Abstract
The high prevalence of middle ear disease with related hearing loss in Kabuki syndrome requires the diagnostic and treatment expertise of otologists. This case report describes outcomes and changes in the quality of life of a patient affected by Kabuki syndrome with a history of recalcitrant chronic otitis media and mixed hearing loss who had undergone several unsuccessful surgical procedures before solving his problems by means of subtotal petrosectomy and active middle ear implant. (www.actabiomedica.it)
Keywords: Kabuki syndrome, chronic otitis media, hearing loss, active middle ear implant
Introduction
Kabuki syndrome (KS) is a rare disorder characterized by a constellation of dysmorphic facial features, postnatal growth retardation, developmental delay, and skeletal anomalies (1). Mutations in KMT2D have been identified as the main cause for KS, less frequent are mutations in KDM6A (2). Different otolaryngologic problems, including dysmorphic pinnae, chronic otitis media (COM), hearing loss (HL) and airway problems have been described in KS (3). COM with HL occurs in approximately 70% of patients and can hinder speech acquisition and adequate quality of life (1). Therefore, early evaluation and intervention with the best rehabilitation option should be mandatory. However, treatment of COM and hearing rehabilitation can be challenging in KS. This report described a patient who ultimately solved his ear problems by means of subtotal petrosectomy (SPT) and active middle ear implant, after multiple unsuccessful surgeries. The aim of our study was to contribute to the knowledge about challenges otologists might deal with in treating COM and HL in KS.
Case presentation
The patient is a 16-year old boy born by vaginal delivery at term of an uneventful pregnancy with birth weight 3600 g. At 8 years, KS was diagnosed due to typical phenotypic traits, including elongated eyelid closure, long eyelashes, high palate, depressed nasal tip, prominent ears, and fingertips with fetal aspect; furthermore, he had mild mental retardation. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from the patient and his parents before data collection. Clinical history included otitis media with effusion on both ears with bilateral mixed HL. At the age of 5 years, the patient was fitted with hearing aids. Unfortunately, two years later, he started to experience bilateral recurrent episodes of purulent otorrhea, resistant to medical therapy. At the age of 8 years, the patient underwent tympanoplasty in the left ear but re-perforation of the tympanic membrane with othorrea occurred few months after surgery. Due to recurrent infection, the patient abandoned hearing aids and was fitted with a head band bone-conduction hearing aid until the age of 11 years. Successively, discomfort resulting from the persistent pressure of the aid on the soft tissue and unsatisfactory sound quality prompted the patient to ask for a bone anchored hearing aid (BAHA). At age of 11 years, two attempts to seat a BAHA at the right ear were unsuccessful with loss of the fixture few days after surgery. Despite failures, in November 2016 the patient came to our Department strongly motivated to verify how his HL could be rehabilitated. Otoscopy showed a perforation of the eardrum with granulation tissue on the tympanic remnants in both ears; purulent secretion was present in the right ear. Audiologic evaluation showed a bilateral mixed HL with a mean air-bone gap of 36 dB in the right ear and 31 dB in the left ear (Figure 1). Speech recognition threshold (SRT) was 60 dB in the right ear and 55 dB in the left ear. Speech discrimination score (SDS) was 90% at the most comfortable level (MCL) of 90 dB, on both ears. Temporal bone computed tomography (TBCT) scan showed cortical portion of the mastoid to be characterized by cancellous rather than compact bone, as usually found in this area. Middle ear was filled with soft tissue density and mastoid air cells were obliterated suggesting COM. No inner ear malformations were present. On the basis of clinical and radiological findings, we proposed a STP along with implantation of a Vibrant Soundbridge (VSB; Medel, Innsbruck, Austria) or a Bonebridge (BB; Medel, Innsbruck, Austria) in order to overcome COM as well as HL. After extensive counseling about the two devices, the patient decided for BB. In January 2017, SPT was performed with external auditory canal and Eustachian tube closure; the cavity was obliterated with abdominal fat. A retrosigmoidal positioning of the implant was chosen on the basis of preoperative radiologic measures (Figure 2). No intraoperative or postoperative complications occurred. The device was activated 3 weeks after surgery. Two months after device activation, pure tone threshold in presence of contralateral noise was 30 dB in the right ear and 40 dB in the left ear in the free-field condition. SRT was of 25 dB in the right ear and 35 dB in the left ear and SDS of 100% at MCL of 50 dB, on both ears. With 1-year follow-up, clinical course and postoperative imaging (Figure 3) confirmed successful eradication of the disease.
