Abstract
Introduction:
Parkinson’s disease (PD) is a common disease of unknown etiology. Even though accurate information on the epidemiology of PD is critical for defining appropriate health policies, epidemiological data on Parkinson’s disease (PD) in Italy are often defined as scant or conflicting. Our study attempted to provide an overview on the prevalence of (PD) by means of a systematic review and metanalysis of existing data.
Material and methods:
We searched into two different databases (PubMed and EMBASE), focusing on studies reporting the prevalence of PD in Italy. Data were extracted using a standardized assessment form, and results of such analyses were systematically reported, summarized and compared.
Results:
A total of 16 studies were eventually included in the analyses, with a prevalence rate of 193.7/100,000. Available reports were heterogeneous both in design and in eventual figures, and also prevalence estimates were affected by substantial heterogeneity. Interestingly, prevalence rates ranged from 37.8/100,000 inhabitants in subjects aged 0 to 64 years, to 578.7 in age group 65 to 75 years, and 1235.7 in age group 75 years or older. PD was significantly associated with male sex, but only in older age groups (i.e. Odds Ratio, OR 1.37 95%CI 1.22-1.53, and OR 1.31, 95%CI 1.21-1.42 for age groups 65-74 years and 75 years or more, respectively).
Discussion and conclusion:
While the observed variations in prevalence rates may result from environmental or genetic factors, differences in methodologies for case ascertainment and diagnostic criteria may have significantly affected our estimates. As a consequence, the comparability of existing studies is limited.
Keywords: Parkinson’s disease, Parkinsonism, prevalence, epidemiology, occurrence
Introduction
Parkinson disease (PD) is a common progressive, neurodegenerative disorder in adult population (1), characterized by four cardinal motor signs (i.e. tremor, rigidity, bradykinesia/akinesia and postural instability) and non-motor symptoms such as depression/psychosis, and autonomic and gastrointestinal dysfunction (1–3), that considerably impair the quality of life of PD patients.
Despite the main pathological feature of PD is well defined (i.e. the loss of dopaminergic neurons), current understanding of its etiology remains incomplete. In facts, while genetic factors have been strongly identified within PD pathogenesis (e.g. SNCA A53T gene mutation; upregulation of alpha-synuclein; impairment of the mitochondrial function following mutations of genes PINK-1 and Parkin), evidence regarding environmental (i.e. residential exposure to certain pesticides, rural living, but also exposure to waste incinerators fumes and industrial pollutants), and occupational factors (i.e. manganese, trichloroethylene, carbon monoxide) remains disputed (2,4–8). In facts, discerning between PD (or, more appropriately, Primary Parkinsonism) and secondary parkinsonisms is still difficult (1–3,9).
Even though imaging techniques can assist an appropriate assessment of suspected cases, PD diagnosis remains essentially based on clinical assessment (1,2,9,10). As a consequence, epidemiological data are often strikingly heterogeneous: even though variability in the occurrence of PD is usually explained by means of environmental and genetic factors, it is reasonable that other differences, such as methodological diversity and reliability of primary diagnosis, may play a significant role, complicating comparisons across studies (2,3,10–12). For example, a previous study summarizing European prevalence rates identified figures ranging from 65.6/100,000 in Sardinia, to 12,500/100,000 for German institutionalized patients (3). That said, available figures suggest that the prevalence of PD in high-income countries may be generally estimated at 0.3% for the entire population, and about 1% in people over 60 years of age (1,2,10,12).
Interestingly enough, previous studies suggested that prevalence data for Italian population may be even more heterogenous, possibly reflecting both methodological and demographic issues (1–3). As a consequence, also estimates for the PD burden are particularly conflicting, ranging from 230,000 (following the public statement of the Italian Ministry of Health) to 600,000 cases. While the longevity of the Italian population steadily increases, the high financial burden associated with the chronic management of PD urges for accurate information about its actual epidemiology.
This survey will therefore provide an overview of the prevalence of PD in Italy, focusing on the methodologies used in the reported studies.
Materials and Methods
This systematic review has been conducted following the PRISMA (Prepared Items for Systematic Reviews and Meta-Analysis) guidelines (13). We searched into two different databases (PubMed and EMBASE) for relevant studies to 31/12/2019, without any chronological restriction. The search strategy was a combination of the following keywords (free text and Medical Subject Heading [MeSH] terms): («Parkinson» OR «Parkinson’s disease» OR «Parkinsonism») AND («Italy» OR «Italian») AND («epidemiology» OR «prevalence» OR «frequency») (Figure 1). Records were handled using a references management software (Mendeley Desktop Version 1.19.5, Mendeley Ltd 2019), and duplicates were removed.
Figure 1.
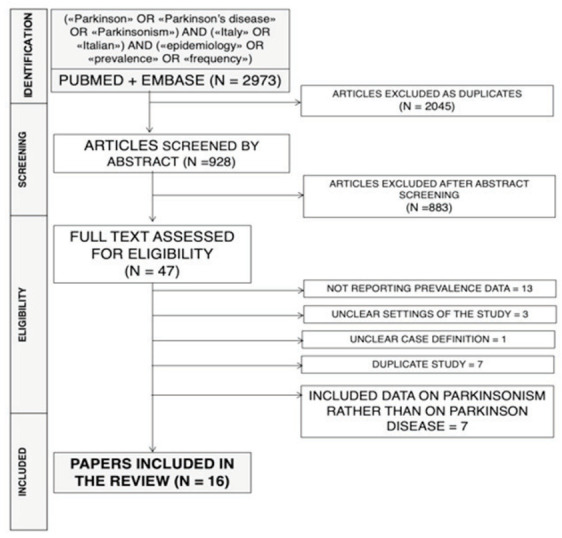
PRISMA flow diagram including keywords employed for the inquiry (i.e. «Parkinson» OR «Parkinson’s disease» OR «Parkinsonism») AND («Italy» OR «Italian») AND («epidemiology» OR «prevalence» OR «frequency»)).
Articles eligible for review were original research publications available online or through inter-library loan. Articles had to be written in Italian, English, German, French or Spanish, the languages spoken by the investigators. Studies included were national and international reports, case studies, cohort studies, case-control studies and cross-sectional studies. Only article reporting diagnostic criteria for PD cases, the number of prevalent cases, or crude prevalence rates, were eligible for the full review. Articles were excluded if: (1) full text was not available; (2) articles were written in a language not understood by reviewers; (3) reports lacked significant timeframe (i.e. the prevalence year); (4) reports lacked geographical settings; (5) diagnostic criteria hinted towards a parkinsonism rather than PD.
