Abstract
Emerging and re-emerging viruses represent an important challenge for global public health. In the 1960s, coronaviruses (CoVs) were recognized as disease agents in humans. In only two decades, three strains of CoVs have crossed species barriers rapidly emerging as human pathogens resulting in life-threatening disease with a pandemic potential: severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002, Middle-East respiratory syndrome coronavirus (MERS-CoV) in 2012 and the recently emerged SARS-CoV-2. This narrative review aims to provide a comprehensive overview of epidemiological, pathogenic and clinical features, along with diagnosis and treatment, of the ongoing epidemic of new coronavirus disease 2019 (COVID-19) in the pediatric population in comparison to the first two previous deadly coronavirus outbreaks, SARS and MERS. Literature analysis showed that SARS-CoV, MERS-CoV and SARS-CoV-2 infections seem to affect children less commonly and less severely as compared with adults. Since children are usually asymptomatic, they are often not tested, leading to an underestimate of the true numbers infected. Most of the documented infections belong to family clusters, so the importance of children in transmitting the virus remains uncertain. Like in SARS and MERS infection, there is the possibility that children are not an important reservoir for novel CoVs and this may have important implications for school attendance. While waiting for an effective against SARS-CoV-2, further prevalence studies in paediatric age are needed, in order to clarify the role of children in different age groups in the spread of the infection. (www.actabiomedica.it)
Keywords: Coronavirus, Middle-East respiratory syndrome (MERS), Severe Acute respiratory syndrome (SARS), COVID-19, children, clinical manifestations
1. Introduction
Coronaviruses (CoVs) are enveloped, positive-sensed, single-stranded RNA viruses with a genome ranging from 26 to 32 kilobases (kb) in size, capped and polyadenylated (1). They take their name from crown-like morphology with extended, petal-shaped surface projections seen on electron microscopy, corresponding to large surface spike glycoprotein (2). CoVs are part of the order Nidovirales, family Coronaviridae, whose two subfamilies include Orthocoronavirinae and Torovirinae. Based on the difference in protein sequences, the CoV subfamily Orthocoronavirinae is further classified into four genera: alpha (alpha-CoV), beta (beta-CoV), gamma (gamma-CoV), and delta CoVs (delta-Co V) (3). CoVs are host-specific and are known to infect humans as well as several other vertebrates including mammals and birds, causing diverse clinical syndromes, typically respiratory, enteric, neurologic and hepatic disorders (4). High mutation and recombination rates may allow cross-species transmission of coronaviruses (5). Classification of different types of CoVs is shown in Figure 1.
Figure 1.
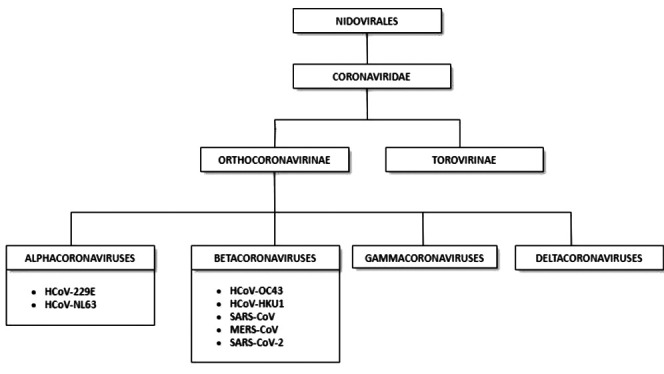
Classification of different types of human coronaviruses within the family Coronaviridae, subfamily Orthocoronavirinae, and the respective genera: alpha-, beta-, gamma-, and deltacoronaviruses
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection marks the third outbreak of human coronaviruses worldwide in the last 20 years, after severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and Middle-East respiratory syndrome coronavirus (MERS-CoV) in 2013. All these three outbreaks have mainly involved the adult population, with few pediatric cases reported globally and modest to scarce knowledge over specific epidemiological and clinical features in children. This narrative review focused on recent global pandemic of novel coronavirus 2019 (COVID-19) compared with past SARS and MERS outbreaks. Systematic searches were performed in PubMed, Embase, Cochrane Library, Scopus, Google Scholar and ClinicalTrials.gov up to June 30th 2020. Language was restricted to English. Search terms included coronavirus, COVID-19, SARS-CoV-2, 2019-nCoV, SARS-CoV, MERS-CoV in combination with children. Case reports, case series, original research studies, review articles, letters to the editor, randomised controlled trials (RCTs), non-RCTs and cohort studies (prospective or retrospective) published were included.
2. Severe Acute Respiratory Syndrome (SARS)
2.1 Background and epidemiology
SARS is an infectious disease caused by a strain of coronavirus (SARS-CoV). The disease first started as an outbreak of atypical pneumonia in the Guangdong Province of southern China in November 2002. Up to December 2003 a worldwide total of 8,098 cases of probable SARS and 774 deaths (9.6%) were recorded (6). In Hong Kong, 1,755 adult and paediatric patients were infected by the coronavirus. A total of 121 children aged <18 years were registered in the e-SARS database of the Hospital Authority of Hong Kong, accounting for 7% of all patients notified. The exact number of children affected by SARS worldwide is unknown as the age ranges shown were not available or incomplete for some of the affected countries (7). It is estimated that children <18 years of age only accounted for about 5% of the total affected. The Stockman et al. review identified 6 case series reporting 135 pediatric SARS patients (80 laboratory-confirmed, 27 probable and 28 suspected) from Canada, Hong Kong, Taiwan and Singapore. Despite a high mortality rate in the adult population (9.6–16.7%) there were no fatalities in the paediatric age group. The clinical course of SARS in paediatric patients is different compared with adults. Young children, in particular, appeared to had a milder form of the disease than 12 years of age who was similar to that in adults. Most children reported were previously healthy and there was no sex predominance. The majority of young children and teenage patients had a definitive contact history with adult SARS patients, usually an immediate family member. Transmission among children or from children to adult contacts was uncommon but possible, although there is only one published report of transmission of SARS virus from a pediatric patient to adults (8).
