Abstract
Hashimoto encephalopathy (HE) is a rare but controversial entity encompassing a variety of neuropsychological presentations in the setting of autoimmune thyroid disease. HE, mostly described in adults, with a female-to-male ratio of 4:1, is a relatively rare entity in the pediatric population and probably under recognized as a cause of acute encephalopathy in children and adolescents. A number of pathogenetic mechanisms have been suggested. Female prevalence, presence of autoantibodies, fluctuating course, and response to immunomodulatory therapy suggest the autoimmune nature of the disease. Existing diagnostic criteria for adults require modification to be applied to children and adolescents, who differ from adults in their clinical presentations, clinical findings, autoantibody profiles, treatment response, and long-term outcomes. A combination of neurological findings, positive antithyroid autoantibodies, and responsiveness to steroids is diagnostic of HE. We add a new case of HE in an adolescent girl and review the current HE literature. (www.actabiomedica.it)
Keywords: Hashimoto encephalopathy, adolescent, thyroiditis
Introduction
Hashimoto encephalopathy (HE) is an autoimmune condition with varied neurological and psychiatric features, associated with high serum antithyroid autoantibodies (ATAs). It was first described by Brain et al. in 1966 (1). Its prevalence is 2.1/100.000; however, it is uncommon in the pediatric population (2). Clinical presentation of HE varies from stroke-like signs, seizures including status epilepticus, amnesic syndrome, ataxia, myoclonus, cognitive impairment, and dementia to psychiatric manifestations (3,4). The diagnosis of HE is clinical and based on the highly variable neurological conditions, the detection of ATAs in serum, and the exclusion of other potential etiologies. A clinical response to corticosteroid therapy is supportive of the diagnosis. The importance of early diagnosis and appropriate treatment is paramount (5).
The intention of this article is to raise awareness of HE, a potential cause of a various neurological and psychiatric condition in pediatric age. So, we report a particular case of HE in an adolescent girl which presented with altered cognitive status and behavioral changes and we carried out a full review of the literature on epidemiology, clinical, diagnosis and treatment regimens in HE.
Case report
A 12-year-old, previously healthy girl, was admitted to our hospital for an acute history of headache, vomiting, tremors, dysarthria, spatio-temporal disorientation, hyposthenia of the lower limbs and slightly blurred vision. The parents also noticed mood worsening, with alternating phases of depression and irritability few weeks before.
On clinical examination, she presented dysphonia, difficulty in maintaining the upright position with Romberg slightly positive. There were no meningeal signs or focal deficit. Her pupils were symmetrical and bilaterally reacting normally to light stimulus. There were no signs of meningeal irritation. She had uncontrolled emotional outbursts like purpose less laughing. Initial blood tests including blood counts, renal and liver function tests, C-reactive protein, erythrocyte sedimentation rate, serum ammonia, and blood gas analysis were normal. Cranial computed tomography (CT) was negative for pathologies. Results of autoimmune, toxics and infectious markers and cerebrospinal fluid (CSF) studies for bacterial and viral infection were negative. A mild increase of protein levels in CSF was present. Thyroid function tests were also within normal limits: free T3 titer was 4.07 pg/mL (normal: 2.3-4.2 pg/ml); free T4 titer was 1.13 ng/dL (normal: 0.89-1.76 ng/dl) and TSH titer was 5.04 μUI/mL (normal: 0.55- 4.78 IU/ml). High levels of antithyriod antibodies were noted, with anti-thyroglobulin (TG-Ab) 176.90 UI/mL (normal: 0-100 IU/ml) and anti-thyroid-peroxidase (TPO-Ab) 11.853.00 UI/mL (normal: 0-100 U/ml). Thyroid ultrasound: thyroid gland with subverted ecostructure with presence of hyperechogenic shoots which determine pseudo-nodular character in both lobes (marked vascularization is appreciated at color-doppler as for inflammatory alterations). CSF antithyroid antibodies were positive (TPO-Ab 28.00 IU/ml, TG-Ab 15 IU/ml).
Brain magnetic resonance imaging (MRI) showed hyperintense spots in white matter of frontal-parietal lobe, bilateral, more evident on the right in FLAIR sequence without enhancement of the focal lesions on T1 post contrast sequence (Figure 1). Electroencephalography (EEG) showed bilateral diffuse slow wave activity, without epileptiform activity, suggestive of encephalopathy.