Two independent reviewers (GG and LV) reviewed titles, abstracts, and articles. Titles were screened for relevance to the subject. Any articles reporting original studies, which did not meet one or more of the exclusion criteria, were retained for full-text review. The investigators independently read full-text versions of eligible articles. Disagreements were resolved by consensus between the two reviewers; where they did not reach consensus, input from a third investigator (MR) was obtained. Further studies were retrieved from reference lists of relevant articles and consultation with experts in the field.
Data abstracted included:
Settings of the study: prevalence year, Italian region, level of assessment (i.e. community, province, region);
Source of information (i.e. patient records, either institutional or maintained by general practitioners or neurologists; door-to-door interviews; institutional databases);
Screening procedures, including: clinical assessment of patients or patient records; diagnostic questionnaires; diagnosis-related groups compatible (DRG) with PD diagnosis from institutional databases; previous prescriptions of antiparkinsonian drug(s).
Reported diagnostic criteria;
Total number of prevalent PD cases, in total, by gender (M/F), and by reported age groups;
Number of reference population, both in general of by gender and age groups.
We first performed a descriptive analysis to report the characteristics of the included studies. Crude PD prevalence figures were initially calculated: if a study did not include raw data, either as number of prevalent cases, or referent population (either in general or by age groups), such figures were either reverse-calculated from available data, or obtained from the Italian National Institute of Statistics (ISTAT) site DEMO (http://demo.istat.it/). When two or more studies reported about a shared population (e.g. a study included community level data, or provincial data, that were then included a in regional study), available local-area data were removed from the larger study in order to avoid duplication of estimates. DEMO includes official Italian demographic data for the timeframe 1974 – 2019, at various geographical levels (i.e. national, regional, provincial, local communities). Pooled prevalence (prevalent cases/100,000 inhabitants) estimates were then calculated by means of a random effect model (in order to cope with the presumptive heterogeneity in study design), in general, and by age groups (i.e. 0-64 years; 65-74 years, ≥75 years) for all studies that allowed such stratification. Estimates of the association of PD diagnosis with male sex were similarly assessed as Odds Ratios (OR) with their correspondent 95% Confidence Intervals (95%CI).
I2 statistic was then calculated to quantify the amount of inconsistency between included studies; it estimates the percentage of total variation across studies that is due to heterogeneity rather than chance. I2 values ranging from 0 to 25% were considered to represent low heterogeneity, from 26% to 50% as moderate heterogeneity and above 50% as substantial heterogeneity, being pooled using a fixed-effects model because of the reduced number of samples eventually included.
To investigate publication bias, contour-enhanced funnel plots were initially generated: publication bias was evaluated by testing the null hypothesis that publication bias does not exist by means of the regression test for funnel plot asymmetry. The null hypothesis was rejected if the p-value is less than 0.10.
All calculations were performed in R (version 3.6.1; R Core Team, 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) and RStudio (version 1.2.5019) software by means of meta package (version 4.9-9), functions metaprop for pooling of HD prevalence, and metabin for comparison of prevalence data by gender. The meta package is an open-source add-on for conducting meta-analyses.
Results
Initially, 2973 entries were identified: as 2045 of them were duplicated across the sources, a total of 928 entries were initially screened. After applying the inclusion and exclusion criteria (Figure 1), 47 articles were assessed for eligility, with the subsequent removal of 13 articles not reporting actual prevalence data, 3 articles not reporting the settings of the study (or reporting it in unclear geographical/chronological terms), 1 article exhibiting unclear case definition. Similarly, 7 articles that eventually duplicated results of similar researches, and 7 further reports including data on parkinsonism rather than on PD were excluded from the analyses.
Eventually, 16 paper published between 1978 and 2019 fulfilled inclusion and exclusion criteria, being analyses and summarized (14–29) (Table 1).
Table 1.
Prevalence estimates of Primary Parkinson’s Disease (PD) in Italy. Notes: DRG = diagnosis-related groups; GP = General practitioner; APD = antiparkinsonian drug.
| Reference | Region | Level | Prevalence years | Study design | Screening procedures | Diagnostic criteria | Assessed Population | Cases |
Crude rate (/100,000 inhabitants) |
|||
| Clinical | Questionnaire | DRG | Anti-PD drugs | |||||||||
| Rosati et al. 1978 (14) | Sardinia | Province (Sassari) | 1971 | Patient record | YES | - | - | - | Two signs or more | 397,891 | 302 | 75.9 |
| Rosati et al. 1979 (15) | Sardinia | Province (Nuoro) |
1972 | Patient record | YES | - | - | - | Two signs or more | 273,421 | 182 | 66.6 |
| Rosati et al. 1980 (22) | Sardinia | Region | 1972 | Patient record | YES | - | - | - | Two signs or more | 1,473,800 | 967 | 65.5 |
| D’Alessandro et al. 1986 (23) | Emilia – Romagna | Community (San Marino) | 1986 | Patient record | YES | - | - | - | Two signs or more | 22,322 | 34 | 152.3 |
| Morgante et al. 1992 (24) | Sicily | Community (Terrasini, Santa Teresa di Riva, Riposto). |