2.2 Clinical manifestations
Fever was the most frequent symptom associated with non-specific features, including lethargy, rhinorrhoea, headache, dizziness, myalgia. Nausea, abdominal pain, diarrhoea and vomiting, febrile convulsions were less common. The clinical course of SARS in the majority of paediatric patients followed a ‘biphasic’ rather than the ‘triphasic’ pattern as described in adult cases (9). Phase 1 was associated with an increase in viral load and the patient presented fever and other systemic features. This phase usually ended a few days after the onset of fever. Phase 2, the immune-pathological phase was marked by persistent or recurrent fever, hypoxaemia and progression of pneumonia with new infiltrations in chest radiographs. The deterioration in clinical condition corresponds to the period when the body viral load decreases and progression of pulmonary disease is caused by exaggerated host immunological reaction. Progression to ARDS, the third phase as in adults, is only seen in a very small number of children, predominantly adolescents. The outcomes in children 6 months after the onset of the disease have been favourable. Mild abnormalities were detected by high resolution computerized tomography and pulmonary function testing in 34 and 11% of patients, respectively. One report has described osteonecrosis in children who received steroids as part of a treatment regimen for SARS, although osteonecrosis has been a recognized risk of corticosteroid treatment of other pediatric illnesses including lymphoblastic leukaemia (10).
2.3 Laboratory features
The most consistent haematological finding was lymphopenia, which was found in over 90% of patients (11). Other haematological abnormalities were thrombocytopenia and coagulopathy with elevated D-dimer levels. Anaemia was rarely found at presentation and was only detected in <5% of children (12). The study of Ng et al. suggested that pediatric SARS patients have markedly elevated circulating IL-1 levels, which suggests selective activation of the caspase-1–dependent pathway. Other key pro-inflammatory cytokines, IL-6 and TNF-α, showed only mildly elevated levels at the initial phase of the illness. The activation of predominant type 1 T-helper lymphocyte (Th1)-mediated immune response facilitates viral clearance and may explain the rapid recovery of the paediatric cases (13).
2.4 Diagnostic test
Real-time polymerase chain reaction (RT-PCR) was first used for detection of SARS-CoV during the early phase of the outbreak. Although the test was specific, the sensitivity was about 60% (14). The method was applied to nasopharyngeal aspirates, nose and throat swabs, saliva, sputum, endotracheal aspirates, bronco-alveolar lavage, stool, urine, plasma and serum. In Toronto experience about 25 children with a presumptive diagnosis of suspect or probable SARS, the nasopharyngeal swab specimens tested negative using RT-PCR so the authors suggested that the quantity of virus in the upper airway of young children during the early symptomatic phase of SARS is extremely low (15). In this report the nasopharyngeal sampling was performed only at the time of admission. The virus was also detected in stool sample of 37% of subject between days 5 and 7 of illness. According to these data, the authors suggested that the sensitivity of RT-PCR could be improved by paying careful attention to type of specimen and timing of collection. One-step real-time quantitative RT-PCR was developed for SARS-CoV RNA quantification to detect SARS-CoV RNA in the plasma of patients.
About serology, most patients seroconverted within 2–3 weeks after onset of illness. However, some people did not develop detectable antibodies until 28 days after onset. IgM seropositivity was lost by about 12 weeks, while IgG titres peaked at 4 weeks and remained elevated until at least 12 weeks. IgG usually remained detectable after resolution of the illness but the duration of persisting protective neutralising antibodies and their boosting response remains unknown. Li et al. suggested that the SARS-specific IgG antibodies persisted for a long time, but the SARS-specific IgM remained measurable for a much shorter period, suggesting that IgG antibody to SARS-CoV may represent the primary humoral immune response protecting patients against SARS (16).
The principal radiographic abnormality of SARS in children is ill-defined airspace shadowing, which presented as ground-glass opacities and/or unifocal, lobar or multifocal areas of consolidation to be no predominant distribution pattern of consolidation in children, opacities are most often peripheral or mixed central and peripheral in location. Widespread ground-glass opacities and diffuse patchy consolidations representing progression to ARDS were evident in a small number of case. High-resolution computed tomography (CT) scanning of the chest was performed in children with initial negative or equivocal chest radiographs (17).
2.5 Treatment
Corticosteroids and ribavirin were the most commonly used therapies during the 2003 outbreak. Interferon-alpha and combination of protease inhibitors (eg, ritonavir and lopinavir) were also administered. In the pediatric setting, a combination of antibiotics for treatment of community-acquired pneumonia caused by usual and by atypical pathogens, corticosteroids and short-term intravenous or oral ribavirin was well-tolerated, without serious adverse events such as haemolytic anemia, although there was no clear evidence of the therapeutic efficacy (18).
3. Middle-East Respiratory Syndrome (MERS)
3.1 Background and epidemiology
MERS-CoV was identified in June 2012, when a patient died from a severe form of respiratory infection in Jeddah, Saudi Arabia (19). According to its latest report (January 2020), a total of 2,519 laboratory-confirmed cases worldwide were reported, including 866 fatalities, with an associated mortality rate of 34.3%. Overall, 27 countries were involved globally. Most cases were identified across the Arabian Peninsula and particularly in Saudi Arabia (2,121), which also recorded the majority of deaths (788), but other countries in Asia, Africa, Europe and USA were affected as well (20). To our knowledge, MERS mainly affected the adult population, with a median age at infection of 50 years (21). However, along with all the previously identified human CoVs, MERS-CoV could also infect the pediatric population. As of November 2015, 35 cases of MERS-CoV were reported in children (age <18 years), accounting for around 2% of all cases (21,22). An epidemiologic investigation involving in-hospital patients, health care providers and their family contacts listed 625 children, of whom only 1.2% tested positive for MERS-CoV (23). Moreover, although children are well known to be a source of viral respiratory infections, they did not seem to play the role of “reservoir” in the diffusion of MERS-CoV. In a large sample of children <2 years old hospitalised in Amman, Jordan, from March 2010 to September 2012, none of the collected specimen tested positive for MERS-CoV, suggesting that this novel coronavirus was not actively circulating in young children in the 30-month-period before and after its outbreak in the country (24). Conversely, most of the 35 reported pediatric cases were household contacts of adult cases (25).