Figure 1.
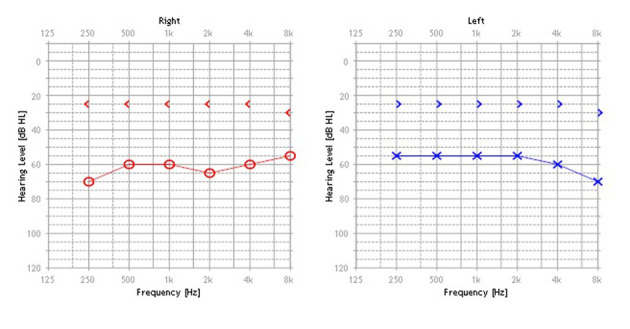
Initial pure tone audiogram
Figure 2.
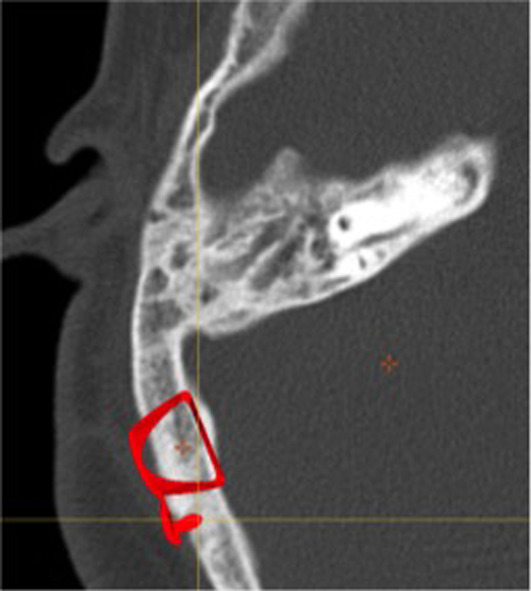
CT scan, axial view. Preoperative measures
Figure 3.
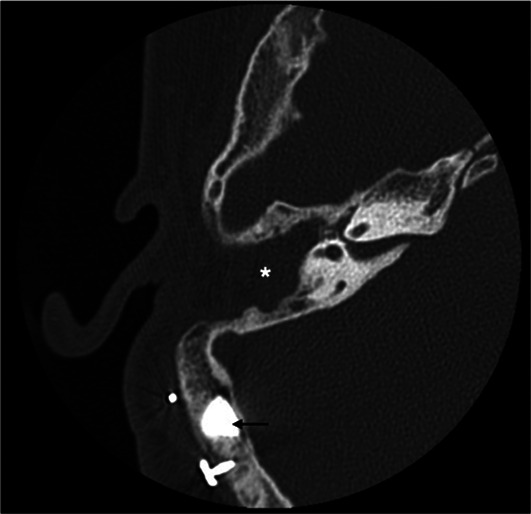
One-year postoperative CT. Device in retrosigmoidal postion (black arrow) and surgical cavity completely obliterated by abdominal fat
Discussion
A high prevalence of COM and HL has been reported in patients affected by KS (4). Conductive HL appears to be the most frequent hearing impairment, followed by a sensorineural type (5). It has been conjectured that the high incidence of middle ear disease may be related to Eustachian tube dysfunction (3).
As in other syndromes characterized by cranio-facial anomalies, treatment of COM in KS may present difficult challenges to otologists. Peterson-Falzone et al. (3) described a child who had recurrent ear disease ultimately requiring bilateral mastoidectomies and amplification for what should have been simply medically-manageable otitis media. Similarly, recalcitrant othorrea in our patient ultimately required SPT for what should have been simply COM. Hearing rehabilitation was also challenging because chronic ear infection prevented conventional hearing aids fitting and 2 attempts to seat a BAHA were unsuccessful. Although such finding has not been reported in the literature, we have hypothesized that cancellous rather than compact bone in the cortical portion of the mastoid was the cause of precocious loss of the fixtures. Therefore, a device for which osseointegration was not needed has been proposed to the patient.