1987 | Door-to-Door | YES | YES | - | - |
Two signs or more in untreated patients; one sign or more in treated patients. Exclusion of all other possible causes of parkinsonism. |
24,396 | 63 | 280.7 |
| Beghi et al. 1994 (a) (25) | Lombardy | Community (Arcisate) | 1988 | Patient record (GPs) | YES | - | - | - |
Two signs or more in untreated patients. History of signs/symptoms in treated patients. |
19,900 | 29 | 145.7 |
| Beghi et al. 1994 (b) (25) | Apulia | Community (San Giovanni Rotondo) | 1989 | 8,477 | 16 | 188.7 | ||||||
| Chiò et al. 1998 (26) | Piedmont | Community (Cossato) | 1991 | Institutional database (single) + Patient record (GPs) |
YES | YES | - | YES |
Two signs or more OR previous diagnosis of PD or parkinsonism. Exclusion of drug-related parkinsonism. |
61,830 | 104 | 168.2 |
| Totaro et al. 2005 (27) | Abruzzo | Province (L’Aquila) | 2001 | Institutional database (multiple) | YES | - | YES | YES |
Two signs or more in untreated patients; one sign or more in treated patients. Exclusion of all other possible causes of parkinsonism. |
294,424 | 682 | 231.6 |
| Morgante et al. 2008 (28) | Sicily | Community (Aeolian Islands) | 2001 | Institutional database (multiple) | YES | YES | YES | YES | Previous diagnosis of PD or Parkinsonism. | 13,431 | 14 | 104.2 |
| Zucchi et al. 2011 (29) | Lombardy | Province (Bergamo) | 2008 | Institutional database (multiple) | - | - | YES | YES | Integration of pharmacological records and medical records of the local Health Unit. | 700,328 | 2425 | 346.3 |
| Tominz et al. 2015 (16) | Friuli Venezia Giulia | Province (Trieste) | 2011 | Institutional database (multiple) | - | - | YES | YES | Integration of pharmacological records and medical records of the local Health Unit. | 239,325 | 909 | 379.8 |
| Pupillo et al. 2016 (17) | Nationwide | 2013 | Institutional database (multiple) | YES | - | YES | YES | Integration of pharmacological records and medical records of the referring GPs. Exclusion of patients with less than 1 year of follow-up. | 923,356 | 2204 | 238.7 | |
| Baldacci et al. 2016 (18) | Tuscany | Region | 2010 | Institutional database (multiple) | - | - | YES | YES | At least one hospital discharge diagnosis of PD, OR a specific exemption for PD, OR a minimum of two separate prescription for at least one APD. | 3,167,777 | 10,632 | 335.6 |
| Malaguti et al. 2016 (19) | Trentino -Sud Tyrol | Province (Trento) | 2014 | Institutional database (multiple) | YES | - | YES | YES | Two signs or more reported from at least 3 years without features of a possible alternative diagnosis; documented response to APD. | 536,237 | 1149 | 214.3 |
| Valent et al. 2018 (20) | Friuli Venezia Giulia | Region | 2016 | Institutional database (multiple) | - | - | YES | YES | At least one hospital discharge diagnosis of PD, OR a specific exemption for PD, OR home care for PD, OR nursing home admission with a diagnosis of PD, OR a minimum of 3 separate prescription for at least one APD during at least 6 consecutive months. | 1,221,218 | 4735 | 387.7 |
| Eusebi et al. 2019 (21) | Umbria | Region | 2016 | Institutional database (multiple) | - | - | YES | YES | At least one hospital discharge diagnosis of PD, OR a specific exemption for PD, OR a minimum of 3 separate prescription for at least one APD during at least 6 consecutive months. | 891,181 | 5500 | 617.2 |
Overall, 4 reports included data retrieved at regional level (25.0%) (18,20–22), 6 studies reported figures at provincial level (37.5%) (14–16,19,27,29), 5 studies with 6 estimates at community level (31.3%) (23–26,28), with 1 estimate at national level (5.9%) (Figure 2) (17). The pooled population included a total of 28,445 cases for a total sample size of 9,358,777 people: compared to the demographic estimates for 2019, reference areas would include around 24.1% of total Italian residents. Unfortunately, accurate description of the prevalent PD cases by age groups were retrieved only for 10 studies (11 estimates), being included in further analyses.
Figure 2.
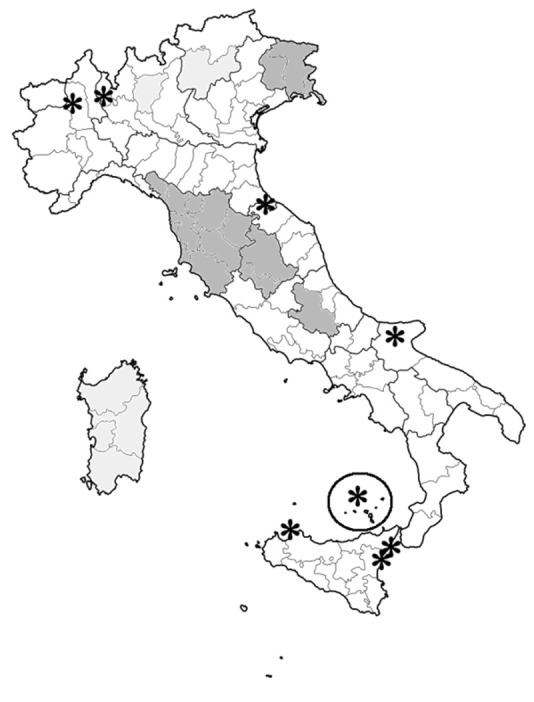
Geographic locations of studies performed on the prevalence of Parkinson’s disease in Italy (1979 – 2019), and included in the meta-analysis. Deep gray = data retrieved at provincial and/or regional level, by sex and age groups; light grey = data retrieved at provincial and/or regional level, cumulative; * = data retrieved at municipal level.
Focusing on the diagnostic assessment, while nearly all earlier reports retrieved PD cases by means of the analysis of patient records (14,15,22,23,25), since 1998 the majority of them were based on the retrospective analysis of institutional databases (16–21,26–29), with only one study identifying PD cases by means of door-to-door analysis (24). Even more recent reports were somewhat heterogenous in terms of diagnostic criteria, with an increasing relevance for reports based on the analysis of prescription history rather than on clinical criteria.
Pooled estimates for PD prevalence are reported in Figure 3, being initially presented by subgroups represented by the three conventional Italian macroregions (i.e. North, Center, South), plus Sardinia. Briefly, individual estimates ranged from 60.2/100,000 inhabitants (95%CI 59.5 to 75.0), in the regional study of Rosati et al. on Sardinian residents (after the removal of data about the otherwise reported provinces of Nuoro and Sassari) (22), to 617.2/100,000 in the regional study of Eusebi et al. on the Central Italian Region of Umbria (21), with a relevant heterogeneity across the studies (I2 = 100%). In facts, pooled prevalence estimates of 193.7/100,000 (95%CI 141.8 to 264.6) included the very low rates of Sardinia (66.8/100,000), very high rates from Central Italy (455.1/100,000), and intermediate figures for Northern (241.3/100,000) and Southern (197.2/100,000) Italy.
Figure 3.