3.2 Clinical features
Data on clinical features of MERS in the pediatric population are scarce. In September 2014, Memish et al. presented 12 laboratory-confirmed pediatric cases (25). Overall, 11 had been reported by Saudi Arabia to the World Health Organization (WHO) between September 1st 2012 and December 2nd 2013 and 1 had been detected in Abu Dhabi, United Arab Emirates. Out of these 12 (age range, 2- to 16-year-old), 9 were asymptomatic, whereas 3 had symptoms. All asymptomatic patients but one were family contacts, none had comorbidities nor required hospitalisation, imaging and/or treatment. One out of the 3 symptomatic patients, an 8-year old boy without underlying diseases, acquired the infection from his parents and only showed mild respiratory symptoms. The other two symptomatic patients had underlying conditions and required hospitalisation. A 14-year-old girl with Down’s disease, was admitted to the hospital with fever and cough; she had no history of travel or contact with animals. She tested positive for MERS-CoV through RT-PCR and underwent treatment with nebulisation therapy along with intravenous diuretics, antibiotics and antivirals (oseltamivir): she recovered and was discharged 7 days after admission. A 2-year-old boy with cystic fibrosis was admitted to the hospital with fever and respiratory distress; no history of travel, contact with animals or confirmed MERS-CoV cases was recorded. He was first treated with intravenous antibiotic therapy for multidrug-resistant Pseudomonas aeruginosa in sputum culture, but his clinical conditions rapidly deteriorated and he was transferred to paediatric intensive care unit (PICU) where he later developed respiratory insufficiency and multiorgan failure, dying 60 days after his first admission. RT-PCR on nasopharyngeal swab had tested positive for both MERS-CoV and H1N1 influenza.
Two WHO Updates (December 2013 and April 2014) reported respectively of an 8-year-old boy and a 4-year-old boy, both with no underlying comorbidities and both presenting with mild respiratory symptoms, who fully recovered without requiring intensive care.
In January 2015, Thabet et al. reported another symptomatic laboratory-confirmed pediatric case in Saudi Arabia (26). A 9-month-old infant, recently diagnosed with nephrotic syndrome and started to therapy with oral prednisolone, entered the hospital for Streptococcus pneumoniae sepsis and was transferred to PICU 5 days after admission in order to receive mechanical ventilation and inotropic support. After an initial improvement, he headed for a deterioration of his clinical conditions, and died 17 days after his first admission. His tracheal aspirate tested positive for MERS-CoV by RT-PCR. They were not able to trace back the source of infection.
According to the data reported above, it appears clear that MERS clinical features in pediatric age were milder than in adult population. Access to PICU was limited and only two fatalities were reported globally. As in other CoVs infections, respiratory symptoms, including fever, cough and shortness of breath, were the most commonly reported.
3.3 Laboratory findings
Data on laboratory findings in children <18 years old with MERS-CoV infection are insufficient due to the small amount of cases in the pediatric population. As we cited above, most pediatric cases were completely asymptomatic and did not undergo any laboratory testing. Collected data on the 9-month-old child suffering from nephrotic syndrome and dying from MERS showed normal white blood cell count with concomitant modest increase in CRP, minor anemia and significant drop in platelet count; his coagulation profile also deteriorated with time, with prolonged prothrombin time (PT) and partial thromboplastin time (PTT) and increased fibrinogen (26). No other laboratory findings were available in children for comparison.
3.4 Diagnostic test
All 35 reported pediatric cases were defined as confirmed cases after testing positive for MERS-CoV from respiratory specimens (27). Respiratory specimens included nasopharyngeal swabs and nasal and/or tracheal aspirates. The molecular method which was applied to such specimen mainly consisted in qualitative and quantitative RT-PCR, thus respecting the WHO case definition for MERS-CoV infection (28). All 5 pediatric patients presenting with fever and/or respiratory symptoms underwent radiological imaging: in 3 out of 5 cases, chest X-rays showed bilateral diffuse infiltrate, relating to more severe clinical conditions and in 2 out of 3 to death. Asymptomatic pediatric patients did not undertake any radiological imagine (26).
3.5 Treatment
No conclusive data are available about therapy for MERS in the pediatric population. As we previously stated, only 35 pediatric cases were reported; out of these cases, only 3 required treatment. Empirically, both antibiotics and antivirals were used, as well as intravenous steroid therapy; inotropic support was attempted in patients accessing PICU, but the outcome was poor (25,26).
4. Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)
4.1 Background and epidemiology
In late December 2019, an outbreak of pneumonia with unknown aetiology was found in Wuhan, Hubei province of China (29). A previously unknown coronavirus was identified as the causative agent and it was given the designation 2019-nCoV (2019 novel coronavirus) (30). COVID-19 is the name given by WHO on February 11th 2020 to the disease caused by the novel coronavirus; the International Committee on Taxonomy of Viruses (ICTV) named the new SARS-CoV-2 (31). Virus spread rapidly to other countries and the WHO classified the outbreak as a pandemic on March 11th 2020 (32).
The complete genomic sequences of SARS-CoV-2 were characterized from early infected individuals. Genome size ranges from 29891 to 29903 nucleotides. Based on sequence analysis SARS-CoV-2 belongs to genus Betacoronavirus (Figure 1) (33,34). SARS-CoV-2 shares 79% nucleotide sequence identity to SARS-CoV and around 50% to MERS-CoV (33). Phylogenetic and comparative analyses showed 96.2% genomic sequence homology with bat CoV RaTG13 collected from Yunna province, China, and 88% identity with bat-SL-CoVZC45 and bat-SL-CoVZXC21, two bat-derived SARS-like CoVs collected in 2018 in Zhoushan, Eastern China (30,33,35). Evolutionary analyses suggested that bats are the most likely natural reservoir of SARS-CoV-2. Intermediate hosts sold at the Huanan Seafood Wholesale Market, with which many of the initial cases of COVID-19 were associated, might have been the intermediate host between bat and human and have been reported to be snakes, minks, pangolins and others (37-39). However, the evolutionary pathway remains to be fully established.
A key stage in the viral replication pathway is binding of viral particles to host surface cellular receptors, playing an important role in determining host and tissue tropism and pathogenesis. The SARS-CoV-2 genome encodes for both structural and non-structural proteins (34,40). Its cell entry is mediated by the transmembrane surface-located spike (S) glycoprotein (41). The S glycoprotein of SARS-CoV-2 can be further divided into two functional subunits: an N-terminal S1 subunit responsible for virus–receptor binding and a membrane-embedded C-terminal S2 subunit mediating viral membrane fusion. S1 is further divided into an N-terminal domain (NTD) and a C-terminal domain (CTD), both of which can function as a receptor-binding entity. In most CoVs, the receptor-binding domain (RBD) is located within the C-terminal domain (CTD) of the S1 domain (2,41-43).
