Abstract
Metformin is a widely used drug that results in clear benefits in relation to glucose metabolism and diabetes-related complications. The global increase in the prevalence of obesity among children and adolescents is accompanied by the appearance and increasing prevalence of insulin resistance, prediabetes, and type 2 diabetes mellitus (T2DM). In addition, children, and adolescents with premature pubarche and polycystic ovary have considerable degree of insulin resistance. The insulin sensitizing actions of metformin encouraged many investigators and physician to use it as the key drug in these conditions for both prevention and treatment. However, long term-controlled studies are still required to assess the degree and duration of effectiveness and safety of using metformin. This review tries to update physicians about the main and the new therapeutic perspectives of this drug. (www.actabiomedica.it)
Keywords: Metformin, insulin resistance, prediabetes, type 2 diabetes mellitus, premature pubarche, polycystic ovary syndrome
Introduction
Metformin (1,1-dimethylbiguanide) is the most widely used drug to treat type 2 diabetes, and is one of two oral antidiabetic drugs reported by the World Health Organization (WHO) in list of essential medicines and is recommended, in conjunction with lifestyle modification (diet, weight control and physical activity), as a first line oral therapy in the guidelines of the American Diabetes Association and European Association of the Study of Diabetes (1).
Unlike most modern drugs, metformin is derived from a natural product used in herbal medicine (plant Galega officinalis) (2). Jean Sterne (3) was the first to investigate dimethylbiguanide (Metformin) for clinical development and proposed the name ‘Glucophage’ (glucose eater) and published his results in 1957. It was established as a safe and effective therapy before detailed mechanistic studies became possible. Despite its clinical use for 60 years, its molecular mechanisms of action remain much debated (4).
It is an effective anti-hyperglycemic agent that inhibits hepatic glucose production, increases peripheral glucose uptake and inhibits small intestinal glucose absorption. Metformin also exerts beneficial effects on circulating lipids and exhibits cardio-protective features in obese patients. In addition, it may also have therapeutic potential in other conditions in which insulin resistance constitutes part of the pathogenesis, including obesity, prediabetes, and polycystic ovary disease and non-alcoholics fatty liver. There are relatively few controlled studies that address therapeutic benefits and safety of metformin in children and adolescents with these disorders. Some have controversial results. This review aims at updating physicians’ information about the mechanisms of action, therapeutic effects, and side effects of using metformin in children and adolescents, with different diseases, which have insulin resistance as important part in their pathogenesis (5,6).
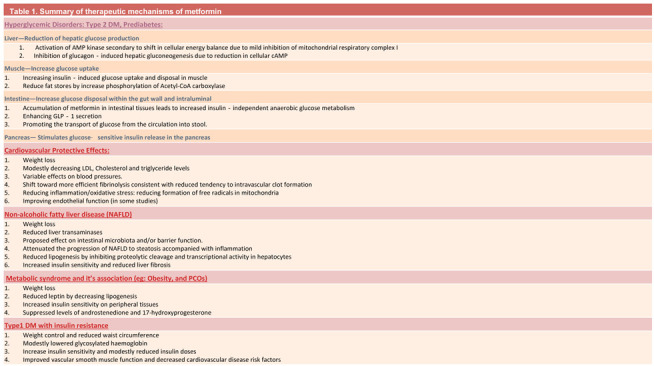
Pharmacokinetics and mechanism(s) of action (Figure 1 and table 1)
Figure 1.
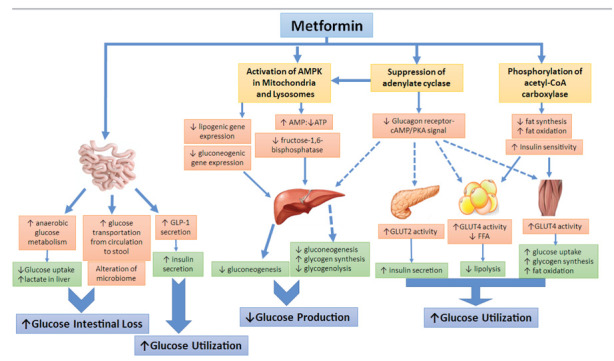
The main cellular actions of metformin in different end-organs
Legend: AMP: Adenosine Monophosphate, ATP: Adenosine Triphosphate, AMPK: Adenosine monophosphate-activated protein kinase, cAMP: Cyclic adenosine monophosphate, PKA: Protein kinase A, GLP-1: Glucagon-like peptide-1, FFA: Free fatty acid, GLUT: Glucose transporter type.
Table 1.
Summary of therapeutic mechanisms of metformin
 |
In humans, following oral dosing of immediate-release metformin, approximately 70% of the dose is absorbed from the small intestine with the remainder passing into the colon before being excreted in feces. The intestinal absorption of metformin may be primarily mediated by plasma membrane monoamine transporter (PMAT/SLC29A4), which is expressed on the luminal side of the enterocytes.
Metformin has an oral bioavailability of 50-60% under fasting conditions and is absorbed slowly. Peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release metformin and four to eight hours with extended-release formulations. Steady state is usually reached in one or two days. Metformin has a half-life of around five hours and undergoes renal excretion with 90% being eliminated within 24 hours (7, 8). Metformin is excreted in urine unchanged, with no metabolites reported. A metformin positron emission tomography (PET) study demonstrated that oral metformin becomes highly concentrated in the intestines, liver, kidneys and bladder (reflecting its route of elimination), with only slow accumulation in muscle (9). The absence of liver metabolism clearly differentiates the pharmacokinetics of metformin from that of other biguanides, such as phenformin. Metformin is undetectable in blood plasma within 24 hours of a single oral dose (10). This elimination is prolonged in patients with renal impairment and correlates with creatinine clearance. There are only scarce data on the relationship between plasma metformin concentrations and metabolic effects (11). Food decreases the extent of and slightly delays the absorption of metformin, as shown by approximately a 40% lower mean peak plasma concentration. The plasma protein binding of metformin is negligible. It should be taken with food to reduce the potential for gastrointestinal side effects.
The therapeutic dose for most patients is in the range of 2 grams per day, suggesting that the effect is not the result of an interaction with a specific protein target (12).