BB is a semi-implantable, active bone conduction implant consisting of an external part, the audio processor, and an implanted part, the bone conduction implant (BCI). The BCI includes a demodulator, a receiver coil and a transducer. Information from the audio processor is sent transcutaneously to the BCI so that the transducer vibrates in a controlled manner, specific to each patient’s hearing needs. For signal transmission, osseointegration of the cortical screws is not crucial (6). BB is intended for patients who have conductive or mixed mild-to moderate HL (pure tone average bone conduction threshold should be better than or equal to 45 dB); BB is also indicated for single-sided deafness. Since the implant is relatively bulky, its main challenge is finding adequate bone depth and space (7). BB needs to be placed around important structures such as the sigmoid sinus and dura; thus, preoperative planning using patients’ TBCT scans is fundamental to choose the best placement option. Depending on the anatomy and on the patient’s history, BB may be placed in sinodural angle or in retrosigmoidal position (8). Radiological and clinical findings of our patient prompted us to place the implant in retrosigmoidal position, away from the cavity. Rehabilitative results allow the patient to regain social hearing and improve his quality of life; furthermore, the patient was satisfied with the sound quality of BB.
Conclusion
Although clinical heterogeneity of KS makes difficult to generalize the results of individual cases, our experience indicates that SPT along with implantation of active middle ear implant is a reliable and effective procedure in treating recalcitrant COM and related HL in syndromes characterized by craniofacial anomalies, such as KS.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Cheon CK, Ko JM. Kabuki syndrome: clinical and molecular characteristics. Korean J Pediatr. 2015;58:317–324. doi: 10.3345/kjp.2015.58.9.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepri FR, Cocciadiferro D, Augello B, et al. Clinical and neurobehavioral features of three novel Kabuki syndrome patients with mosaic KMT2D mutations and review of literatrure. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19010082. Doi: “http://dx.doi.org/10.3390/ijms19010082. ”10.3390/ijms19010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson-Falzone SJ, Golabi M, Lalwani AK. Otolaryngologic manifestations of Kabuki syndrome. Int J Pediatr Otorhinolaryngol. 1997;38:227–36. doi: 10.1016/s0165-5876(96)01443-7. [DOI] [PubMed] [Google Scholar]
- 4.Vesseur A, Cillessen E, Mylanus E. Cochlear implantation in a Patient with Kabuki Syndrome. J Int Adv Otol. 2016;12:129–31. doi: 10.5152/iao.2016.2004. [DOI] [PubMed] [Google Scholar]
- 5.Schrander-Stumpel CT, Spruyt L, Curfs LM, Defllor T, Schrander JJ. Kabuki syndrome: clinical data in 20 patients, literature review, and further guidelines for preventive management. Am J Med Genet A. 2005;132:234–243. doi: 10.1002/ajmg.a.30331. [DOI] [PubMed] [Google Scholar]
- 6.Sprinzl GM, Wolf-Magele A. The bone conduction Bonebridge hearing implant: indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol. 2016;41:131–143. doi: 10.1111/coa.12484. [DOI] [PubMed] [Google Scholar]
- 7.Barbara M, Perotti M, Gioia B, Volpini L, Monini S. Transcutaneous bone-conduction hearing device: audiological and surgical aspects in a first series of patients with mixed hearing loss. Acta Oto-Laryngol. 2013;133:1058–1064. doi: 10.3109/00016489.2013.799293. [DOI] [PubMed] [Google Scholar]
- 8.Law EK, Bhatia KS, Tsang WS, Tong MC, Shi L. CT pre-operative planning of a new semi-implantable bone conduction hearing device. Eur Radiol. 2016;26:1686–1695. doi: 10.1007/s00330-015-3983-x. [DOI] [PubMed] [Google Scholar]