As for SARS-CoV, the human metallopeptidase angiotensin-converting enzyme 2 (hACE2) has been identified as the functional receptor for SARS-CoV-2 (34,44). Both SARS-CoV C-terminal domain (CTD) and SARS-CoV-2 C-terminal domain (CTD) have been recognized as the key region for interaction with the hACE2 receptor. SARS-CoV-2- CTD and SARS-CoV-2-CTD show 73.9% sequence identity, the S2 regions exhibit 90% sequence identity. Engagement of hACE2 by SARS-CoV-2 is 4-fold higher than that observed for the SARS-CoV C-terminal domain (CTD). SARS-CoV-2 spike (S) protein S2 subunit plays a key role in virus-cell membrane fusion and the consequent release of viral genome RNA into cell cytoplasm (45,46).
The pathophysiology of COVID-19 is under investigation. According to available epidemiological data, SARS-CoV-2 infected patients, with or without clinical symptoms, are the main sources of the infection (47).
SARS-CoV-2 is highly infectious. In epidemiology, the basic reproduction number (R0), also called basic reproductive ratio, is an indicator of the contagiousness or transmissibility of infectious agents: it is defined as the number of secondary cases which one case would directly generate in a completely susceptible population, reflecting not only the rate of transmission of the virus, but also the potential and severity of the outbreak of infectious diseases. When R0 is more than 1, the infection can spread between people and there may be an outbreak or epidemic (48,49). R0 value changes during an epidemic being called the effective reproduction number (Re). R0 value of SARS-CoV-2 has been estimated by many researchers, ranging from 1.5 to 6.49 depending on the estimation method used (50-52).
SARS-CoV-2 enters cells via the hACE2 receptor (34,44). HACE2-expressing cells found to be susceptible to infection with SARS-CoV-2 include type II alveolar epithelial cells in lungs, esophagus upper and stratified epithelial cells, enterocytes of the small intestine and the colon, cholangiocytes, myocardial cells, kidney proximal tubule cells, and bladder urothelial cells (53-56).
SARS-CoV-2 is transmitted mainly through respiratory secretions and droplets containing the virus during coughing, sneezing and loud talking; therefore, natural infection mainly occurs through the respiratory route (47,57-59). Prolonged exposure in closed environments to high concentrations of aerosols may facilitate transmission; air-tightness of the environment and the density of viruses per unit volume can also affect the spread of SARS-CoV-2 (59). Virus-containing droplets propelled in the air are further deposited on the mucous membranes of the mouth, nose or eyes of nearby persons; frequent touching of the mouth or nose, direct contact with eye or shaking hands with an infected person are other ways to transmit the virus (60,61). Another major type of indirect contact is via infected fomites (61,62). Decay rates of SARS-CoV-2 has been estimated on different environmental conditions: the longest surface viability has been observed on stainless steel and plastic, with an estimated median half-life of 5.6 hours and 6.8 hours respectively. No viable SARS-CoV-2 was measured after 8 hours on cardboard and after 4 hours on copper (59).
According to the current data, exposure to faecal-contaminated environments may offer a potential route of SARS-CoV-2 infection (55,63,64). SARS-CoV-2 RNA is detectable in stool samples of infected patients with a median duration time between onset of symptoms and the first positive RT-PCR test result for viral of 11 (7-13) days (65,66). Faecal viral shedding in children with COVID-19 can last as long as 2–4 weeks, raising the question whether the gastrointestinal tract may be another site of viral replication (67). Faecal-mucosal transmission of SARS-CoV-2 can occur when touching of mouth, nose or eyes with contaminated hands or through the faecal-aerosol-respiratory pathway with exposition to faecal-contaminated environments (68).
As regards prevalence of SARS-CoV-2 infection in children, there is a lack of information about the exact amount of pediatric cases worldwide. We here report data regarding the Italian experience. Up to May 20th, 4,244 cases of SARS-CoV-2 in the pediatric age (0-17) have been reported in Italy, accounting for 2.1% of all cases (Figure 2) (69).
Figure 2.
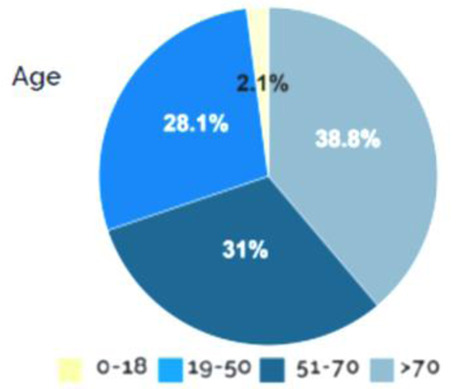
Confirmed COVID-19 cases at pediatric age (0-18), adapted from Istituto Superiore di Sanità (69)
Most pediatric cases belong to family clusters and were identified through family screening (70). The incubation period in children is reported to be slightly shorter than the one in adults, ranging from 1 to 14 days, with 3 to 7 days on average (71).
4.2 Clinical features
Children of all ages have proven to be susceptible to SARS-CoV-2 infection, including newborns and infants. Available data on adults show a significantly higher prevalence in males; accordingly, SARS-CoV-2 infection in children seems to affect predominantly males, although this difference is not statistically relevant and has not been described in other studies (69,72). In two of the main reports of SARS-Cov-2 pediatric cases in China, the median age was 7-year-old and 6.7-year-old respectively (72,73). Table 1, Figure 3 and Figure 4 reassume demographic features of Italian pediatric cases (69).
Table 1.
Distribution of pediatric cases according to age group and sex, adapted from Istituto Superiore di Sanità (69)
| Age group (year-old) | N. cases | % | Females | Males | Not known | % Females | % Males |
| 0-1 | 566 | 13.3 | 254 | 309 | 3 | 45.1 | 54.9 |
| 2-6 | 745 | 17.6 | 344 | 401 | NA | 46.2 | 53.8 |
| 7-17 | 2,933 | 69.1 | 1,464 | 1,469 | NA | 49.4 | 50.1 |
| <18 | 4,244 | 100.0 | 2,062 | 2,179 | 3 | 48.6 | 51.4 |
Figure 3.
