To the Editor,
recent studies indicated a clear decrease in peripheral lymphocytes and natural killer cells (NK) in Covid-19 patients (1). The lymphodepletion induced by Sars-Cov-2 has a pivotal diagnostic role and represents a valid prognostic tool. Total lymphocytes, CD4+ T cells, CD8+ T cells, B cells and Natural Killer (NK) cells decreased in Covid-19 patients and severe cases had a lower level than mild cases. In recovered Covid-19 patients is documented an increase in lymphocytes count and related subsets. No further significant changes are detected in unresponsive patients.
In Covid-19 infected patients a clinical constellation of cytokine storm, respiratory failure and eventually death is reminiscent of a “hyperferritinemic syndrome”. The inflammatory microenvironment may shift the balance to reduce NK cell effector functions in both Covid-19 and inflammatory forms of secondary hyperferritinemic syndrome. Elevated IL-6 and IL-10 levels, as observed in Sars-CoV2-infected patients have the capacity to directly reduce NK cell cytotoxicity and increase the expression of NKG2A, which is important in killing virally infected cells (2).
It has been shown that Sars-CoV2 binding to ACE2 may infect NK cells to suppress their functions, as NK cells express angiotensin converting enzyme 2 (ACE2). Although not published in Covid-19, other RNA viruses that cause acute pulmonary infections promote NK cells apoptosis and reduce their cytotoxicity following their infection.
Sars-CoV2 and the subsequent immune cell inflammatory responses suppress NK cells cytotoxicity which promotes a severe cytokine release syndrome, and inadequate immune responses.
NK/T-cell lymphoma and NK-cell leukemias are aggressive malignancies. NK/T-cell lymphomas are almost exclusively extranodal. Lymphomas occur commonly in the nasal and upper aerodigestive region. Rare cases are disseminated with lymphoadenopathy, hepatosplenomegaly, and a leukemic phase. Neoplastic cells are surface CD3-, cytoplasmatic CD3+, CD56+ cytotoxic molecule positive and Epstein Barr virus (EBV) positive with germline T-cell receptor gene. EBV infection is latent and not lytic in the lymphoma cells, and EBV-DNA is an accurate biomarker of tumor load. Serial EBV-DNA monitoring is useful for assessing response and detecting recurrence during chemiotherapy.
Herein we report an interesting case of a transient remission of refractory NK/T-cell lymphoma during Covid-19 infection and the relapse after Covid-19 resolution.
An african 20-years-old male with a medical history of relapsed/refractory NK/T-cell lymphoma associated with Epstein-Barr virus (EBV) and autoimmune hemolytic anemia (AIHA), presented with a 5-days history of fatigue, fever, dry cough and dyspnea. The NK/T-cell malignancy was refractory to multiple immuno-chemiotherapy protocols, (Rituximab, Pembrolizumab, l-asparaginase, SMILE, DDGP and CHOP chemiotherapy). He was febrile (T: 38°C), polypnoeic, the oxygen saturation in ambient air was 93%. Breath sounds were diminished bilaterally with bi-basilar crackles. The abdomen was tender due to massive hepatosplenomegaly. Chest tomography showed diffuse bilateral sub-pleuric ground-glass opacities. Laboratory tests revealed leukocytosis ( 12600 mm3), severe anemia (Hemoglobin: 1,7 g/dl), thrombocytopenia (PLT: 63000 mm3). The C-reactive protein level was elevated (CRP: 178 mg/l). Haemolytic markers were abnormal: lactic-dihydrogenase (LDH: 3071 U/L), indirect bilirubin (2,1 mg/dl). Oropharyngeal swab for Covid-19 testing resulted positive.
Repeated red- blood cell transfusions and methylprednisolone at rate of 1 mg/kg of body weight per day were administered, other than oxygen, intravenous levofloxacin and supportive therapy. No antiviral or cloroquine drugs were administered.
During the first ten days, despite the administration of eight packs of red blood cells and steroid therapy, there was only partial increase in hemoglobin level, platelets count and hemolytic markers (Fig. 1a). Any further transfusion and steroid therapy was stopped. Curiously, however, there was no worsening of Covid-19 pneumonia signs and symptoms. Unexpectedly on day 11, we noticed a spontaneous steady clinical improvement. The hemoglobin level reached a peak on day 20 (Hb 9.5 gr/dL) (Fig. 1a). Similarly, the hemolytic markers and platelets count followed the same trend. A reduction of WBC count, NK cells and was noticed. Peripheral blood flow cytometry detected inverted CD4/CD8 ratio, remarkable reduction in the clonal NK cells population (CD45-, CD3-, CD5-, CD34-, CD2+,CD7+, CD56+, CD16dim, CD38+), from 70% to 4,2%. There was increased proportion of cells with cytotoxic potential including human leucocyte antigen-antigen D related (HLA-DR) CD8+ T cells and double-positive T-cells (DPT). Likewise, plasma EBV-DNA, a sensitive surrogate of lymphoma load, showed a significant drop in values, from 229876 copies/ml to 495 copies/ml (Fig. 1b). Spleen enlargement was reduced on ultrasound examination (from 22 cm to 16 cm).
Figure 1.