In the liver, it suppresses hepatic gluconeogenesis, and increases peripheral insulin sensitivity in insulin sensitive tissues such as muscle and adipose tissue, and enhances peripheral glucose utilization (4-8). A major long-term, effect of metformin is to increase hepatic insulin sensitivity. Metformin can also antagonize the action of glucagon, thus reducing fasting glucose levels (13). In skeletal muscle, activation of AMP-kinases (AMPK) increases glucose uptake and lipid oxidation. In adipose tissue, activation of AMPK reduces both lipolysis and lipogenesis (14,15). In the heart, metformin increases fatty acids uptake and oxidation, and increases glucose uptake and glycolysis (16).
In enterocytes, metformin increases anaerobic glucose metabolism and utilization. This result in reduced net glucose uptake and increased lactate delivery to the liver. Metformin increases colonic uptake and metabolism of systemic glucose. Metformin may also impact on glucose metabolism by increasing glucagon-like peptide-1 (GLP-1) secretion, an effect that is described for both immediate-release and delayed-release. Recently, it has been shown that it promotes the transport of glucose from the circulation into stool (17). Another potential gut-mediated mechanism of action of Metformin involves alteration of the intestinal microbiome. Metformin-dependent increases in Escherichia spp. and decreases in Intestinibacter spp. were consistently observed in type 2 diabetes associated with metformin use (18).
In addition to the previously discussed mechanisms, many studies suggested a significant effect of metformin through inhibition of appetite probably by increasing the levels of GLP- 1 and by interacting with signaling of other hormones or cytokines (such as ghrelin, leptin and insulin). Metformin enhances the expression of the genes encoding the receptors for both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) in mouse islets and also increases the effects of GIP and GLP-1 on insulin secretion from beta cells. These incretin-sensitizing effects of metformin appear to be mediated by a peroxisome proliferator-activated receptor α-dependent pathway, as opposed to the more commonly ascribed pathway of metformin action involving AMP-activated protein kinase (19,20).
In addition, emerging evidence suggests that metformin could improve obesity-induced meta-inflammation via direct and indirect effects on tissue-resident immune cells in metabolic organs (adipose tissue, the gastrointestinal tract and the liver) (13).
In summary, Metformin has multiple sites of action and multiple molecular mechanisms. It acts directly or indirectly on the liver to lower glucose production, and acts on the gut to increase glucose utilization and loss in the stools, increase GLP-1 and alter the microbiome. At the molecular level, metformin inhibits the mitochondrial respiratory chain in the liver, leading to activation of AMPK, enhancing insulin sensitivity, and reducing the expression of gluconeogenic enzymes. Metformin inhibits hepatic fructose-1,6-bisphosphatase by AMP. These cell and tissue responses are not only a product of dose, but also of treatment duration. Further, pharmacogenetic studies in humans, and careful physiological validation of cell-based metformin studies, are justified to enable a clearer understanding of the key mechanisms that are active in long-term treatment with metformin in humans.
Side-effects and contraindications of metformin
Adverse events were reported to have occurred in all metformin trials, with gastrointestinal side effects the most commonly reported. Moderate-strength evidence indicated that 26% children treated with metformin reported gastrointestinal complaints versus 13% in the control groups (21).
Intestinal side effects (transient abdominal pain, diarrhea, nausea) may occur, but can be minimized in most patients with slow dosage titration over 3 – 4 weeks and instructions to always take the medication with food. The side effects may also be attenuated by the use of extended release formulations (22).
The most common symptoms following overdose appear to include vomiting, diarrhea, abdominal pain, tachycardia, drowsiness, and, rarely, hypoglycemia (16). This gut irritating effect is believed to be mediated through inhibition of serotonin reuptake transporter (SERT)-mediated intestinal reuptake of serotonin resulting in increased intestinal motility and water retention (10).
There is little to no risk of hypoglycemia with monotherapy and hypoglycemia is very uncommon but has been reported in combination regimens, likely due to metformin potentiating other therapeutic agents.
In a 5-year chart review of metformin exposure cases that were reported to the American Association of Poison Control Center (AAPCC), hypoglycemia was reported in 112 (2.8%) of 4072 cases and it was referred to decreased caloric intake, heavy exercise, or sulfonylurea co-ingestion. A pediatric clinical trial compared Metformin to a sulfonylurea (glimepiride). Metformin showed similar efficacy to glimepiride with lower occurrence of hypoglycemia (23).
However, the main alarming effect of metformin is lactic acidosis, although it is a rare condition (24).
Members of the American Diabetes Association and European Association for the Study of Diabetes (25), report that metformin seems safe unless eGFR falls to <30 mL/min per 1.73 m2.
The National Institute for Health and Clinical Excellence specifies that metformin be stopped if serum creatinine exceeds 150 μmol/L (1.7 mg/dL) or eGFR is < 30 mL/min per 1.73 m2 (26).
In contrast, the Canadian Diabetes Association practice guidelines recommend caution with eGFR <60 mL/min per 1.73 m2 and contraindicating its use with eGFR < 30 mL/min per 1.73 m2 (27).
Inzucchi et al. (28) analyzed publications related to the topic between January 1950 and June 2014. They showed that lactate levels were frequently normal in patients receiving metformin with mild CKD (eGFR 60-90 mL/min) or moderate (30-60 mL/min).
Long-term use of metformin has been associated with increased homocysteine levels and malabsorption of vitamin B12. Malabsorption of vitamin B12 occurs with metformin in 30% of diabetic subjects. The earliest manifestations are numbness and paresthesia in the feet, which, unless the vitamin B12 deficiency is corrected, can be followed by weakness, ataxia, sphincter disturbance, and changes in mental status. B12 deficiency –associated macrocytic anemia, is often preceded by the development of neuropathy. While the anemia of vitamin B12 deficiency is reversible, the progress of the neuropathy is only arrested and not reversed with initiation of vitamin B12 therapy. Periodic monitoring of B12 should be considered (29,30).
In summary, metformin appears to be safe in children and adolescents with little risk of hypoglycemia and/or lactic acidosis. The main side effects are gastrointestinal that can be minimized in most patients by using slow dosage titration. However, metformin should not be given to patients with severe renal impairment, hepatic disease, cardiac or respiratory insufficiency, or who are receiving radiographic contrast materials. Metformin should be temporarily discontinued during a gastrointestinal illness.
Therapeutic perspectives in children and adolescents
1. Prediabetes
New criteria defining prediabetes includes the presence of one or more of the following, impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and HbA1c of 5.7–6.4% (31). Prediabetes is a serious health condition which increases the risk of developing T2D, heart disease and stroke. The progression from prediabetes to diabetes is related to insulin resistance and β-cell dysfunction. In people with IGT or recently diagnosed diabetes, youth have lower insulin sensitivity, hyper responsive β-cells, and reduced insulin clearance compared with adults. The impact of responses to glucose-lowering interventions including metformin remains to be determined (32).