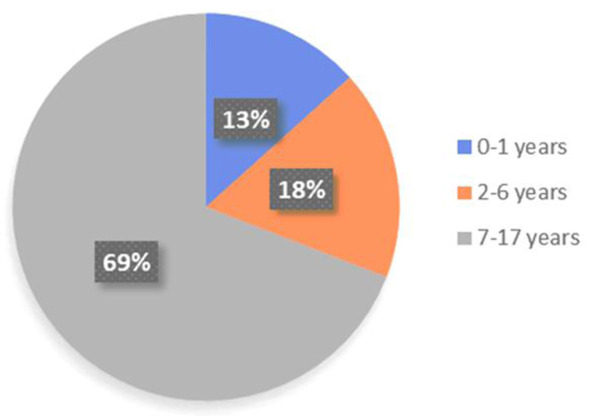
Distribution in group ages, adapted from Istituto Superiore di Sanità (69)
Figure 4.
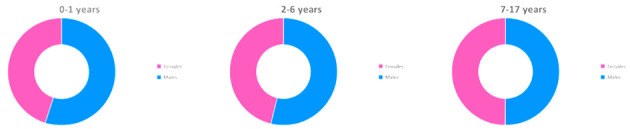
Female versus male pediatric cases in different age groups, adapted from Istituto Superiore di Sanità (69)
Since the start of the pandemic, it has been known that clinical presentation in the pediatric age includes fever and cough, as reported in a small series of 6 cases detected in January in Wuhan, China, all presenting with such symptoms (74). Following reports have confirmed this early finding. Overall, the most commonly reported symptoms are fever and cough, with reported rates ranging between 40% and 80%, according to different authors (73,75,76). Other common symptoms are tachypnoea (28.7%), rhinorrhoea (7.6%), pharyngeal erythema (46.4%) and sinus tachycardia (42.1%) (73). Differently from adult population, all pediatric records show a higher prevalence of gastrointestinal symptoms: diarrhoea and vomiting have been described in 10% to 15% of cases (77).
In addition, attention has been recently raised to a likely correlation between SARS-CoV-2 infection and hyperinflammatory status resembling atypical Kawasaki disease. The National Health Service (NHS) in UK has given national and international alert after dealing with an unprecedented cluster of children presenting with hyperinflammatory shock. An initial report included 8 children, presenting with high fever (>39°C) for 4 to 5 days, abdominal pain with vomiting and diarrhoea, rash and conjunctivitis (78). The majority developed some degree of heart involvement, requiring access to PICU and inotropic support with amines. Overall, 7 out of 8 recovered and are now subjects to ongoing follow-up for coronary abnormalities; one developed arrhythmia with refractory shock and died from a major cerebrovascular infarct. All cases initially tested negative for SARS-CoV-2, but 4 out of 8 were likely to have COVID-19 after exposure to close family contacts. Moreover, no other infectious agent was found in 7 out of 8; in one Adenovirus and Enterovirus were isolated. At subsequent analysis, 2 children tested positive for SARS-CoV-2 (including the one who died, whose test was performed post-mortem). The association between SARS-CoV-2 infection and Kawasaki disease shock syndrome appears to be promising, but greater evidence is currently missing.
Overall, all available data suggest that COVID-19 clinical presentation in children is milder than that in the adult population. As of today, Dong et al. collected the largest number of cases at pediatric age: based upon clinical features, laboratory testing and chest X-ray imaging, they defined the severity of Covid-19, thus identifying 5 stages of severity (72). Out of 2,134 children, more than 90% were completely asymptomatic or presented with mild or moderate disease. Severe and critical cases accounted for 5.2% and 0.6%, respectively. Moreover, the authors further divided children based upon age and found that severity was highest in younger children (0-5 years old) and especially in those < 1-year-old (10.6%), slowly decreasing with increasing age. In the Chinese experience, access to intensive care units has been required for approximately 2% of pediatric cases, with much higher rates in adult (79). Similar rates have been reported by the American CDC, which has recorded a 29% rate of hospitalisation and a 0.58% to 2% rate of access to PICU (80).
As regards mortality, mortality rate in children has been much lower than the one that has been described in adults. The first reported death was a 10-month-old child presenting with intussusception, later complicating with multiorgan failure and dying 4 weeks after admission (73). A second fatality was reported in China, a 14 year old boy who died on February 7th 2020 in Hubei (81). As of March 8th 2020, these remained the only 2 reported fatality in children in China. Accordingly, the US CDC and the Italian ISS have counted 3 and 4 deaths among the reported pediatric cases, respectively. In particular, all 4 fatalities in the Italian registry occurred in younger children (age range 0-9, 1 male and 3 females), with a lethality rate of 0.2% for such age range (Table 2) (69). No further information is available on comorbidities or other risk factors.
Table 2.
Distribution of reported deaths according to age group, adapted from Istituto Superiore di Sanità (69)
| Age group (year-old) | Total | ||||
| N. cases | % cases per age group | N. deaths | % deaths per age group | Lethality (%) | |
| 0-9 | 1,851 | 0.8 | 4 | 0 | 0.2 |
| 10-19 | 3,312 | 1.5 | 0 | 0 | 0 |
4.3 Laboratory findings
Data on laboratory findings in SARS-CoV-2 infection in children are scarce. As we depicted above, most pediatric cases have been asymptomatic or mildly to moderately symptomatic and therefore have not required any laboratory testing. One large meta-analysis has collected data from 12 different studies, for a total amount of 66 cases (82). In most cases, the authors did not find any relevant laboratory findings, with normal leukocyte count (69.2%). Neutrophilia and neutropenia were rare (4.6% and 6% respectively). CRP and PCT were increased in 13.6% and 10.6% respectively. Early in February 2020, a report of 20 pediatric cases suggested that PCT seemingly increases in children with Covid-19, miming bacterial infections, but this finding has not been confirmed in any of the following studies (77).