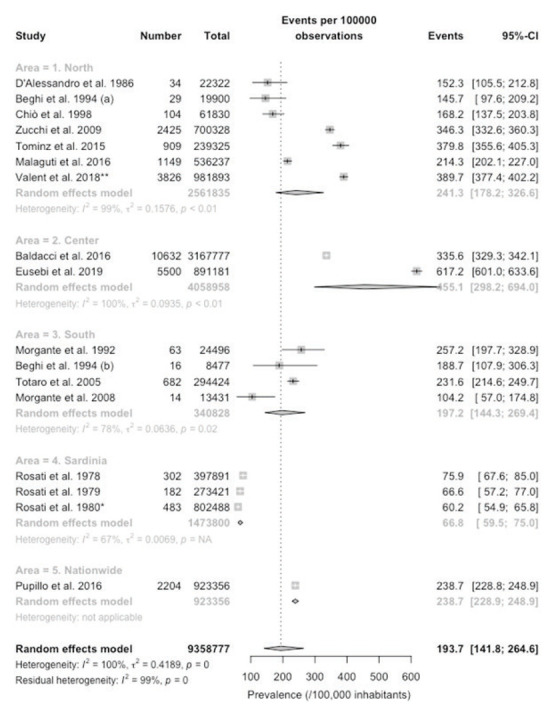
Forest plot of retrieved studies on the prevalence of Parkinson’s Disease. Prevalence data are reported as cases/100,000 inhabitants with their correspondent 95% confidence intervals (95%-CI). Notes: (a) data on the community of Arcisate; (b) data on the community of San Giovanni Rotondo; * = after removal of cases and population reported from Rosati et al. 1978 and Rosati et al. 1979; ** = after removal of Tominz et al. 2015.
When prevalence rates were assessed by age groups, an increasing trend was clearly evident, with a pooled prevalence rate of 37.8/100,000 (95%CI 25.2 to 56.5) in subjects aged 0 to 64 years (Figure 4), that increased to 578.7/100,000 (95%CI 373.5 to 895.5) in the age group 65 to 74 years (Figure 5), and to 1235.7 (806.9 to 1888.1) in age group 75 years or more (Figure 6). Still, it should be stressed that heterogeneity was substantial, with I2 values ranging from 99% to 100% in the three estimates.
Figure 4.
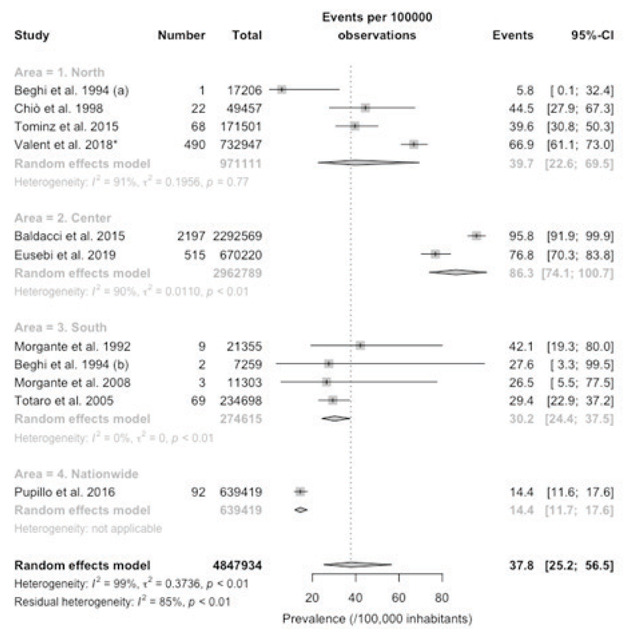
Forest plot of retrieved studies on the prevalence of Parkinson’s Disease, in age group 0 to 64 years. Prevalence data are reported as cases/100,000 inhabitants with their correspondent 95% confidence intervals (95%-CI). Notes: (a) data on the community of Arcisate; (b) data on the community of San Giovanni Rotondo; * = after removal of Tominz et al. 2015.
Figure 5.
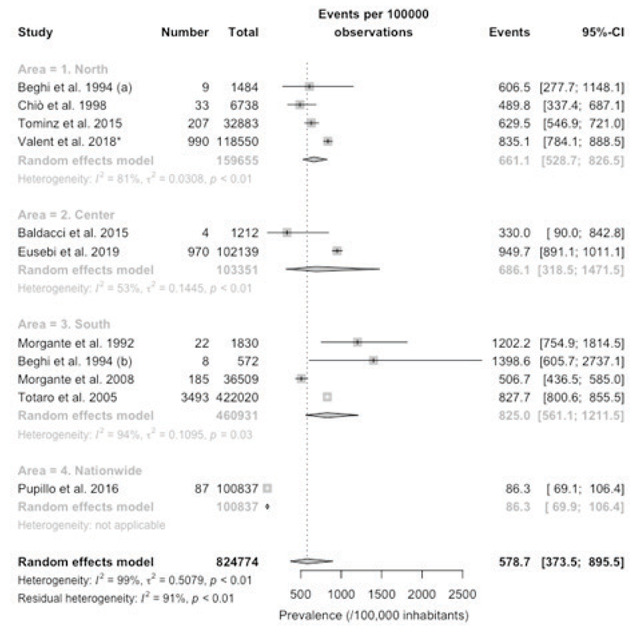
Forest plot of retrieved studies on the prevalence of Parkinson’s Disease, in age group 65 to 74 years. Prevalence data are reported as cases/100,000 inhabitants with their correspondent 95% confidence intervals (95%-CI). Notes: (a) data on the community of Arcisate; (b) data on the community of San Giovanni Rotondo; * = after removal of Tominz et al. 2015.
Figure 6.
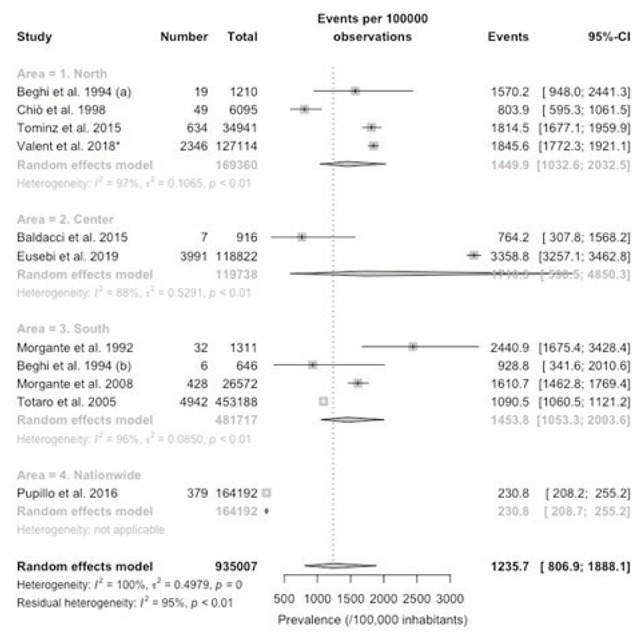
Forest plot of retrieved studies on the prevalence of Parkinson’s Disease, in age group 75 years or more. Prevalence data are reported as cases/100,000 inhabitants with their correspondent 95% confidence intervals (95%-CI). Notes: (a) data on the community of Arcisate; (b) data on the community of San Giovanni Rotondo; * = after removal of Tominz et al. 2015.