Controversy still exit regarding the benefit of metformin to improve glycemic abnormality or to delay the onset of DM in individuals with prediabetes. Results from several clinical trials in the prediabetes population, including children, adolescents, and adults, have indicated that metformin can delay or halt the progression from prediabetes to diabetes (33-35).
In summary, metformin can be considered in selected high-risk patients with prediabetes who are not successfully treated with lifestyle and weight-loss medications and who remain glucose intolerant, as well as for patients with additional CVD risk factors including hypertension, dyslipidemia, or polycystic ovarian syndrome; for patients with a family history of DM in a first-degree relative and/or for patients who are obese.
2. Type 2 Diabetes Mellitus (T2DM)
Thirty-eight million children under the age of 5 were overweight or obese in 2019 and over 340 million children and adolescents aged 5-19 were overweight or obese in 2016 (36). This increased prevalence was accompanied by the appearance and increasing prevalence of type 2 diabetes mellitus (T2DM). Depending on geographic locations, T2DM accounted for 8-45% of new cases of childhood diabetes (37). T2DM is not a benign disease and can be more hazardous and lethal compared to T1DM in children (38).
Metformin has been approved by the Food and Drug Administration (FDA) for children with T2DM aged 10 years and older. The ADA clinical practice and the international Society of Pediatric Diabetes (ISPAD) guidelines (39) recommended metformin as the initial pharmacologic treatment of choice in the incidentally diagnosed or metabolically stable patients (HbA1c <8.5% and asymptomatic) adolescents with T2DM, if renal function is normal. It is used with a concomitant initiation of lifestyle modification program, including nutrition and physical activity (40,41) (Table 2). Figure 2 shows the glycemic effect of 2 months treatment with oral metformin in a newly diagnosed obese adolescent with T2DM.
Table 2.
The ADA recommendations regarding metformin use (Ref.41)
| 1. Initial pharmacologic treatment of youth with T2D should include metformin and insulin alone or in combination, depending on degree of hyperglycemia and metabolic disturbances, and presence or absence of ketosis/ketoacidosis. |
| 2. Metabolically stable patients (HbA1c < 8.5 and no symptoms) should be started on metformin. |
| 3. Patient can be started on 500-1000 mg (or 850 mg when this is the lowest available dose) daily x 7- 15 days, then titration of the dose can be done weekly over 3-4 weeks, depending on patient tolerance, to a maximal dose of 1000 mg BID or 850 mg TID. |
| 4. In patients with ketosis/Ketonuria/ketoacidosis, treatment with subcutaneous or intravenous insulin should be initiated to rapidly correct the metabolic abnormality. Metformin can be started along with insulin, once acidosis is resolved. Transition onto metformin monotherapy can usually be achieved safely over 2-6 weeks. |
| 5. Subsequent treatment 1) If the patient fails to reach target HbA1c of < 7% (<47.5 mmol/mol) within 4 months on metformin monotherapy, addition of basal insulin should be strongly considered. 2) If target is not attained on the combination metformin and basal insulin (up to 1.5 U/kg), prandial insulin should be initiated and titrated to reach target HbA1c < 7%. |
Figure 2.
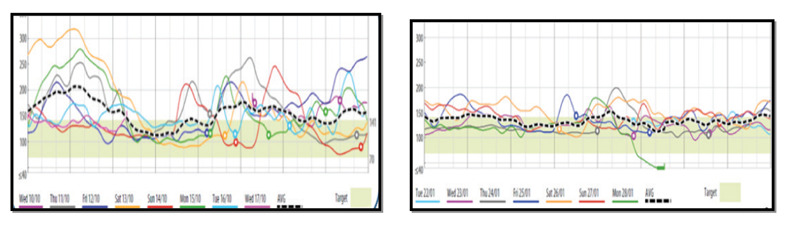
Glycemic effect of 2 months treatment with metformin in an obese adolescent with Type 2 DM (Continuous Glucose Monitors ; CGMS)
Table 2. The ADA recommendations regarding metformin use (Ref.41)
In many studies metformin proved to be both safe and effective for treatment of pediatric patients with T2D (age 10 to 16 years) (42).The treatment was associated with decreased HbA1c and improved glycemic control in half of the participants. Metformin plus rosiglitazone was significantly better than metformin monotherapy (43).
However data from the TODAY study (Treatment Options for T2DM in Adolescents and Youth) tested three approaches: metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle intervention (44,45). The 3.9-year clinical trial showed that metformin plus rosiglitazone was the most effective treatment for T2DM, followed by metformin plus lifestyle intervention and then metformin alone (44,45). A more recent TODAY study showed the effectiveness of metformin plus short-term diabetes education in a large ethnically/racially and geographically diverse population of adolescents with recent-onset T2DM who completed at least 8 weeks of treatment. This study provided short-term improvements in glycemic control and cardio metabolic risk factors in a large adolescent T2DM population (46).
3. Obesity, insulin resistance and prediabetes
a. Prevention of obesity and metabolic syndrome in obese children and adolescents
Rates of childhood overweight and obesity have nearly tripled in the U.S. and western countries over the last 30 years. Concomitant with the rise in prevalence of childhood obesity there has been a corresponding increase in the incidence insulin resistance, prediabetes or impaired glucose tolerance (IGT), T2DM, hypertension, hyperlipidemia, non-alcoholic fatty liver disease (NAFLD) and polycystic ovary syndrome (47).
It has been hypothesized that high insulin blood levels, due to the high concentration of glucose, may excessively stimulate specific insulin-dependent pathways, resulting in acanthosis nigricans, ovarian hyperandrogenism (PCOS), lipodystrophy, accelerated or impaired linear growth, autoimmunity, and muscle cramps (48).
Arslanian et al. (49) compared obese adolescents and adults with IGT, showed a greater insulin resistance in adolescents than adults, despite similar degrees of adiposity and glycemic status. This finding could give an explanation to the less improvement in insulin sensitivity in response to metformin and faster decline of β-cell function registered in youth than in adults with T2DM.
Lifestyle intervention is recommended as the primary treatment for childhood obesity. Reduction of excessive weight gain has a favorable effect on blood pressure, glycemia, and lipid metabolism. However, the long-term outcomes of lifestyle interventions for childhood obesity carried out in a clinical-practice setting have varied widely. Low rate of success in many trials promoted an interest in pharmacological interventions and bariatric surgery to prevent diabetes among obese children and adolescents (50).