4.4 Chest computed tomography (CT)
The benchmark for the diagnosis of SARS-CoV-2 infection in adults, along with naso-pharyngeal swab, is chest CT scan. It is now established that sensitivity of CT scan is even greater than that of RT-PCR on respiratory or blood specimens, enhancing the role of imaging in diagnosing patients with clinical manifestations of COVID-19 but negative samples (83). As regards pediatric patients, a few studies have examined the use of chest CT scan. Along with CT features in adults, ground glass opacities (GOO) represent the most commonly seen sign. GOO has been detected in a third of 171 children with diagnosed SARS-Cov-2 infection reported by Lu et al (73). This is consistent with data reported by other authors. Li et al. reviewed a case series of 5 patients and found patchy GOO in 3 (84). Remarkably, 2 out of 3 patients with positive chest CT scan were asymptomatic, proving that the correlation between clinical and radiological findings remains often unclear. All 3 scans normalised at follow-up, held between 5 and 7 days after initial chest CT. Xia et al. obtained CT scan of 20 pediatric patients (age 0-14 years old) with SARS-Cov-2 infection confirmed by RT-PCR on pharyngeal swab (77). At an early stage, 16 out of 20 patients had positive chest CT scan, with unilateral (6/20, 30%) or bilateral (10/20, 50%) pulmonary lesions. Overall, 4 patients had imaging within range. Accordingly, the most common radiological sign was GOO (12/20, 60%), followed by consolidation with surrounding halo sign (10/20, 50%), fine mesh shadow (4/20, 20%) and tiny nodules (3/20, 15%). All children showed subpleural lesions with localised inflammatory infiltration.
Compared to those in adults, CT characteristics are more diverse and less severe, accordingly with slighter clinical manifestations. Therefore, CT shows localised ground glass opacities (GOO) extent, lower GOO attenuation and relatively rare interlobular septal thickening (85). As for the application of CT in children, experts agree that its use in Covid-19 should be most limited due to both the slighter clinical manifestations and the greater radiological risk in this specific age group. At the same time though, CT cannot be usefully replaced by chest X-ray, that has not shown enough sensitivity nor specificity.
4.5 Treatment
As of today, there is no standard therapy to treat COVID-19 in children. Therapeutic strategies have been borrowed from experience with adults. Both antivirals and anti-inflammatory drugs (e.g. corticosteroids and/or hydroxychloroquine) have been used in critical symptomatic cases, but the efficacy of such treatment remains to be established.
5. Conclusions
SARS-CoV, MERS-CoV and SARS-CoV-2 infections seem to affect children less commonly and less severely as compared with adults. Since children are often asymptomatic, they are often not tested, leading to an underestimate of the true numbers infected. Most of the documented infections belong to family clusters, so the importance of children in transmitting the virus remains uncertain. Like in SARS and MERS infection, there is the possibility that children are not an important reservoir for novel CoVs and this may have important implications for school attendance (86).
In paediatric age, clinical features seem similar for all CoVs: fever, rhinitis, otitis, pharyngitis as well as bronchitis, pneumonia and gastrointestinal symptoms. The latter are much more common in children than adults. About laboratory findings, the white blood cell count is typically normal or reduced with decreased neutrophil and/or lymphocyte counts, especially in SARS-CoV infection. There is no laboratory data available regarding MERS. Thrombocytopenia may occur. C-reactive protein and procalcitonin levels are often normal. In severe cases, elevated liver enzymes lactate dehydrogenase levels, as well as an abnormal coagulation and elevated D-dimers have been reported (87,88).
On chest radiography, children mostly show bilateral patchy airspace consolidations often at the periphery of the lungs, peri-bronchial thickening and ground-glass opacities. Chest CT changes observed in children infected with SARS-CoV-2 include bilateral multiple patchy, nodular GOO, speckled ground-glass opacities and/or infiltrating shadows in the middle and outer zone of the lung or under the pleura (89,84).
There are no studies on treatment outcomes for MERS-CoV. In the absence of specific antiviral drugs for CoVs, broad-spectrum antiviral drugs, such as interferon alpha and beta or ribavirin in association with corticosteroid were used for the treatment of SARS-CoV also in children. No approved protocol is currently available on the treatment of COVID-19 in children, although studies on the use of remdesivir will soon be performed. Cases of use in combination of antibiotics and antivirals are reported in the literature. The use of face masks, implementation of hygiene and social distancing measures are recommended.
No deaths in children have been reported for SARS-CoV. Two cases of death by MERS are reported. Some cases of deaths among children infected by SARS-CoV-2 are reported, but we currently do not have adequate information on comorbidities.
In the last weeks, an apparent increase of children with Kawasaki-like disease has been reported. (90) (91). These children present an incomplete or atypical clinical disease characterized by a tendency to a rapid evolution towards a macrophage activation syndrome with the need for second-line treatments and in some cases with the need for hospitalization in PICU. A part of these children present, or presented in the weeks prior to onset, a positive swab for SARS-CoV-2 infection or otherwise had contact with affected patients. It is not yet clear if it is a true Kawasaki disease induced by viral infection or if the forms that are being observed are instead a systemic inflammatory manifestation of the infection, similar but not entirely identical to one classic Kawasaki disease. The cytokine storm typical of COVID-19 actually has a substantial overlap with that of Kawasaki disease, with high levels of IL-1, IL-6 and TNF-alpha, and the presence of circulating activated macrophages can also characterize both diseases (92,93).