Association of PD with male sex was then assessed, in general and by age group, and results are reported in Figure 7. In summary, while overall estimates testified a substantial association of PD status with male sex was reported only in the study of Baldacci et al (OR 2.06, 95%CI 1.99 to 2.13)(18), in older age groups a stronger association was identified (pooled OR 1.37, 95%CI 1.22 to 1.53 and OR 1.31, 95%CI 1.21 to 1.42 for age 65 to 74 years and 75 years or more, respectively), with lower heterogeneity, i.e. I2 48% for age group 65 to 74, and 58% for age group 75 or more.
Figure 7.
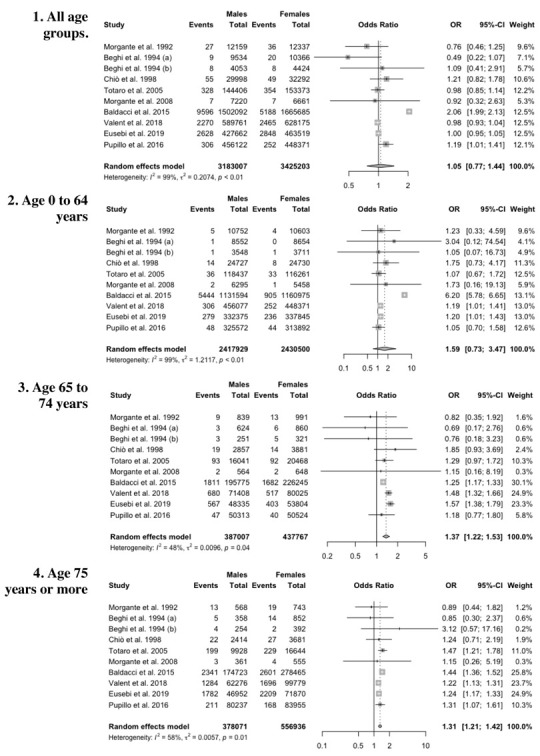
Forest plot of retrieved studies on the prevalence of Parkinson Disease, in the whole study population (1), in age groups 0-64 year-old (2), 65-74 year-old (3), 75 year-old or more (4): association of cases with male gender are assessed as Odds Ratios (ORs) with their correspondent 95% confidence intervals (95%-CI). Notes: (a) data on the community of Arcisate; (b) data on the community of San Giovanni Rotondo.
The presence of publication bias was then evaluated using funnel plots and regression test for funnel plot asymmetry. Each point in funnel plots represents a separate study and asymmetrical distribution indicates the presence of publication bias. First, studies’ effect sizes were plotted against their standard errors: the visual evaluation of the funnel plot suggested the absence of a significant publication bias, as the graph appeared substantially symmetrical (Figure 8). Subjective evidence from the funnel plot was confirmed by the regression test (Z= -0.702, p-value = 0.483).
Figure 8.
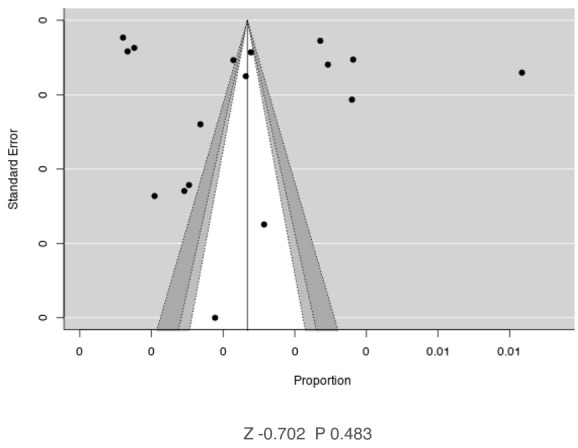
Contour-enhanced funnel plots of available studies on the Italian prevalence of Parkinson disease.
Discussion
This study attempted to summarize available prevalence studies on PD in Italy. In order to obtain the larger base of evidence available, we forcibly included studies of very heterogenous quality and design, published between 1978 and 2019. Obviously, such approach resulted in high heterogeneity across the retrieved studies. The resulting pooled prevalence estimate of 193.7/100,000 was substantially lower than that previously reported in the nationwide study of Pupillo et al., i.e. 238.7/100,000 (95%CI 228.8 to 248.9) (17), but somehow similar to other reports from Western Europe previously summarized by von Campenhausen et al. in 2005 (2,3,10). Similarly, when reporting prevalence rates by age groups with the Italian census of 2019, a cumulative disease burden of 175,972 prevalent cases was estimated, that is around 25% less than that usually acknowledged by the Italian Ministry of Health (i.e. 230,000 cases in 2017).
The heterogeneity of reported estimates may find several explanations. First at all, diagnostic criteria for PD and methodologies applied for case ascertain have radically changed over the years, with increasing role for studies based on inquiries of institutional databases (i.e. use of certain combinations anti-parkinsonian drugs in subjects with individual clinical stories compatible with a diagnosis of PD): even though such search strategy was found sufficiently accurate when a comparison with real-world data was available (18,20,21,29), subsequent estimates are often limitedly comparable with field studies.
Second, because of its tormented history, and following millennia of migratory influxes, the genetic background of the Italian peninsula is usually acknowledged as strikingly heterogenous (30,31). Even though PD is usually understood as a multifactorial disorder, the genetic background is indisputably a major player in its natural history (2,9,10), either decreasing or increasing individual susceptibility to behavioral, environmental, or even occupational risk factors. Not coincidentally, the lowest prevalence rates were identified in studies based in a very peculiar region as Sardinia (14,15,22), and also the study on the residents of Aeolian island reported low prevalence rates (28).