Addressing obesity and insulin resistance by drug treatment represents a rational strategy for the prevention of T2DM. Pediatric randomized, controlled trial studies have shown improvement in BMI, fasting serum glucose, fasting insulin, homeostasis model assessment-estimated insulin resistance (HOMA-IR) and lipid profile in patients on metformin therapy for exogenous obesity associated with insulin resistance.
Many studies have confirmed that, in the short term, metformin combined with standardized lifestyle intervention could reduce body weight and improve insulin sensitivity in obese children and adolescents. Nevertheless, many investigations have focused on the effects of metformin on weight loss, but lack of attention was paid to the effects of insulin resistance, despite it was one of the outcomes for these studies. Meanwhile, different studies have different views on whether metformin could improve insulin resistance in obese children and adolescents (51-55).
Recent reviews including a total of 15 randomized controlled trials with metformin treatment (1000–2000 mg/day) over 6 months in children and/or adolescents with obesity and hyperinsulinemia reported that more than 50% of the studies showed a greater reduction in body mass index (BMI) with metformin versus controls (average reduction of -1.3 kg/m2) and about 25% of the studies showed a significant reduction in HOMA-IR in the metformin versus control group (average reduction of -0.6) (56,57). Furthermore, low-dose metformin (850 mg/day) in young children with obesity and risk markers for metabolic syndrome was efficacious, well tolerated, and had potential long-term benefits in the improvement of body composition and inflammation markers (58).
b. Metformin in children who were born SGA and had rapid catch up in weight and central obesity.
Children born small-for-gestational-age (SGA) who experience rapid postnatal catch-up in weight are at risk for central adiposity and hyperinsulinemia. Metformin reduced central adiposity and improved insulin sensitivity in non-obese catch-up SGA children (59).
In conclusion, metformin offers significant potential to intervene to reduce or reverse the metabolic, endocrine, and cardiovascular changes associated with obesity in children and adolescents. Metformin effects were more apparent in studies that lasted only 6 months compared to those that continued for a year or more, suggesting a decline in effectiveness over time. Patients with insulin resistance and higher BMI appeared to have a better benefit. Therefore, metformin appears to be a valuable and useful drug for obese adolescents with prediabetes and insulin resistance but definitely it is not the answer to the obesity epidemic. Larger, long-term controlled studies across different populations are needed to establish the role of metformin as therapy for obesity and cardio-metabolic risk in young people.
4. Type 1-diabetes (T1DM) associated to insulin resistance
Insulin resistance of puberty is well documented in both non diabetic and diabetic adolescents. During puberty increased body fat and BMI correlate strongly with insulin resistance. In adolescents with Type 1-diabetes (T1DM), insulin resistance plays a role in complicating glycemic control and potentially increasing cardiovascular disease (CVD) risk (60-62).
In addition, overweight and obesity are now prevalent among youth with T1DM, and a recent study demonstrated that youth with T1D are more likely to be overweight than their peers without the condition (63). Others have noted more than a twofold increase of overweight and obesity in youth with T1DM since the 1990s when the Diabetes Control and Complications Trial (DCCT) availed the benefits of tight glucose control to reduce the complications of T1DM. Both puberty and obesity are potential factors of increasing insulin resistance and increasing cardio metabolic risks in adolescents with T1DM (64,65).
The addition of metformin to insulin therapy in an attempt to improve insulin sensitivity, support weight control, and reduce insulin dose requirements in patients with T1DM has been assessed in some studies and systematic reviews (66).
A double-blind placebo-controlled study was conducted to assess the effects of metformin on metabolic parameters when added to insulin therapy in 74 pubertal adolescents (ages: 13-20 yr.) with T1DM . Participants were randomized to receive either metformin or placebo for 6 months. Compared to placebo group, metformin caused significant decrease in total daily insulin dose, BMI Z-score and waist circumference at 3 and 6 months compared to baseline, even among normal-weight participants. In the placebo group, total insulin dose and systolic blood pressure increased significantly at 3 months and total insulin dose increased significantly at 6 months. No significant change was observed in HbA1c at any time point between metformin and placebo groups or within either group (67).
In adolescents with poorly controlled T1DM, the effect of metformin added to insulin therapy was reviewed. Two studies confirmed that metformin treatment modestly lowered HbA1c in adolescents with T1DM who had poor metabolic control. Improvements in insulin sensitivity, body composition or serum lipids were not documented in either study. One study showed a decrease in insulin dosage by 10%. Adverse gastrointestinal side effects were reported in both studies and hypoglycaemia in one study (68).
In a metanalysis, five double-blind randomized controlled trials (RCTs) that included 301 adolescents with T1DM were identified. Metformin plus insulin was associated with reduced HbA1c levels, total daily insulin dosage, body mass index (BMI), and body weight. However, the subgroup analysis demonstrated that HbA1c levels were not significantly changed in overweight/obese adolescents and were significantly reduced in the general patients. On the contrary, BMI and body weight were significantly reduced in overweight/obese adolescents but not in the general patients. Metformin was associated with higher incidence of adverse events. The authors concluded that among adolescents with T1DM, administering adjunctive metformin therapy in addition to insulin was associated with improved HbA1c levels, decreased total daily insulin dosage, BMI, and body weight (69).
In summary, adjunctive metformin therapy to insulin in children and adolescents with T1DM showed variable clinical results. The ADA reported that there is insufficient evidence to support the routine use of adjunctive medical therapies including metformin in children with T1DM (70) .
5. Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease (NAFLD) is recognized as the most common cause of liver disease in obese children and adolescents. NAFLD includes a wide spectrum of histologic abnormalities ranging from hepatic steatosis to non-alcoholic steato-hepatitis (NASH) that may progress to cirrhosis, and subsequent end stage liver disease and hepatocellular carcinoma (71).
The prevalence of dysglycemia in youth with NAFLD is higher than in those without NAFLD. In a multicenter cohort of youth with NAFLD, primarily of Hispanic descent, a third of the children with NAFLD had abnormalities in glucose metabolism; 23.4% had prediabetes and 6.5% had T2DM. Moreover, T2DM in youth is associated with greater NAFLD histologic severity than in adults, which may imply a heightened risk of progression to fibrosis, cirrhosis, and hepatic failure. In addition, dyslipidemia is a frequent in children with NAFLD. NASH resolution and histologic improvement are associated with improvements in some forms of dyslipidemia (72,73).
