Abstract
Platypnea-orthodeoxia syndrome (POS) is a clinical entity characterized by positional dyspnoea (platypnea) and arterial desaturation (orthodeoxia) that occurs when sitting or standing up and usually resolves by lying down. POS may result from some cardiopulmonary disorders or from other miscellaneous aetiologies. We report a case of POS in a patient after fibrotic evolution of SARS-CoV-2 interstitial pneumonia associated with pulmonary embolism. The patient did not have any evidence of an intracardiac/intrapulmonary shunt.
Keywords: Platypnea, Orthodeoxia, Pulmonary fibrosis, SARS-CoV-2
Introduction
Platypnea-orthodeoxia syndrome (POS) is a clinical entity characterized by positional dyspnoea (platypnea) and arterial desaturation (orthodeoxia) that occurs when sitting or standing up and usually resolves by lying down. The drop in oxygen saturation is considered as significant, when PaO2 falls greater than 4 mmHg or SaO2 than 5 % from supine to an upright position (1). POS is a very rare finding; its real prevalence is not known (1).
Although it is not well understood, the cause of arterial blood desaturation and hypoxaemia is the blend of deoxygenated venous blood into the oxygenated arterial blood via a pathologic shunt. A certain percentage of shunting (up to 5%) can be found even in healthy individuals due to the presence of the smallest cardiac veins (Thebesian veins) and due to the particular anatomy of the bronchial arteries (2). Concerning POS, the shunt is to be referred to intracardiac or extracardiac abnormalities and to miscellaneous aetiologies (1,3-4).
In the context of the intracardiac aetiologies, POS typically occurs in the presence of right heart failure or elevated right-sided filling pressures (1). The most common underlying anatomical condition is the patent foramen ovale (PFO). In addition to PFO, intracardiac shunt leading to POS has been reported from either an atrial septal defect (ASD) or an atrial septal aneurysm (ASA) (1, 3-4).
The most common causes of orthodeoxia-platypnea in the extracardiac aetiologies are intra-pulmonary arteriovenous malformations, lung parenchymal disease and cirrhosis leading to hepatopulmonary syndrome (1, 3-5). The aetiology was not found in a significant percentage of cases (1, 3-4).
In the context of the miscellaneous causes, POS can be due to Amiodarone lung toxicity, Parkinson’s disease, diabetic neuropathy, organophosphorous poisoning, radiation-induced bronchial stenosis and traumatic bronchial rupture (1, 3-5).
We report a case of POS in a patient after fibrotic evolution of SARS-CoV-2 interstitial pneumonia associated with pulmonary embolism (PE). The patient did not have any evidence of an intracardiac/intrapulmonary shunt.
Case Presentation
A 76-year-old woman presented to our hospital’s emergency room with fever, cough and dyspnoea. Her medical history was notable for non-smoking habit, mild aortic and tricuspid insufficiency and hiatal hernia. Her arterial blood gas (ABG) analysis showed PaO2 59 mmHg, PaCO2 32 mmHg and pH 7.49. ECG showed nonspecific repolarization abnormalities. The patient had a positive swab for SARS-CoV-2, then performed a chest CT scan, which showed an interstitial pneumonia with 35% score (Fig. 1).
Figure 1.
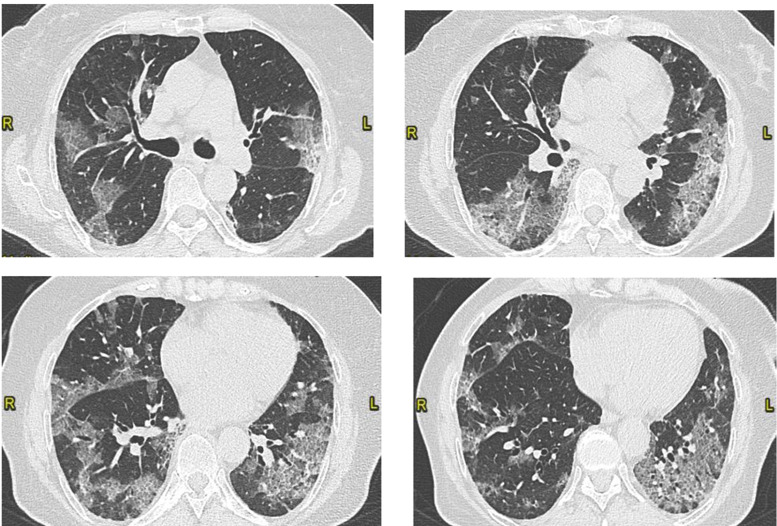
Bilateral ground glass opacities with patchy distribution. Visual score of 35% (CT on March 28, 2020).