Third, Italy is also heterogenous in terms of economic development: not only northern regions are usually characterized by a highly developed industrial sectors, but the very same industrial or agricultural activities may be performed in strikingly different settings, with consequent differences in occupational and/or residential exposures, and possible heterogeneity in the occurrence of PD in exposed people (32–36).
Our study identified a clear trend across age groups, with prevalence rates increasing more than ten times from 37.8/100,000 in subject aged less than 65 years, to 578.7/100,000 in subjects aged 65 to 74 years, eventually doubling in older groups (≥ 75 years). Such trend was not unexpected, as PD is also usually acknowledged as strikingly age-dependent (2,3,10,11,17), and our estimates were quite similar to those reported by the European study from von Campenhausen et al (2,3,10).
On the contrary, the clear and significant association of male sex with PD diagnosis in older age groups (i.e. 65 to 74 years, and ≥ 75 years), while not totally unexpected, is somewhat conflicting with more doubtful evidence usually reported by epidemiological studies (2,10). Several explanations of conflicting association between sex and PD have been suggested, including neuroprotective effects of estrogens, but all remains controversial (2). More precisely, an increasing number of original field studies and subsequent systematic reviews and meta-analyses suggest that PD (or more appropriately parkinsonisms) may be elicited or even caused by exposures to occupational or environmental toxicants (e.g. heavy metals, pesticides, etc.) (4,6,32,37), and such evidences can lead to two opposite interpretations. On the one hand, we can deduce the increasing prevalence of PD in older age subjects, and particularly of male sex, as a consequence of cumulative, life-time exposure to the aforementioned risk factors, that are usually more frequently associated with occupations and work tasks performed by personnel of male sex (32,38,39). On the other hand, similarly to other multifactorial, work-related disorders (e.g. musculoskeletal disorders) (40–43), higher prevalence rates would be precisely expected in younger age groups, as occupational/residential exposures would anticipate the eventual diagnosis in a favorable background. Similarly, it should be stressed that while some earlier reports hinted towards higher rates in regions characterized by either agricultural (e.g. Apulia compared to Lombardy) (25,44,45), or highly developed industrial background (e.g. provinces of Bergamo and Brescia) (29,32), not only the prevalence rates reported from highly developed agricultural areas such as the Autonomous Province of Trento were relatively low, with similar occurrence in males and females (19), but most of available studies reported about parkinsonism rather than on PD (25,29,32,44,45), being therefore excluded from our analyses, and also hinting to a similar but distinctive series of neurological disorders.
Despite their potential interest, both for public health and clinical professionals, our results should be cautiously interpreted, for several reasons. In first place, as the studies were quite heterogenous, we cannot rule out that new reports may significantly modify eventual estimates, particular if involving areas characterized by genetic and/or geographical specificities (e.g. Alpine regions; mountainous enclaves, etc.). Second, studies based on the retrospective analysis of institutional databases may have failed to ascertain the actual prevalence of PD, either as unable to retrieve all diagnosis, or incorporating secondary parkinsonisms rather than PD cases (16,18,20,21). Third, even for accurate estimates, an original diagnostic bias cannot be totally ruled out. In other words, as the diagnosis of PD remains largely clinical, and no screening procedures have been made available, only subjects complaining one or more of the cardinal symptoms, appropriately interacting with a physician deserving to him/her patient an appropriate suspicion index (i.e. the general practitioner, or a medical specialist, including neurologists, psychologists, psychiatrics, or even professionals of sleep medicine), had a significant probability to obtain the diagnosis of PD, being therefore incorporated in the estimates we retrieved and analyzed. In this regard, it is noteworthy that the only door-to-door study we were able to analyze (24), was characterized by relatively high prevalence estimates (i.e. 257.2/100,000), nearly the double of those reported by the same Authors in the Aeolian island ten years after the first survey (i.e. 104.2/100,000) (28). Even though the latter study may have been significantly influenced by the genetic background of the study population, the role of the study design should not be undermined.
Conclusions
In summary, we identified a pooled prevalence rate of PD in Italy of 193.7/100,000 inhabitants. Such figures are well below previous estimates, and hint toward a disease burden of around 175,972 prevalent cases, i.e. one quarter less than previously suspected and usually reported by the Italian Health Ministry. Interestingly, we found both a significant age-dependent trend, with higher rates in older groups, and a relatively strong association of PD with male sex, but only in older age groups. Despite its limits, on the one hand our study stresses the importance of promoting large, appropriately designed population studies in order to guarantee a better definition of the actual epidemiology of PD in Italy; on the other hand, it also highlights how retrospective studies based on institutional databases and deprived of an accurate analysis (either preventive of retrospective) of potential cases by well-trained professionals may elicit doubtful or even unreliable epidemiologic assessments.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Balestrino R, Schapira AH V. Parkinson Disease. Eur J Neurol. 2020;27(1):27–42. doi: 10.1111/ene.14108. DOI: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- 2.De Lau LML, Breteler MMB. The epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. DOI: 10.1016/S1474-4422(06)70471–9. [DOI] [PubMed] [Google Scholar]