While waiting for an effective against SARS-CoV-2, although the infection in children seems to have a mild course compared to adults, further prevalence studies in paediatric age are needed, also to determine the role of children in different age groups in the spread of the infection.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Lai MM. Coronavirus: organization, replication and expression of genome. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. doi:10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- 2.Masters PS, Perlman S, et al. Coronaviridae. In: Knipe DM, Howley PM, Cohen JI, editors. Fields Virology. 6th ed. Vol. 2. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2013. p. 825. [Google Scholar]
- 3.Woo PC, Huang Y, Lau SK, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804–1820. doi: 10.3390/v2081803. doi:10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James Maclachlan N, Edward J Dubovi. Fenner’s Veterinary Virology. 2017 Chapter 24, p. 435. [Google Scholar]
- 5.Su S, Wong G, Shi W, et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. doi:10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at http://www.who.int/csr/sars/country/table20030923/en/print.html ; last accessed March 30 2020. [Google Scholar]
- 7.World Health Organisation. Severe Acute Respiratory Syndrome Surveillance Team. Personal Communication [Google Scholar]
- 8.Chan WM, Kwan YW, Wan HS, Leung CW, Chiu MC. Epidemiologic linkage and public health implication of a cluster of severe acute respiratory syndrome in an extended family. Pediatr Infect Dis J. 2004;23(12):1156–1159. [PubMed] [Google Scholar]
- 9.Hui DS, Sung JJ. Severe acute respiratory syndrome. Chest. 2003;124(1):12–15. doi: 10.1378/chest.124.1.12. doi:10.1378/chest.124.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CW, Chiu WK, Chan CC, Chow EY, Cheung HM, Ip PL. Osteonecrosis in children with severe acute respiratory syndrome. Pediatr Infect Dis J. 2004;23(9):888–890. doi: 10.1097/01.inf.0000137570.37856.ea. doi:10.1097/01.inf.0000137570.37856.ea. [DOI] [PubMed] [Google Scholar]
- 11.Chiu WK, Cheung PC, Ng KL, et al. Severe acute respiratory syndrome in children: experience in a regional hospital in Hong Kong. Pediatr Crit Care Med. 2003;4(3):279–283. doi: 10.1097/01.PCC.0000077079.42302.81. doi:10.1097/01.PCC.0000077079.42302.81. [DOI] [PubMed] [Google Scholar]
- 12.Leung CW, Kwan YW, Ko PW, et al. Severe acute respiratory syndrome among children. Pediatrics. 2004;113(6):e535–e543. doi: 10.1542/peds.113.6.e535. doi:10.1542/peds.113.6.e535. [DOI] [PubMed] [Google Scholar]
- 13.Ng PC, Lam CW, Li AM, et al. Inflammatory cytokine profile in children with severe acute respiratory syndrome. Pediatrics. 2004;113(1 Pt 1):e7–e14. doi: 10.1542/peds.113.1.e7. doi:10.1542/peds.113.1.e7. [DOI] [PubMed] [Google Scholar]
- 14.Leung CW, Chiu WK. Clinical picture, diagnosis, treatment and outcome of severe acute respiratory syndrome (SARS) in children. Paediatr Respir Rev. 2004;5(4):275–288. doi: 10.1016/j.prrv.2004.07.010. doi:10.1016/j.prrv.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bitnun A, Allen U, Heurter H, et al. Children hospitalized with severe acute respiratory syndrome-related illness in Toronto. Pediatrics. 2003;112(4):e261. doi: 10.1542/peds.112.4.e261. doi:10.1542/peds.112.4.e261. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Chen X, Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med. 2003;349(5):508–509. doi: 10.1056/NEJM200307313490520. doi:10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 17.Tsou IY, Loh LE, Kaw GJ, Chan I, Chee TS. Severe acute respiratory syndrome (SARS) in a paediatric cluster in Singapore. Pediatr Radiol. 2004;34(1):43–46. doi: 10.1007/s00247-003-1042-2. doi:10.1007/s00247-003-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung CW, Li CK. PMH/PWH interim guideline on the management of children with SARS. HK J Paediatr. 2003;8:168–9. [Google Scholar]
- 19.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia [published correction appears in N Engl J Med. 2013 Jul 25;369(4):394] N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. doi:10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organisation. Middle East respiratory syndrome coronavirus (MERS-CoV) – The Kingdom of Saudi Arabia. Available at: https://www.who.int/csr/don/24-february-2020-mers-saudi-arabia/en/ ; last accessed March 4 2020. [Google Scholar]
- 21.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. doi:10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartenfeld M, Griese S, Uyeki T, Gerber SI, Peacock G. Middle East Respiratory Syndrome Coronavirus and Children. Clin Pediatr (Phila) 2017;56(2):187–189. doi: 10.1177/0009922816678820. doi:10.1177/0009922816678820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20(5):469–474. doi: 10.1111/1469-0691.12562. doi:10.1111/1469-0691.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khuri-Bulos N, Payne DC, Lu X, et al. Middle East respiratory syndrome coronavirus not detected in children hospitalized with acute respiratory illness in Amman, Jordan, March 2010 to September 2012. Clin Microbiol Infect. 2014;20(7):678–682. doi: 10.1111/1469-0691.12438. doi:10.1111/1469-0691.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33(9):904–906. doi: 10.1097/INF.0000000000000325. doi:10.1097/INF.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 26.Thabet F, Chehab M, Bafaqih H, Al Mohaimeed S. Middle East respiratory syndrome coronavirus in children. Saudi Med J. 2015;36(4):484–486. doi: 10.15537/smj.2015.4.10243. doi:10.15537/smj.2015.4.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. doi:10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organisation. Middle East respiratory syndrome coronavirus: case definition for reporting to WHO. Available at: https://www.who.int/csr/disease/coronavirus_infections/case_definition/en/ ; last accessed March 4 2020. [Google Scholar]
- 29.World Health Organisation. WHO Statement Regarding Cluster of Pneumonia Cases in wuhan, China. Available at: https://www.who.int/china/news/detail/09-01-2020-who-statement ; last accessed March 4 2020. [Google Scholar]
- 30.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Coronavirus disease (COVID-19) outbreak. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 ; last accessed March 4 2020. [Google Scholar]
- 32.World Health Organisation. WHO Virtual press conference on COVID-19. March 11, 2020. Available at: https://www.who.int/docs/defaultsource/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final11mar2020.pdf?sfvrsn=cb432bb3_2 ; last accessed March 16 2020. [Google Scholar]
- 33.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. doi:10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. doi:10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren LL, Wang YM, Wu ZQ, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. doi:10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Q, Li M, Wang C, Wang P, Fang Z, Tan J, Wu S, Xiao Y, Zhu H. Host and infectivity prediction of Wuhan 2019 novel coronavirus using deep learning algorithm. bioRxiv. 2020 [Google Scholar]
- 37.Ji W, Wang W, Zhao X, Zai J, Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao KP, Zhai JP, Feng YY, et al. Isolation and Characterization of 2019 -nCoV-like Coronavirus from Malayan Pangolins. bioRxiv. 2020 02.17.951335. [Google Scholar]
- 39.Lam T, Shum M, Zhu HZ, et al. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. bioRxiv. 2020 02.13.945485. [Google Scholar]