The first-line treatment of NAFLD is currently based on diet and lifestyle modifications. Gradual weight loss (5-10%), calorie-restricted diet, and regular physical exercise lead to a decrease in the incidence of metabolic syndrome, improvement in liver enzyme profile, and resolution of hepatic steatosis. Lifestyle changes also had a significant impact on steatosis, reducing the risk by 61%. However, treatment is more focused on those with NASH because they are at highest risk for progressive liver disease (74,75).
A pharmacological treatment in patients with NAFLD is not universally accepted yet. However, given that insulin resistance plays a key role in the pathogenesis of NAFLD, many studies have evaluated the use of insulin sensitizers (metformin and thiazolidinediones) as a possible treatment for this disease (76).
Several clinical trials have supported the beneficial role of metformin in patients with NAFLD.
Mazza A et al. (77) reviewed 6 open-label trials that have evaluated the liver histology modification together with serum aminotransferase levels and insulin resistance markers’ amelioration in NAFLD patients treated with metformin alone or in association with other drugs. All these studies reported an improvement in the indices of insulin resistance: five studies reported a reduction in liver function test values, and one reported a non-significant increase of these values. In terms of histological improvement, only three trials showed significant differences in inflammation, steatosis, and fibrosis after treatment with metformin.
More recently, a metanalysis of 6 RCTs involving 307 non-diabetic individuals with NAFLD compared the effect of taking metformin compared to controls. Metformin significantly reduced BMI and serum aspartate aminotransferase (AST) with marginal improvement of body weight. The authors emphasized the clinical importance of metformin administration for improving liver function and body composition in non-diabetic NAFLD patients (78).
On the other hand, a metanalysis of randomized clinical trials comparing metformin with placebo or other interventions for treating NAFLD, that included 417 patients, showed that metformin failed to improve any pooled outcome (79).
In conclusion, lifestyle intervention remains the cornerstone of NAFLD treatment. Non-conclusive studies suggested that metformin, when associated to hypocaloric diet and weight control, might add a little benefit in the treatment of children and adolescents with NAFLD irrespective of its effects as insulin sensitizer. However, the heterogeneity of these studies still prevents from reaching firm conclusions about treatment guidelines. ADA did not recommend adjuvant pharmacological treatment of children and adolescents with NAFLD (80). Nevertheless, metformin, because of its metabolic effects and its safety profile, remains a hopeful drug in NAFLD therapy, especially in patients who meet the diagnostic criteria of metabolic syndrome. Larger randomized controlled trials of sufficient duration using clearly defined histological endpoints are needed to fully assess the efficacy of this drug in modifying the natural history of NAFLD in children and adolescents.
8. Premature pubarche
Premature adrenarche (PA) has been linked with unfavorable metabolic features including hyperinsulinism, dyslipidemia, and later-appearing ovarian hyperandrogenism and it may be associated with the metabolic syndrome and this may precede the development of clinical ovarian androgen excess in adolescence. The recent discoveries of two novel monogenic causes of early onset androgen excess, apparent cortisone reductase deficiency and apparent DHEA sulpho-transferase deficiency, support the notion that PA may represent a forerunner condition for PCOs (81,82).
Miller et al. (83) suggested a “unified theory of adrenarche, PCOS and hyperinsulinemia”, and Ibáñez et al. (84) suggested that the underlying mechanism may be abnormal regulation or increased expression of the CYP17A1 androgen synthesis enzyme in the adrenals and ovaries. The researchers suggested precocious treatment with metformin and lifestyle changes aimed at reducing hyperinsulinemia, as some authors have suggested as a treatment for PCOS (85,86). The early recognition of girls at risk of developing hyperinsulinemia androgen excess might enable prevention in childhood (84-86).
In adolescents with premature pubarche, intervention with metformin, as an insulin sensitizing agent, either as mono-therapy or in combination with the anti-androgen flutamide at low doses, appeared to have some beneficial effects on abdominal adiposity, androgen levels and indices of insulin resistance (87,88).
Girls born with low birth weight (LBW) and precocious pubarche are more insulin resistant with evidence of increased cardiovascular risk and have an increased incidence of a polycystic ovarian phenotype in young adulthood. Early metformin therapy, for 4- 5 years, proved valuable in delaying menarche, augmenting height and reducing total, visceral, and hepatic adiposity and lowering circulating levels of androgens and leptin in these girls compared to late therapy (89,90).
In a study of adolescents with hyperandrogenism and T1DM, metformin treatment significantly decreased serum androgens compared to placebo. Metformin therapy did not, however, significantly affect clinical parameters, such as hirsutism, ovulation, and glycemic control; but therapy duration of only 9 months is generally thought to not be long enough to impact on hirsutism (91).
In summary, there is not enough evidence to recommend metformin as first-line therapy for women with PCOS but adding metformin to other PCOS treatment seems an optimal option
7. Polycystic Ovary Syndrome (PCOs)
Polycystic ovary disease (PCOs) is a common endocrine condition which is rapidly gaining epidemic proportions. PCOs affects5–10% of females in the reproductive age-group and is characterized by hyperandrogenism and amenorrhea or oligomenorrhea secondary to chronic anovulation. PCOs is the most common cause of menstrual dysfunction and hyperandrogenism in adolescents (92).
In a recent review and meta-analysis included 12 studies and 149,477 participants the prevalence of PCO in adolescents based on the Rotterdam criteria was 11.04% (95% CI: 6.84-16.09%), based on the National Institute of Health criteria and was 8.03% (95% CI: 6.24-10.01%) based on Androgen Excess and Polycystic Ovary Syndrome Society (93).
The prevalence of PCOS is significantly higher in adolescent girls with obesity compared with adolescent girls without overweight/obesity. Metabolic dysfunction constitutes an important risk associated with PCOs, and it can manifest at an early age. One-third of adolescents with PCOs meet criteria for the metabolic syndrome including (obesity, dyslipidemia, hypertension, and glucose intolerance) compared with approximately 5% of adolescents from the general population . In adolescent girls with PCOS and obesity, there is increased insulin resistance that, when combined with impaired β-cell function, predispose to prediabetes and T2DM (94,95).
Pau et al. (96) conducted an open-Label, Interventional Study showed on women with PCOS (n = 36) who received metformin. They reported that the use of metformin resulted in decreased weight, waist and hip circumferences, and diastolic blood pressure. In addition, it improved glucose effectiveness (not insulin sensitivity) and decreased androgen levels. Furthermore, 61% of the women with PCOs had an improved ovulatory response after metformin treatment.