She was hospitalized in the Emergency Unit and was treated with Hydroxychloroquine, Darunavir/cobicistat and oxygen therapy. After a week because of the worsening of respiratory failure, she performed a second chest CT which showed a bilateral segmental PE and a worsening of the interstitial pneumonia with 60% score (Fig. 2). Therapy with Fondaparinux, empirical antibiotic (Piperacillin/tazobactam), steroids and helmet non-invasive mechanical ventilation (CPAP) was hence undertaken, Tocilizumab was also administered.
Figure 2.
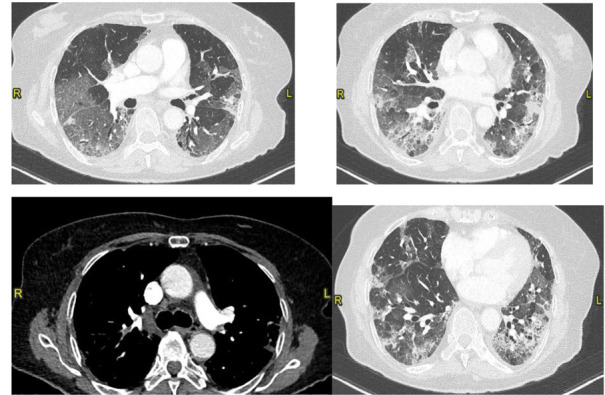
Bilateral segmental pulmonary embolism involving the branch for the posterior segment of the right upper lobe, anterior segmental branch of the left upper lobe and the branch for the dorsal segment of the left lower lobe. Worsening of ground glass opacities (visual score of 60%) (CT on April 5, 2020)
After two weeks, she performed a control chest CT scan, which showed a quite complete resolution of the PE with focal segmental thrombosis in the left upper lobe and the resolution of the ground glass with the peribronchial consolidating areas of organized appearance with contextual traction bronchiectasis (Fig. 3). Because of the persistence of the respiratory failure requiring oxygen therapy and in order to undertake a rehabilitation program, she was transferred to our Unit.
Figure 3.
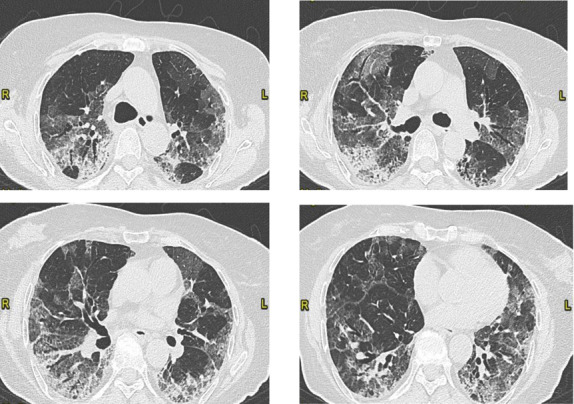
Fibrotic evolution of interstitial pneumonia. Decrease of the ground glass opacities with peribronchial consolidating areas of organized appearance with contextual traction bronchiectasis. Visual score of 60%. (CT on April 20, 2020)
Upon admission to our ward, the patient was no longer feverish and was being treated with low-dose oral steroids as well as with anticoagulant treatment (Fondaparinux). Next, she progressively complained of breathlessness while sitting, standing or walking, even with supplemental oxygen and showed orthodeoxia (SpO2 99% on 4 l/min O2 by nasal cannula in the supine position vs SpO2 84% on 4 l/min O2 by nasal cannula while in the seated position); the fall in SpO2 was associated with simultaneous increase in respiratory rate. She also had basal inspiratory crackles on chest auscultation. Bubble-contrast echocardiography in recumbent, sitting and upright positions revealed no intracardiac or intrapulmonary shunts. Signs of mild pulmonary hypertension (PAPs 45 mmHg) were found and a diuretic treatment with Torasemide was initiated.