- 40.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. doi:10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu G, Wang Q, Gao GF. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23(8):468–478. doi: 10.1016/j.tim.2015.06.003. doi:10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. doi:10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du L, Yang Y, Zhou Y, Lu L, Li F, Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017;21(2):131–143. doi: 10.1080/14728222.2017.1271415. doi:10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. doi:10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Zhang Y, Wu L, et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181(4):894–904.e9. doi: 10.1016/j.cell.2020.03.045. doi:10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia S, Zhu Y, Liu M, et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein [published online ahead of print, 2020 Feb 11] Cell Mol Immunol. 2020:1–3. doi: 10.1038/s41423-020-0374-2. doi:10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. doi:10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2(1):23–41. doi: 10.1177/096228029300200103. doi:10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- 49.Ridenhour B, Kowalik JM, Shay DK. Unraveling R0: considerations for public health applications. Am J Public Health. 2014;104(2):e32–e41. doi: 10.2105/AJPH.2013.301704. doi:10.2105/AJPH.2013.301704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report-46, 7th March 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4 ; last accessed March 16 2020. [Google Scholar]
- 51.Lai A, Bergna A, Acciarri C, Galli M, Zehender G. Early phylogenetic estimate of the effective reproduction number of SARS-CoV-2 [published online ahead of print, 2020 Feb 25] J Med Virol. 2020 doi: 10.1002/jmv.25723. 10.1002/jmv.25723. doi:10.1002/jmv.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa021. taaa021. doi:10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. doi:10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. doi:10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. Preprint at https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1. (2020) [Google Scholar]
- 56.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Preprint at https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1. (2020) [Google Scholar]
- 57.Lee PI, Hsueh PR. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV [published online ahead of print, 2020 Feb 4] J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.02.001. doi:10.1016/j.jmii.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. doi:10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. doi:10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. doi:10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun CB, Wang YY, Liu GH, Liu Z. Role of the Eye in Transmitting Human Coronavirus: What We Know and What We Do Not Know. Front Public Health. 2020;8:155. doi: 10.3389/fpubh.2020.00155. Published 2020 Apr 24. doi:10.3389/fpubh.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. doi:10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020 [published online ahead of print, 2020 Apr 10] Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200885. 10.3201/eid2607.200885. doi:10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. doi:10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients [published online ahead of print, 2020 Apr 3] J Med Virol. 2020 doi: 10.1002/jmv.25825. 10.1002/jmv.25825. doi:10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 66.Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. doi:10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai J, Xu J, Lin D, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features [published online ahead of print, 2020 Feb 28] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa198. ciaa198. doi:10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient [published online ahead of print, 2020 Mar 4] JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. doi:10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Istituto Superiore di Sanità. Epidemia COVID-19. Aggiornamento nazionale. 20 maggio 2020 – ore 16.00. Available at: https://www.epicentro.iss.it/coronavirus/bollettino/bollettino-sorveglianza-integrata-covid-19_20-maggio-2020 ; last accessed May 24 2020. [Google Scholar]
- 70.Yang P, Liu P, Li D, Zhao D. Corona Virus Disease 2019, a growing threat to children? J Infect. 2020;80(6):671–693. doi: 10.1016/j.jinf.2020.02.024. doi:10.1016/j.jinf.2020.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. doi:10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong Y, Mo XI, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;16:16. doi:10.1542/peds.2020-0702. [Google Scholar]
- 73.Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. doi:10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Zhang Q, Chen J, et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370–1371. doi: 10.1056/NEJMc2003717. doi:10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng F, Liao C, Fan QH, et al. Clinical Characteristics of Children with Coronavirus Disease 2019 in Hubei, China. Curr Med Sci. 2020;40(2):275–280. doi: 10.1007/s11596-020-2172-6. doi:10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai J, Xu J, Lin D, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features [published online ahead of print, 2020 Feb 28] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa198. ciaa198. doi:10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55(5):1169–1174. doi: 10.1002/ppul.24718. doi:10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. doi:10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. doi:10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Centers for Disease Control and Prevention. Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020. doi: 10.15585/mmwr.mm6914e4. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6914e4.htm#F2_down ; last accessed May 24 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 Among Children in China [published online ahead of print, 2020 Mar 16] Pediatrics. 2020 e20200702. doi:10.1542/peds.2020-0702. [Google Scholar]
- 82.Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019 [published online ahead of print, 2020 Mar 16] Clin Chem Lab Med. doi: 10.1515/cclm-2020-0272. 2020;/j/cclm.ahead-of-print/cclm-2020-0272/cclm-2020-0272.xml. doi:10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 83.Fang Y, Zhang H, Xie J, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR [published online ahead of print, 2020 Feb 19] Radiology. 2020 doi: 10.1148/radiol.2020200432. 200432. doi:10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020;50(6):796–799. doi: 10.1007/s00247-020-04656-7. doi:10.1007/s00247-020-04656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duan YN, Zhu YQ, Tang LL, Qin J. CT features of novel coronavirus pneumonia (COVID-19) in children [published online ahead of print, 2020 Apr 14] Eur Radiol. 2020:1–7. doi: 10.1007/s00330-020-06860-3. doi:10.1007/s00330-020-06860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. doi:10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hon KL, Leung CW, Cheng WT, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361(9370):1701–1703. doi: 10.1016/S0140-6736(03)13364-8. doi:10.1016/s0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus [published online ahead of print, 2020 Feb 5] World J Pediatr. 2020:1–7. doi: 10.1007/s12519-020-00345-5. doi:10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsou IY, Loh LE, Kaw GJ, Chan I, Chee TS. Severe acute respiratory syndrome (SARS) in a paediatric cluster in Singapore. Pediatr Radiol. 2004;34(1):43–46. doi: 10.1007/s00247-003-1042-2. doi:10.1007/s00247-003-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus [published online ahead of print, 2020 Feb 5] World J Pediatr. 2020:1–7. doi: 10.1007/s12519-020-00345-5. doi:10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case [published online ahead of print, 2020 Apr 7] Hosp Pediatr. 2020 doi: 10.1542/hpeds.2020-0123. hpeds.2020-0123. doi:10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 92.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure [published online ahead of print, 2020 Apr 17] Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. S1931-3128(20)30236-5. doi:10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe Covid-19 patients. Natl Sci Rev. 2020 Mar 13 doi: 10.1093/nsr/nwaa041. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]