In 22 adolescents aged 13 to 18 years with hyperinsulinemia and PCO, Participants were randomly assigned to take a 12-week course of either metformin or placebo. Metformin significantly lowered total testosterone concentrations, increased the likelihood of menses, and improved high-density lipoprotein cholesterol levels without affecting measures of insulin sensitivity or body weight (97). Two small randomized, placebo-controlled clinical trials were performed on 79 obese adolescent women with PCOs. Results showed that metformin, in combination with lifestyle modification and oral contraceptives reduced central adiposity, decreased total testosterone, and increased HDL, but did not enhance overall weight reduction (98). Fifteen obese adolescents with PCOs and impaired glucose tolerance received 3 months of metformin (850 mg, twice daily) therapy. Results showed that metformin treatment of those adolescents was beneficial in improving glucose tolerance and insulin sensitivity, in lowering insulinemia, and in reducing elevated androgen levels. Moreover, metformin therapy was associated with attenuation of the adrenal steroidogenic response to ACTH (99).
Al Khalifah RA et al. (100) conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the use of metformin versus oral contraceptive pills (OCPs) for the treatment of PCOs in adolescents, aged 11 to 19 years. Metformin resulted in greater BMI reduction and it was associated with decreased dysglycemia prevalence and improved total cholesterol and low-density lipoprotein levels. Metformin and OCPs were similar in terms of impact on hirsutism.
However, randomized controlled studies and several meta-analyses have changed the picture and put the drug that was once heralded as “magic” in a much contracted place with modest effects on body weight and adiposity, and posed controversial issue on its efficiency in PCOS patients (101,102).
Recommendations from the international evidence-based guideline for the assessment and management of PCOs advised that metformin in additional to lifestyle, could be considered in adolescents with a clear diagnosis of PCOs or with symptoms of PCOs before the diagnosis is made. They advised that combination of metformin shall be given with the combination of COCP for treatment of adolescents with PCOs with BMI > 25 kg/m2 where COCP and lifestyle changes do not achieve desired goals (103).
In summary, currently, metformin appears to offer reasonable benefit to obese adolescents with PCOs, but greater evidence of benefit is required to encourage its wide use.
Conclusions
Metformin is currently approved and widely prescribed for adolescent patients with T2D and PCOs. Adolescents during their pubertal growth spurt have higher insulin resistance compared to other periods in life. Many conditions and metabolic disorders have been shown to be linked to increased insulin resistance including T2DM, obesity and PCOs. The interest in metformin therapy has dramatically increased as the population-based cohort studies showed it can decrease insulin resistance and decline the risk of developing the metabolic syndrome and associated cardiovascular and cerebral morbidities in children and adolescents with high insulin resistance. The clinical trial data and clinical experience over several decades have demonstrated its safety and variable degree of efficacy in these conditions. This review discussed the results of recent studies (in the past 20 years) including systemic reviews , metanalyses as well as RCT and controlled studies on clinical use of metformin and its potential benefits and side effects in the management of obese children and adolescents with T2DM, obesity, NAFLD and those with prediabetes and with premature pubarche and PCOs. Long-term controlled translational and clinical trials need to be continued and expanded to determine if these indications are genuine and reliable and to assess the degree and duration of effectiveness.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patade GR, Marita AR. Metformin: A Journey from countryside to the bedside. J ObesMetab Res. 2014;1:127–130. [Google Scholar]
- 3.Sterne J. Du nouveau dans les antidiabetiques.La NN dimethylamineguanylguanide (N.N.D.G.) (The newantidiabetic NNdimethylamineguanylguanide) Maroc Med. 1957;36:1295–1296. [Google Scholar]
- 4.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilov AV, Abdelaziz SI, AlShammary A, et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab Res Rev. 2019;35(7):e3173. doi: 10.1002/dmrr.3173. doi:10.1002/dmrr.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, Xie C, Wu H, Jones KL, Horowitz M, Rayner CK. Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes. Diabetes Obes Metab. 2017;19:290–293. doi: 10.1111/dom.12812. [DOI] [PubMed] [Google Scholar]
- 7. Bristol-Myers .U.S. Food and Drug Administration. Glucophage (metformin hydrochloride tablets) Label Squibb Information; August 27, 2008. [Google Scholar]
- 8.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22:820–827. doi: 10.1097/FPC.0b013e3283559b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gormsen LC, Sundelin EI, Jensen JB, et al. In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J Nucl Med. 2016;57:1920–1926. doi: 10.2967/jnumed.116.177774. [DOI] [PubMed] [Google Scholar]
- 10.Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr Diabetes. 2017;18:10–16. doi: 10.1111/pedi.12473. [DOI] [PubMed] [Google Scholar]
- 13.Foretz M, Guigas Biollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi S, Katahira H, Ozawa S, Nakamichi Y, Tanaka T, et al. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2005;289:643–649. doi: 10.1152/ajpendo.00456.2004. [DOI] [PubMed] [Google Scholar]
- 15.Bogachus LD, Turcotte LP. Genetic downregulation of AMPK-alpha isoforms uncovers the mechanism by which metformin decreases FA uptake and oxidation in skeletal muscle cells. Am J Physiol Cell Physiol. 2010;299:1549–1561. doi: 10.1152/ajpcell.00279.2010. [DOI] [PubMed] [Google Scholar]
- 16.Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294:2497–2506. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 17.McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copeland KC, Silverstein J, Moore KR, et al. American Academy of Pediatrics. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131:364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):17–32. doi: 10.1111/j.1399-5448.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 21.McDonagh MS, Selph S, Ozpinar A, Foley C. Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 2014;168:178–184. doi: 10.1001/jamapediatrics.2013.4200. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes A. Children and Adolescents: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S126–S36. doi: 10.2337/dc18-S012. [DOI] [PubMed] [Google Scholar]
- 23.Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care. 2007;30:790–794. doi: 10.2337/dc06-1554. [DOI] [PubMed] [Google Scholar]