To further investigate the respiratory gas exchange, the patient wearing a reservoir mask on 15 L/min of O2 breathed for 20-min in a supine position and then for further 20-min in a sitting position. ABGs were performed at the end of the 20-min period of supine position (PaO2 133 mmHg, PaCO2 51 mmHg and pH 7.430) and at the end of 20-min period of sitting position (PaO2 46 mmHg, PaCO2 44 mmHg and pH 7.470). The estimated rates of wasted ventilation in relation to the cardiac output in supine and in sitting positions were calculated as previously described (6) and were 19 % and 23 %, respectively.
A ventilation/perfusion (V/Q) scan was performed both in supine and sitting position using a 99mTc-labelled aerosol (Technegas®) to evaluate ventilation and labelled macroaggregates of human albumin (99mTc-MAA) for perfusion imaging. Planar and SPECT/CT images were acquired (7). Analysis of regional aerosol deposition was performed dividing lung images into regions (ROIs). The recommended method for dividing lung into an ‘‘outer’’ (O) and ‘‘inner’’ (I) zone was previously described (8). The aerosol deposition pattern was obtained from the ratio of outer lung deposition to inner lung deposition (O/I).
The V/Q scan in supine position showed a widespread reduced deposition of the radioactive aerosol in the left lung and a prevailing deposition of the radioactive aerosol at the base of the right lung (Right Outer/Inner ratio: 2.59, Left Outer/Inner ratio: 2.87; Left upper/lower lobe ratio: 1.88, Right upper/lower ratio: 0.49). In the planar images there was no evidence of significant alterations in pulmonary perfusion bilaterally (Fig. 4).
Figure 4.
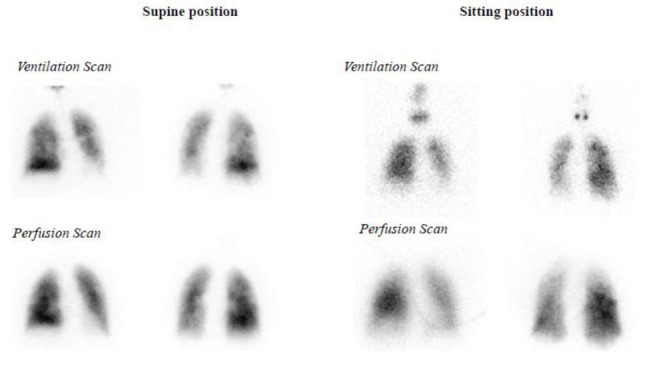
A ventilation/perfusion (V/Q) scan in supine and sitting position. For more details, see the text.
In sitting position, ventilation images were captured only as planar images in the anterio-posterior view for a rapid oxygen desaturation of the patient and showed a widespread reduced deposition of the radioactive aerosol in the left lung and a heterogeneously reduced deposition of the radioactive aerosol in the right lung (Right Outer/Inner ratio: 1.71, Left Outer/Inner ratio: 1.41; Left upper/lower lobe ratio: 2, Right upper/lower ratio: 1). Perfusion was reduced in the upper third of the right lung and a widespread reduced perfusion was revealed in the left lung (Fig. 4).
The quantitative analysis of the V/Q scans in terms of kilocounts per second (kc/s) is shown in table 1.
Table 1.
Quantitative analysis of ventilation/perfusion scan in supine and in sitting position.
| Supine | Sitting | Change | ||||
| Right | Left | Right | Left | Right | Left | |
| Ventilation | ||||||
| Upper | 20 kc/s | 30 kc/s | 3 kc/s | 2 kc/s | -85 % | -93% |
| Middle | 51 kc/s | 36 kc/s | 8 kc/s | 3 kc/s | -84 % | -92% |
| Lower | 62 kc/s | 10 kc/s | 3 kc/s | 1 kc/s | -95 % | -90% |
| Total | 133 kc/s | 76 kc/s | 14 kc/s | 6 kc/s | -89% | -92% |
| Perfusion | ||||||
| Upper | 36 kc/s | 44 kc/s | 20 kc/s | 16 kc/s | -44 % | -64% |
| Middle | 112 kc/s | 71 kc/s | 57 kc/s | 25 kc/s | -49 % | -65% |
| Lower | 91 kc/s | 33 kc/s | 26 kc/s | 16 kc/s | -71 % | -51% |
| Total | 239 kc/s | 148 kc/s | 103 kc/s | 57 kc/s | -57% | -61% |
When the patient switched from supine to sitting position, a reduction in kc/s both of the ventilation scan and the perfusion scan was observed. The percent change of the ventilation scan was greater than that of perfusion.