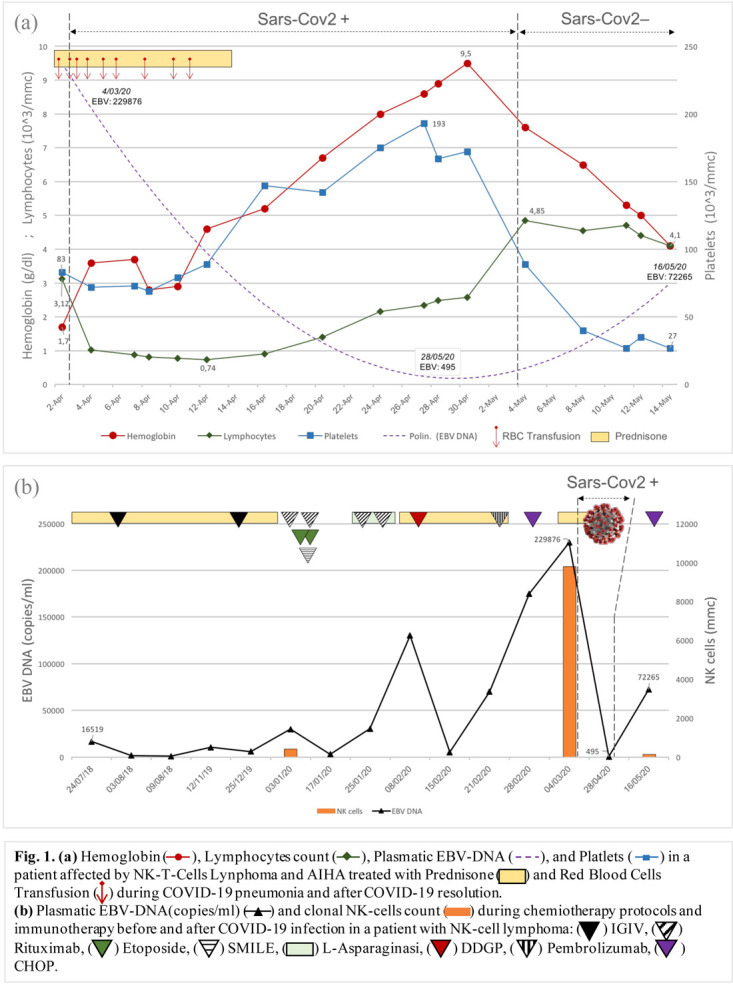
The unexpected clinical and laboratory improvement suggested a remission of NK lymphoma during Covid-19 infection, as if the Sars-CoV2 had played some oncolytic activity. On day 34, the subsequent recovery from Covid-19 infection detected by negative oropharyngeal swab was followed by NK/T-cell lymphoma relapse. Recurrence of hemolytic anemia, fever, spleen enlargement and rise of NK cells count was observed. Furthermore, plasma EBV-DNA raised from less than 500 copies/ml to more than 72250 copies/ml (Fig1b).
Antitumor immunomodulatory effects causing lymphodepletion is well recognized when oncolytic viruses was engineered to express interleukin-2 (IL-2) and tumor necrosis factor alpha (TNFa) in adoptive T cell therapy (ACT) strategies. Oncolytic viruses used instead of lymphodepleting preconditionating high-dose chemiotherapy can achieve antitumor effect resulting in lymphocytic cells depletion. Similarly, Sars-Cov2 induced a release of large amount of pro-inflammatory cytokines, including interleukin 6 (IL-6), TNF-a, IL-2 (3). IL-2 and TNF-a can recruit NK and T cells to the tumor when produced from engineered oncolytic viruses.
The temporal sequence in this case suggests but not prove, that SARS-CoV2 was a possible causal factor in the decrease number and exhaustion of NK cells resulting in an evident improvement of clinical and laboratoristic features related to NK/T cell lymphoma.
In conclusion, our observation indicates that Covid-19 infection might have played a crucial role in transient remission of NK/T cell lymphoma as shown by reduction of NK neoplastic cells and plasmatic EBV-DNA drop. Tumor associated infection (EBV-related) and possibly preexisting autoimmunity (AHIA) could compound propoensity for spontaneous remission (4,5).
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 Pneumonia. J Infect Dis. 2020 Mar 30 doi: 10.1093/infdis/jiaa150. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020 Mar 17 doi: 10.1038/s41423-020-0402-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos JM, Cervera-Carrascon V, Havunen R, et al. Adenovirus Coding for Interleukin-2 and Tumor Necrosis Factor AlphaReplaces Lymphodepleting Chemotherapy in Adoptive T Cell Therapy. Mol Ther. 2018 Sep 5;26(9):2243–2254. doi: 10.1016/j.ymthe.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snijder J, Mihyawi N, Frolov A, et al. Spontaneous remission in diffuse large cell lymphoma: a case report. J of Med Case Reports. 2019;13:28. doi: 10.1186/s13256-018-1937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musto P, et al. Spontaneus remission in Acute Myeloid Leukaemia: A role for endogenous production of Tumour necrosis factor and Interleukin-2. Br J Haematol. 1994 Aug;87(4):879–80. doi: 10.1111/j.1365-2141.1994.tb06761.x. [DOI] [PubMed] [Google Scholar]