- 24.Bodmer M, Meier C, Krähenbühl JSS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31:2086–91. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consen sus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hur KY, Kim MK, Ko SH, et al. Metformin Treatment for Patients with Diabetes and Chronic Kidney Disease: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement. Diabetes Metab J. 2020;44:3–10. doi: 10.4093/dmj.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Canadian Diabetes Association (CDA) Clinical Practice Guideline Pharmacologic Management of Type 2 Diabetes. CDA website. http://guidelines.diabetes.ca/Browse/Chapter 13 . [Google Scholar]
- 28.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a sys tematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101(4):1754–1761. doi: 10.1210/jc.2015-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes A. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S73–S85. doi: 10.2337/dc18-S008. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes A. Classification and diagnosis of Diabetes: Standards of medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 32.RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care. 2018;41(8):1696–1706. doi: 10.2337/dc18-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: II. Observations Using the Oral Glucose Tolerance Test. Diabetes Care. 2018;41(8):1707–1716. doi: 10.2337/dc18-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khokhar A, Umpaichitra V, Chin VL, Perez-Colon S. Metformin use in children and adolescents with Prediabetes. Pediatr Clin North Am. 2017;64:1341–1353. doi: 10.1016/j.pcl.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight . [Google Scholar]
- 37.Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9:235–239. [PMC free article] [PubMed] [Google Scholar]
- 38.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: Type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeitler P, Arslanian S, Fu J, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(Suppl. 27):28–46. doi: 10.1111/pedi.12719. [DOI] [PubMed] [Google Scholar]
- 40.Copeland KC, Silverstein J, Moore KR, Prazar GE, Raymer T, et al. American Academy of Pediatrics. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131:364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 41.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2648–2668. doi: 10.2337/dci18-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: A randomized controlled trial. Diabetes Care. 2002;25:89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 43.Group TS, Zeitler P, Epstein L, et al. Treatment options for type 2 diabetes in adolescents and youth: A study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatric Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.TODAY Study Group. Zeitler P, Hirst K, Pyle L, Linder B, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narashimhan S, Weinstock R. Youth-onset of type 2 diabetes mellitus: lessons learned from the TODAY Study. Mayo Clin Proc. 2014;89:806–816. doi: 10.1016/j.mayocp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler P, White Study Group Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes. 2016;17:212–221. doi: 10.1111/pedi.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bloomgarden ZT. Insulin resistance syndrome and nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1518–1523. doi: 10.2337/diacare.28.6.1518. [DOI] [PubMed] [Google Scholar]
- 48.Levy-Marchal C, Arslanian S, Cutfield W, et al. Insulin resistance in children: Consensus, perspective, and future directions. J Clin Endocrinol Metabol. 2010;95:5189–5198. doi: 10.1210/jc.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance : who is worse off ? Pediatr Diabetes. 2017;19:205–211. doi: 10.1111/pedi.12562. [DOI] [PubMed] [Google Scholar]
- 50.Ranucci C, Pippi R, Buratta L, et al. Effects of an Intensive Lifestyle Intervention to Treat Overweight/ Obese Children and Adolescents. Biomed Res Int. 2017;2017:8573725. doi: 10.1155/2017/8573725. doi:10.1155/2017/8573725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tagi VM, Giannini C, Chiarelli F. Insulin Resistance in Children. Front Endocrinol (Lausanne) 2019;10:342. doi: 10.3389/fendo.2019.00342. doi:10.3389/fendo.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lentferink YE, Knibbe CAJ, van der Vorst MMJ. Efficacy of Metformin Treatment with Respect to Weight Reduction in Children and Adults with Obesity: A Systematic Review. Drugs. 2018;78:1887–1901. doi: 10.1007/s40265-018-1025-0. [DOI] [PubMed] [Google Scholar]
- 53.Kay JP, Alemzadeh R, Langley G, D’Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–1461. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 54.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–2080. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 55.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339–348. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 56.Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11(11):CD012436. doi: 10.1002/14651858.CD012436. Published 2016 Nov 29. doi:10.1002/ 14651858.CD012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lentferink YE, Knibbe CAJ, van der Vorst MMJ. Efficacy of Metformin Treatment with Respect to Weight Reduction in Children and Adults with Obesity: A Systematic Review. Drugs. 2018;78:1887–1901. doi: 10.1007/s40265-018-1025-0. [DOI] [PubMed] [Google Scholar]
- 58.Bassols J, Martínez-Calcerrada JM, Osiniri I, et al. Effects of metformin administration on endocrine-metabolic parameters, visceral adiposity and cardiovascular risk factors in children with obesity and risk markers for metabolic syndrome: A pilot study. PLoS One. 2019;14(12):e0226303. doi: 10.1371/journal.pone.0226303. doi:10.1371/ journal.pone.0226303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Díaz M, Bassols J, López-Bermejo A, de Zegher F, Ibáñez L. Metformin treatment to reduce central adiposity after prenatal growth restraint: a placebo-controlled pilot study in prepubertal children. Pediatr Diabetes. 2015;16:538–545. doi: 10.1111/pedi.12220. [DOI] [PubMed] [Google Scholar]
- 60.Cardenas-Vargas E, Nava JA, Garza-Veloz I, et al. The Influence of Obesity on Puberty and Insulin Resistance in Mexican Children. Int J Endocrinol. 2018;2018:7067292. doi: 10.1155/2018/7067292. doi:10.1155/2018/7067292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelsey MM, Zeitler PS. Insulin Resistance of Puberty. Curr Diab Rep. 2016;16(7):64. doi: 10.1007/s11892-016-0751-5. doi:10.1007/ s11892-016-0751–5. [DOI] [PubMed] [Google Scholar]
- 62.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37:2843–2863. doi: 10.2337/dc14-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu LL, Lawrence JM, Davis C, Liese AD, et al. , SEARCH for Diabetes in Youth Study Group.Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 64.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26:2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 65.Purnell JQ, Zinman B, Brunzell JD. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) Study. Circulation. 2013;127:180–187. doi: 10.1161/CIRCULATIONAHA.111.077487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, Petrie JR. The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia. 2010;53:809–820. doi: 10.1007/s00125-009-1636-9. [DOI] [PubMed] [Google Scholar]