The patient was then treated for 4 weeks with high doses of steroids, which were then gradually reduced. A control chest TC after 5 weeks showed a complete resolution of the PE and fibrotic pattern with basal subpleural predominance and traction bronchiectasis (Fig. 5).
Figure 5.
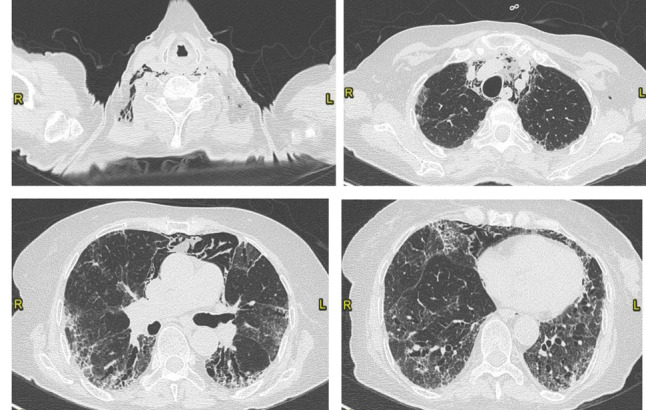
Complete resolution of the pulmonary embolism. Mild reduction of the ground glass opacities with patchy distribution, persistence of the basal fibrotic pattern, with traction bronchiectasis. Presence of pneumomediastinum. Visual score of 50% (CT on June 5, 2020).
At discharge, the patient still complained of platypnea and orthodeoxia (SpO2 97% on 2 l/min O2 by nasal cannula in the supine position vs SpO2 87% on 2 l/min O2 by nasal cannula while in the seated position). She was discharged with long term oxygen therapy, anticoagulant treatment with warfarin, diuretic and low dose azithromycin every other day. She was also referred to our interstitial lung disease (ILD) outpatient clinic in order to follow up and to evaluate a possible anti-fibrotic treatment.
Discussion
We report a case of a patient with POS secondary to fibrotic evolution of interstitial pneumonia by SARS-CoV-2 and pulmonary embolism. Bubble-contrast echocardiography excluded intracardiac or intrapulmonary shunts. On the other hand, V/Q scan and ABG data suggest that POS was due to ventilation-perfusion mismatching. Notably, both ventilation and perfusion decreased when the patient switched from lying to sitting, but the reduction in ventilation was remarkably greater than the corresponding reduction in perfusion, accordingly the wasted ventilation rate in relation to the cardiac output increased when patient was in the upright position. Furthermore, the patient exhibited a poor response to a high FIO2 when she was in recumbent position and a still poorer when she was upright.
Platypnea-orthodeoxia is a very rarely identified clinical syndrome: its real prevalence is not known. It was first identified in 1949 and was initially termed “orthostatic cyanosis” (9). The terms “platypnea” and “orthopnea” were coined in 1969 (10) and 1976 (11), respectively, to describe breathlessness and arterial desaturation worsening in the upright position and improving when supine.
The condition of POS and its pathophysiology have remained somewhat mysterious. The condition is probably under-recognized because upright and supine vital signs are not routinely collected in most clinical visits. A review of literature by Agrawal et al. (1) found 216 articles on PubMed with the keywords “Platypnea” and/or “Orthodeoxia” from January 1949 to November 2016. A total of 261 relevant articles were screened. 111 articles not related to true platypnea or orthodeoxia were excluded. Therefore, a total of 150 articles with 239 patients were analysed. The causes of POS in these patients were classified as intracardiac abnormalities, extracardiac abnormalities and miscellaneous aetiologies. Intracardiac communication between the two atria was the most common cause of POS in 208 of 239 (87%) patients (1). A PFO was the most common reported site of an intracardiac shunt. In addition to PFO, intracardiac shunt leading to POS has been reported from either ASD or an ASA. Extracardiac causes of POS included lung parenchymal diseases (4 %) and intra-pulmonary arterio-venous malformations (9 %). In hepatopulmonary syndrome, acquired pulmonary arteriovenous fistulae, which stem from liver disease, allow the requisite right-to-left shunting that is accentuated in the upright position (5).