- 3.Von Campenhausen S, Bornschein B, Wick R, Bötzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson’s disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473–90. doi: 10.1016/j.euroneuro.2005.04.007. DOI: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Ball N, Teo W-P, Chandra S, Chapman J. Parkinson’s Disease and the Environment. Front Neurol. 2019;10:218. doi: 10.3389/fneur.2019.00218. DOI: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbaz A, Carcaillon L, Kab S, Moisan F. The scientific bases to consider Parkinson’s disease an occupational disease in agriculture professionals exposed to pesticides in France. J Epidemiol Community Heal. 2014;70(4):319–21. doi: 10.1136/jech-2015-205455. DOI: 10.1136/jech-2015-205455. [DOI] [PubMed] [Google Scholar]
- 6.Vlaar T, Kab S, Schwaab Y, Fréry N, Elbaz A, Moisan F. Association of Parkinson’s disease with industry sectors: a French nationwide incidence study. Eur J Epidemiol. 2018;33(11):1101–11. doi: 10.1007/s10654-018-0399-3. DOI: 10.1007/s10654-018-0399-3. [DOI] [PubMed] [Google Scholar]
- 7.Moisan F, Spinosi J, Delabre L, Gourlet V, Mazurie JL, Bénatru I, et al. Association of parkinson’s disease and its subtypes with agricultural pesticide exposures in men: A case-control study in France. Environ Health Perspect. 2015;123(11):1123–9. doi: 10.1289/ehp.1307970. DOI: 10.1289/ehp.1307970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Signorelli C, Riccò M, Vinceti M. [Waste incinerator and human health: a state-of-the-art review] Ann Ig. 2008;20(3):251–277. [PubMed] [Google Scholar]
- 9.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5(1):75–86. doi: 10.1016/S1474-4422(05)70285-4. DOI: 10.1016/S1474-4422(05)70285–4. [DOI] [PubMed] [Google Scholar]
- 10.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–72. doi: 10.1016/S1474-4422(16)30230-7. DOI: 10.1016/S1474-4422(16)30230–7. [DOI] [PubMed] [Google Scholar]
- 11.Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–5. doi: 10.1007/s00702-017-1686-y. DOI: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 12.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: A review of the evidence. Eur J Epidemiol. 2011;26(S1):S1–S58. doi: 10.1007/s10654-011-9581-6. DOI: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. DOI: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosati G, Granieri E, Pinna L, Aiello I, De Bastiani P, Tola R, et al. Parkinson’s Disease. Prevalence and Incidence in the Province of Sassari, North Sardinia. Acta Neurol. 1978;33(3):201–7. [PubMed] [Google Scholar]
- 15.Rosati G, Granieri E, Pinna L, Devoto MC. The Frequency of Parkinson’s Disease in the Province of Nuoro (Sardinia) Acta Neurol. 1979;1(4):303–8. [PubMed] [Google Scholar]
- 16.Tominz R, Marin L, Mezzarobba S. [Estimate of the Prevalence of Parkinson’s Disease by Using Electronic Health Archives] Recenti Prog Med. 2015;106(2):97–102. doi: 10.1701/1790.19496. DOI: 10.1701/1790.19496. [DOI] [PubMed] [Google Scholar]
- 17.Pupillo E, Cricelli C, Mazzoleni F, Cricelli I, Pasqua A, Pecchioli S, et al. Epidemiology of Parkinson’s Disease: A Population-Based Study in Primary Care in Italy. Neuroepidemiology. 2016;47(1):38–45. doi: 10.1159/000448402. DOI: 10.1159/000448402. [DOI] [PubMed] [Google Scholar]
- 18.Baldacci F, Policardo L, Rossi S, Ulivelli M, Ramat S, Grassi E, et al. Reliability of administrative data for the identification of Parkinson’s disease cohorts. Neurol Sci. 2015 May 1;36(5):783–6. doi: 10.1007/s10072-015-2062-z. DOI: 10.1007/s10072-015-2062-z. [DOI] [PubMed] [Google Scholar]
- 19.Malaguti MC, Vanacore N, Ferrari S, Piffer S, Pertile R, Roni R, et al. An unexpected higher prevalence of Parkinson’s disease in females than in males in the province of Trento (Italy): a clues for the etiopathogenesi. Park Relat Disord. 2016;22:e29. DOI: 10.1016/j.parkreldis.2015.10.025. [Google Scholar]
- 20.Valent F, Devigili G, Rinaldo S, Del Zotto S, Tullio A, Eleopra R. The epidemiology of Parkinson’s disease in the Italian region Friuli Venezia Giulia: a population-based study with administrative data. Neurol Sci. 2018;39(4):699–704. doi: 10.1007/s10072-018-3273-x. DOI: 10.1007/s10072-018-3273-x. [DOI] [PubMed] [Google Scholar]
- 21.Eusebi P, Franchini D, De Giorgi M, Abraha I, Montedori A, Casucci P, et al. Incidence and prevalence of Parkinson’s disease in the Italian region of Umbria: a population-based study using healthcare administrative databases. Neurol Sci. 2019 Aug 1;40(8):1709–12. doi: 10.1007/s10072-019-03872-w. DOI: 10.1007/s10072-019-03872-w. [DOI] [PubMed] [Google Scholar]
- 22.Rosati G, Granieri E, Pinna L, Aiello I, Tola R, De Bastiani P, et al. The Risk of Parkinson Disease in Mediterranean People. Neurology. 1980;30(3):250–5. doi: 10.1212/wnl.30.3.250. DOI: 10.1212/wnl.30.3.250. [DOI] [PubMed] [Google Scholar]
- 23.D’Alessandro R, Gamberini G, Granieri E, Benassi G, Naccarato S, Manzaroli D. Prevalence of Parkinson’s Disease in the Republic of San Marino. Neurology. 1987;37(10):1679–82. doi: 10.1212/wnl.37.10.1679. DOI: 10.1212/wnl.37.10.1679. [DOI] [PubMed] [Google Scholar]
- 24.Morgante L, Rocca WA, Di Rosa AE, De DP, Grigoletto F, Meneghini F, et al. Prevalence of Parkinson’s disease and other types of parkinsonism: a door-to-door survey in three Sicilian municipalities. The Sicilian Neuro-Epidemiologic Study (SNES) Group. Neurology. 1992;42(10):1901–7. doi: 10.1212/wnl.42.10.1901. DOI: 10.1212/wnl.42.10.1901. [DOI] [PubMed] [Google Scholar]
- 25.Beghi E, Monticelli ML, Sessa A, Simone P. The prevalence of parkinsonism in Italy: An epidemiological survey of the disease in general practice. Mov Disord. 1994;9(4):403–8. doi: 10.1002/mds.870090405. DOI: 10.1002/mds.870090405. [DOI] [PubMed] [Google Scholar]
