatectomy vs. radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol. 2019;76:586–95. doi: 10.1016/j.eururo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Sineshaw HM, Gray PJ, Efstathiou JA, et al. Declining use of radiotherapy for adverse features after radical prostatectomy: Results from the National Cancer Database. Eur Urol. 2015;68:768–74. doi: 10.1016/j.eururo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Wallis CJ, Cheung P, Herschorn S, et al. Complications following surgery with or without radiotherapy or radiotherapy alone for prostate cancer. Br J Cancer. 2015;112:977–82. doi: 10.1038/bjc.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kneebone A, Pearse M, Fraser-Browne C, et al. A phase 3, multicenter, randomized trial comparing adjuvant vs. early salvage radiotherapy following a radical prostatectomy: Results of the TROG 0803 and ANZUP “RAVES” trial. ASTRO Annual Meeting; Sep 16, 2019; Chicago. 2019. [DOI] [Google Scholar]
- 14.Parker C, Clarke N, Cook A, et al. Timing of radiotherapy after radical prostatectomy: First results from the RADICALS-RT randomized controlled trial [ NCT00541047]. European Society of Medical Oncology Annual Congress 2019; Sep 27, 2019; Barcelona, Spain. 2019. [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35:3097–3104. doi: 10.1200/JCO.2017.73.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartor O, Flood E, Beusterien K, et al. Health-related quality of life in advanced prostate cancer and its treatments: Biochemical failure and metastatic disease populations. Clin Genitourin Cancer. 2015;13:101–12. doi: 10.1016/j.clgc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Spratt DE. The Finnish randomized trial of adjuvant radiotherapy vs. observation after prostatectomy: Almost a trial of adjuvant vs. late salvage radiotherapy. Eur Urol. 2019;76:596–8. doi: 10.1016/j.eururo.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 21.Van der Kwast TH, Bolla M, Van Poppel H, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25:4178–86. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 22.Bhindi B, Carlson RE, Mason RJ, et al. Long-term followup of a matched cohort study evaluating the role of adjuvant radiotherapy for organ-confined prostate cancer with a positive surgical margin. Urology. 2017;109:145–52. doi: 10.1016/j.urology.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Abdollah F, Suardi N, Cozzarini C, et al. Selecting the optimal candidate for adjuvant radiotherapy after radical prostatectomy for prostate cancer: A long-term survival analysis. Eur Urol. 2013;63:998–1008. doi: 10.1016/j.eururo.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–54. doi: 10.1200/JCO.2016.67.9647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Risk of bias assessment for using Cochrane collaborative tool
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinded outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| ARO-96-02 | Low | Low | High | High | Low | Low | Low | Low |
| EORTC 22911 | Low | Low | High | High | Low | Low | Moderate | Moderate |
| SWOG 8794 | Low | Low | High | High | Low | Low | High | High |
| FP-FINROG-0301 | Low | Low | High | High | Low | Low | Low | Low |
Supplementary Table 2.
Specific sources of potential bias in randomized adjuvant radiation trials
| Feature predisposing to bias | Inclusion of patients with postoperatively detectable PSA | Low rates of salvage treatment in control arms | Very late use of salvage radiation (PSA >1.0 ng/ml) |
|---|---|---|---|
| SWOG-8794 | Yes | Yes | Yes |
| EORTC-22911 | Yes | Yes | Yes |
| ARO-96-02 | No | Unknown | Unknown |
| FinnProstate | Yes | No | No |
PSA: prostate-specific antigen.