To the best of our knowledge, the association of POS with ILD has been rarely reported (12,13). Parenchymal lung diseases with preferential involvement of lung bases can present as POS through severe V/Q mismatch. In upright position orthodeoxia may, therefore, reflect obligate gravitational perfusion to basal alveolar units with severe derangements in ventilation and gas exchange. In our patient, basal part of both lungs were predominantly affected by the interstitial pneumonia’s fibrotic evolution. Accordingly, standing posture may flow the blood to poorly ventilated lower parts, thereby inducing physiological shunting since deoxygenated blood matches fibrotic zones of the lung. Furthermore, in our patient the physiological shunt as well as the wasted ventilation are amplified by the disproportionate reduction of ventilation as related to the reduction of perfusion, when position changes from supine to seated occur. However, it remains unclear why these blood gas derangements induced by the posture do not occur in all other cases of ILD and pulmonary fibrosis or in other causes of predominant basal lung disease. In this context, we may speculate that in our case fine and diffuse vascular changes following PE occurred and deeply upset the vascular bed physiology. Finally, an open question is whether or not anti-fibrotic drugs, such as Pirfenidone or Nintedanib, could resolve POS.
In conclusion, POS is a quite rare syndrome and should be considered in the differential diagnosis of dyspnoea and refractory hypoxaemia. It is crucial to determine its aetiology in order to the adequate management.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Agrawal A, Palkar A, Talwar A. The multiple dimensions of Platypnea-Orthodeoxia syndrome: A review. Respir Med. 2017;129:31–38. doi: 10.1016/j.rmed.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Charan NB, Thompson WH, Carvalho P. Functional anatomy of bronchial veins. Pulm Pharmacol Ther. 2007;20:100–103. doi: 10.1016/j.pupt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.De Vecchis R, Baldi C, Ariano C, et al. Platypnea-orthodeoxia syndrome: Orthostatic dyspnea and possible pathophysiological substrates. Herz. 2017;42(4):384–389. doi: 10.1007/s00059-016-4479-4. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues P, Palma P, Sousa-Pereira L. Platypnea-orthodeoxia syndrome in review: defining a new disease? Cardiology. 2012;123(1):15–23. doi: 10.1159/000339872. [DOI] [PubMed] [Google Scholar]
- 5.Lee C-H, Cheng S-T. Shortness of breath while sitting up: hepatopulmonary syndrome. CMAJ. 2011;183:80. doi: 10.1503/cmaj.090576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brambilla I, Pizzamiglio R. ABC dei test di funzionalità respiratoria. Masson Italia Ed Milano. 1979:137–141. [Google Scholar]
- 7.Bajc M, Schümichen C, Grüning T, et al. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur J Nucl Med Mol Imaging. 2019;46:2429–2451. doi: 10.1007/s00259-019-04450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman S, Bennett WD, Biddiscombe M, et al. Standardization of Techniques for Using Planar (2D) Imaging for Aerosol Deposition Assessment of Orally Inhaled Products. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2012:S-10-S-28. doi: 10.1089/jamp.2012.1Su4. [DOI] [PubMed] [Google Scholar]
- 9.Burchell HBHHJ, Wood EH. Reflex orthostatic dyspnea associated with pulmonary hypotension. Am. J. Physiol. 1949;159:563–564. [Google Scholar]
- 10.Altman M, Robin ED. Platypnea (diffuse zone I phenomenon?) N. Engl. J. Med. 1969;281:1347–1348. doi: 10.1056/NEJM196912112812408. [DOI] [PubMed] [Google Scholar]
- 11.Robin ED, Laman D, Horn BR, Theodore J. Platypnea related to orthodeoxia caused by true vascular lung shunts. N. Engl. J. Med. 1976;294:941–943. doi: 10.1056/NEJM197604222941711. [DOI] [PubMed] [Google Scholar]
- 12.Takhar R, Biswas R, Arora A, Jain V. Platypnoea-orthodeoxia syndrome: novel cause for a known condition. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-201284. doi:10.1136/bcr-2013–201284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenholder MF, Russell MD, Knight E, Rajagopal KR. Orthodeoxia: a new finding in interstitial fibrosis. Am Rev Respir Dis. 1986;136:170–173. doi: 10.1164/ajrccm/136.1.170. [DOI] [PubMed] [Google Scholar]