- 26.Chiò A, Magnani C, Schiffer D. Comparison of Tracer Methodology and Clinical Ascertainment of Cases. Mov Disord. 1998;13(3):40. doi: 10.1002/mds.870130305. DOI: 10.1002/mds.870130305. [DOI] [PubMed] [Google Scholar]
- 27.Totaro R, Marini C, Pistoia F, Sacco S, Russo T, Carolei A. Prevalence of Parkinson’s disease in the L’Aquila district, central Italy. Acta Neurol Scand. 2005;112(1):24–8. doi: 10.1111/j.1600-0404.2005.00426.x. DOI: 10.1111/j.1600-0404.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- 28.Morgante L, Nicoletti A, Epifanio A, Contrafatto D, Savica R, Lanzafame S, et al. Prevalence of Parkinson’s disease and other types of parkinsonism in the Aeolian Archipelago, Sicily. Park Relat Disord. 2008;14(7):572–5. doi: 10.1016/j.parkreldis.2007.11.010. DOI: 10.1016/j.parkreldis.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Zucchi A, Ferraro B, Imbalzano G, Brembilla G, Sileo C. Economia e Management Il network Parkinson Bergamo . organizzativo-assistenziali a rete Economia e Management. Sanità Pubblica e Priv. 2011;6:43–52. [Google Scholar]
- 30.Squitieri F, Griguoli A, Capelli G, Porcellini A, D’Alessio B. Epidemiology of Huntington disease: First post-HTT gene analysis of prevalence in Italy. Clin Genet. 2016;89(3):367–70. doi: 10.1111/cge.12574. DOI: 10.1111/cge.12574. [DOI] [PubMed] [Google Scholar]
- 31.Frontali M, Malaspina P, Rossi C, Jacopini AG, Vivona G, Pergola MS, et al. Epidemiological and linkage studies on Huntington’s disease in Italy. Hum Genet. 1990;85(2):165–70. doi: 10.1007/BF00193190. DOI: 10.1007/bf00193190. [DOI] [PubMed] [Google Scholar]
- 32.Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, et al. High Prevalence of Parkinsonian Disorders Associated to Manganese Exposure in the Vicinities of Ferroalloy Industries. Am J Ind Med. 2007;50(11):788–800. doi: 10.1002/ajim.20494. DOI: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- 33.Riccò M, Bragazzi NL, Vezzosi L, Balzarini F, Colucci E, Veronesi L, et al. Knowledge , Attitudes , and Practices on Tick-Borne Human Diseases and Tick-Borne Encephalitis Vaccine among Farmers from North-Eastern Italy ( 2017 ) J Agromedicine. 2020;25(1):73–85. doi: 10.1080/1059924X.2019.1659204. DOI: 10.1080/1059924X.2019.1659204. [DOI] [PubMed] [Google Scholar]
- 34.Riccò M, Cattani S, Veronesi L, Colucci ME. Knowledge, attitudes, beliefs and practices of construction workers towards tetanus vaccine in northern Italy. Ind Health [Internet] 2016;54(6):554–563. doi: 10.2486/indhealth.2015-0249. DOI: 10.2486/indhealth.2015-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riccò M. Air temperature exposure and agricultural occupational injuries in the autonomous province of trento (2000-2013, north-eastern Italy) Int J Occup Med Environ Health. 2018;31(3):317–331. doi: 10.13075/ijomeh.1896.01114. DOI: 10.13075/ijomeh.1896.01114. [DOI] [PubMed] [Google Scholar]
- 36.Riccò M, Vezzosi L, Mezzoiuso AG. Occupational Eye Injury in the agricultural settings: a retrospective study from North-Eastern Italy. Acta Biomed. 2020;90(4):457–467. doi: 10.23750/abm.v90i4.7602. DOI: 10.23750/abm.v90i4.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kab S, Spinosi J, Chaperon L, Dugravot A, Singh-Manoux A, Moisan F, et al. Agricultural activities and the incidence of Parkinson’s disease in the general French population. Eur J Epidemiol. 2017;32(3):203–16. doi: 10.1007/s10654-017-0229-z. DOI: 10.1007/s10654-017-0229-z. [DOI] [PubMed] [Google Scholar]
- 38.Riccò M, Vezzosi L, Bragazzi NL, Balzarini F. Heat-Related Illnesses among Pesticide Applicators in North-Eastern Italy (2017) J Agromedicine. 2020;25(1):52–64. doi: 10.1080/1059924X.2019.1606745. DOI: 10.1080/1059924X.2019.1606745. [DOI] [PubMed] [Google Scholar]
- 39.Riccò M, Vezzosi L, Gualerzi G. Health and Safety of Pesticide Applicators in a high income agricultural setting: a knowledge, attitude, practice, and toxicity study from North-Eastern Italy. J PREV MED HYG. 2018;59(3):e200–e211. doi: 10.15167/2421-4248/jpmh2018.59.3.934. DOI: 10.15167/2421-4248/jpmh2018.59.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riccò M, Cattani S, Signorelli C. Personal risk factors for carpal tunnel syndrome in female visual display unit workers. Int J Occup Med Environ Health. 2016;29(6):927–936. doi: 10.13075/ijomeh.1896.00781. 10.13075/ijomeh.1896.00781. [DOI] [PubMed] [Google Scholar]
- 41.Ricco M, Signorelli C. Personal and occupational risk factors for carpal tunnel syndrome in meat processing industry workers in northern italy. Med Pr. 2017;68(2):199–209. doi: 10.13075/mp.5893.00605. DOI: 10.13075/mp.5893.00605. [DOI] [PubMed] [Google Scholar]
- 42.Petit A, Bodin J, Delarue A, Escatha AD, Fouquet N, Roquelaure Y. Risk factors for episodic neck pain in workers : a 5-year prospective study of a general working population. Int Arch Occup Environ Health. 2018;91(3):251–261. doi: 10.1007/s00420-017-1272-5. DOI: 10.1007/s00420-017-1272-5. [DOI] [PubMed] [Google Scholar]
- 43.Roquelaure Y, Bodin J, Ha C, le Manac’h AP, Descatha A, Chastang JF, et al. Personal, biomechanical, and psychosocial risk factors for rotator cuff syndrome in a working population. Scand J Work Environ Heal. 2011;37(6):502–11. doi: 10.5271/sjweh.3179. DOI: 10.5271/sjweh.3179. [DOI] [PubMed] [Google Scholar]
- 44.Govoni V, Granieri E, Tola MR, Casetta I, Monetti VC, Fainardi E, et al. Prevalence of anti-parkinson drugs’ use in Ferrara, Northern Italy, 1988. Acta Neurol Scand. 1994;31:433–8. doi: 10.1111/j.1600-0404.1994.tb02662.x. DOI: 10.1111/j.1600-0404.1994.tb02662.x. [DOI] [PubMed] [Google Scholar]
- 45.Granieri E, Carreras M, Casetta I, Govoni V, Tola MR, Paolino E, et al. Parkinson ’ s Disease in Ferrara, Italy, 1967 through 1987. Arch Neurol. 1991;48:854–7. doi: 10.1001/archneur.1991.00530200096026. DOI: 10.1001/archneur.1991.00530200096026. [DOI] [PubMed] [Google Scholar]