- 67.Nadeau KJ, Chow K, Alam S, et al. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double-blinded placebo-controlled study. Pediatr Diabetes. 2015;16:196–203. doi: 10.1111/pedi.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdelghaffar S, Attia AM. Metformin added to insulin therapy for type 1 diabetes mellitus in adolescents. Cochrane Database Syst Rev. 2009;1:CD006691. doi: 10.1002/14651858.CD006691.pub2. Published 2009 Jan 21. doi:10.1002/ 14651858.CD006691.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W, Yang XJ. The Effect of Metformin on Adolescents with Type 1 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2016;2016:3854071. doi: 10.1155/2016/3854071. doi:10.1155/ 2016/3854071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiang JL, Maahs DM, Garvey KC, et al. Type 1 Diabetes in Children and Adolescents:A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2026–2044. doi: 10.2337/dci18-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 72.Newton KP, Hou J, Crimmins NA, et al. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016;170(10):e161971. doi: 10.1001/jamapediatrics.2016.1971. doi:10.1001/jamapediatrics.2016.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corey KE, Vuppalanchi R, Vos M, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Improvement in liver histology is associated with reduction in dyslipidemia in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2015;60:360–367. doi: 10.1097/MPG.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Utz-Melere M, Targa-Ferreira C, Lessa-Horta B, Epifanio M, Mouzaki M, Mattos AA. Non-Alcoholic Fatty Liver Disease in Children and Adolescents: Lifestyle Change – a Systematic Review and Meta-Analysis. Ann Hepatol. 2018;17:345–354. doi: 10.5604/01.3001.0011.7380. [DOI] [PubMed] [Google Scholar]
- 75.Hung CK, Bodenheimer HC Jr. Current Treatment of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin Liver Dis. 2018;22:175–187. doi: 10.1016/j.cld.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 76.Ozturk ZA, Kadayifci A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J Hepatol. 2014;6:199–206. doi: 10.4254/wjh.v6.i4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. doi: 10.1155/2012/716404. doi:10.1155/2012/716404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jalali M, Rahimlou M, Mahmoodi M, et al. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2020:104799. doi: 10.1016/j.phrs.2020.104799. doi:10.1016/j. phrs. 2020.104799. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2648–2668. doi: 10.2337/dci18-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Idkowiak J, Lavery GG, Dhir V, et al. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165:189–207. doi: 10.1530/EJE-11-0223. [DOI] [PubMed] [Google Scholar]
- 82.Utriainen P, Laakso S, Liimatta J, Jääskeläinen J, Voutilainen R. Premature Adrenarche - A Common Condition with Variable Presentation. Horm Res Paediatr. 2015;83:221–231. doi: 10.1159/000369458. [DOI] [PubMed] [Google Scholar]
- 83.Miller WL. The molecular basis of premature adrenarche: an hypothesis. Acta Paediatr Suppl. 1999;88:60–6. doi: 10.1111/j.1651-2227.1999.tb14405.x. [DOI] [PubMed] [Google Scholar]
- 84.Ibánez L, Ong KK, López-Bermejo A, Dunger DB, de Zegher F. Hyperinsulinaemic androgen excess in adolescent girls. Nat Rev Endocrinol. 2014;10(8):499–508. doi: 10.1038/nrendo.2014.58. [DOI] [PubMed] [Google Scholar]
- 85.Ibánez L, Ong K, Valls C, Marcos MV, Dunger DB, de Zegher F. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab. 2006;91:2888–91. doi: 10.1210/jc.2006-0336. [DOI] [PubMed] [Google Scholar]
- 86.Ibánez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab. 2011;96:E1262–7. doi: 10.1210/jc.2011-0555. [DOI] [PubMed] [Google Scholar]
- 87.Geller DH, Pacaud D, Gordon CM. Misra M; of the Drug and Therapeutics Committee of the Pediatric Endocrine Society. State of the Art Review: Emerging Therapies: The Use of Insulin Sensitizers in the Treatment of Adolescents with Polycystic Ovary Syndrome (PCOS) Int J Pediatr Endocrinol. 2011;2011(1):9. doi: 10.1186/1687-9856-2011-9. doi:10.1186/1687-9856-2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibáñez L, Ong K, Ferrer A, Amin R, Dunger D, de Zegher F. Low-dose flutamide-metformin therapy reverses insulin resistance and reduces fat mass in nonobese adolescents with ovarian hyperandrogenism. J Clin Endocrinol Metab. 2003;88:2600–2606. doi: 10.1210/jc.2002-022002. [DOI] [PubMed] [Google Scholar]
- 89.Ibáñez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertil Steril. 2011;95:727–730. doi: 10.1016/j.fertnstert.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 90.Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. Metformin treatment for four years to reduce total and visceral fat in low birth weight girls with precocious pubarche. J Clin Endocrinol Metab. 2008;93:1841–1845. doi: 10.1210/jc.2008-0013. [DOI] [PubMed] [Google Scholar]
- 91.Codner E, Iñíguez G, López P, et al. Metformin for the treatment of hyperandrogenism in adolescents with type 1 diabetes mellitus. Horm Res Paediatr. 2013;80:343–349. doi: 10.1159/000355513. [DOI] [PubMed] [Google Scholar]
- 92.Blank SK, Helm KD, McCartney CR, Marshall JC. (2008) Polycystic ovary syndrome in adolescence. Ann N Y Acad Sci. 2008;1135:76–84. doi: 10.1196/annals.1429.005. [DOI] [PubMed] [Google Scholar]
- 93.Naz MSG, Tehrani FR, Majd HA, et al. The prevalence of polycystic ovary syndrome in adolescents: A systematic review and meta-analysis. Int J Reprod Biomed (Yazd) 2019;17:533–542. doi: 10.18502/ijrm.v17i8.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 95.Alemzadeh R, Kichler J, Calhoun M. Spectrum of metabolic dysfunction in relationship with hyperandrogenemia in obese adolescent girls with polycystic ovary syndrome. Eur J Endocrinol. 2010;162:1093–1099. doi: 10.1530/EJE-10-0205. [DOI] [PubMed] [Google Scholar]
- 96.Pau CT, Keefe C, Duran J, Welt CK. Metformin improves glucose effectiveness, not insulin sensitivity: predicting treatment response in women with polycystic ovary syndrome in an open-label, interventional study. J Clin Endocrinol Metab. 2014;99:1870–1878. doi: 10.1210/jc.2013-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160:241–246. doi: 10.1001/archpedi.160.3.241. [DOI] [PubMed] [Google Scholar]
- 98.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93:4299–4306. doi: 10.1210/jc.2008-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87:1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 100.Al Khalifah RA, Florez ID, Dennis B, Thabane L, Bassilious E. Metformin or Oral Contraceptives for Adolescents With Polycystic Ovarian Syndrome: A Meta-analysis. Pediatrics. 2016;137(5):e20154089. doi: 10.1542/peds.2015-4089. doi:10.1542/peds.2015–4089. [DOI] [PubMed] [Google Scholar]
- 101.Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1:117–128. doi: 10.1177/2042018810380215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. [published correction appears in Hum Reprod Update. 2016;22:408–409. doi: 10.1093/humupd/dmv063. [DOI] [PubMed] [Google Scholar]
- 103.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