Figure 1.
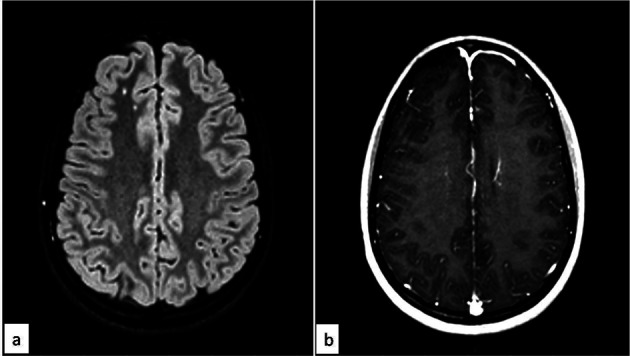
Magnetic Resonance Imaging of the brain, FLAIR sequence (fig. 1a), revealed hyperintense spots in white matter of frontal-parietal lobe, bilateral, more evident on the right. T1 post contrast sequence (fig. 1b) revealed absence of enhancement of the focal lesions
Clinical, MRI, EEG, electromyography and laboratory findings led to the diagnosis of HE, and high-dose methyl-prednisolone was administered intravenously (1 g/day) for the first 3 days followed by oral prednisone for the following 30 days. The treatment induced a rapid disappearance of tremors and dysarthria followed by subsequent improvement of headache and humoral tone.
On 5 years of follow up, the patient is asymptomatic, off steroids, and her thyroid profile is normal.
Written consent for publication of this case report and accompanying images were obtained from the parents of the patient.
Discussion
HE is a rare clinical condition associated with Hashimoto thyroiditis (HT) (1, 6). HE has also been called with acronyms: SREAT (steroid-sensitive encephalopathy associated with autoimmune thyroiditis) (7,8) because affected patients usually have high titers of ATA and respond well to therapy with corticosteroids or NAIM (non-vasculitic autoimmune inflammatory meningoencephalitis) due to the absence of cerebral vasculitis observed in brain biopsies in affected subjects (9). The incidence of HE is higher in female (about 70–88% of female patients) with a female-to-male ratio of 4:1 (10). The risk factors for the development of HE are unclear (11), although few possible risk factors have been mentioned in various case reports such as the effects of adjuvant-like or immune-stimulating agents of exogenous (alpha-interferon, Epstein-Barr virus) (12-14) or endogenous origin (estrogens) (15) and lithium which produces TSH resistance of thyrocytes (16). The average onset is about 40 years old (17).
It is relatively rare in the pediatric population, with about 60 cases described to date and an unknown prevalence (10,18). The majority of pediatric cases are teenagers, with a median age at diagnosis of 14 years (10,19). The pathophysiology of HE is still inconclusive. Three major mechanisms were suspected in pathogenesis of HE: related to vasculitis; related to autoantibody (including those against not only thyroid, but also extra-thyroid antigens); related to toxic/deregulatory influence of some hormones, excessively produced in response to hypothyroidism.
Initially HE was interpreted as a kind of immunopathological vasculitis, that alters the brain microvasculature and procedures local oedema or a vasculitis that results from endothelial inflammation (brain). The single proof for that was a presence of hypoperfusion zones on brain computed tomographic images in some of the cases (20,21). This hypothesis is also supported by the discovery of anti–α-enolaseantibodies, which are abundantly expressed in endothelial cells and have been found in other vasculitic diseases such as Kawasaki disease (22).
An autoimmune pathogenesis of HE has been assumed on the basis of the presence of ATAs and the response of some patients to steroids or other immunotherapy. In that possible mechanism, ATAs could attack antigens that are shared by the thyroid and the brain: TG-Ab recognize antigens of cerebral vasculature, TSH-RAb bind to cortical neurons, and TPO-Ab localize on astroglial cells of cerebellum and cerebral cortex (23,24). However, there is not a relationship between the extent of ATAs elevation and the severity of HE. Rather than playing a direct role in the pathophysiology of HE, it is suggested that thyroid-associated anti-TPO is a hallmark of HE (25).
The hypothesis of toxic effects of thyrotropin-releasing hormone (TRH) is based on the idea that the encephalopathic features of HE are caused by an increase in cerebral TRH (26).TRH is released by the hypothalamus and stimulates TSH production in the pituitary, which subsequently stimulates thyroid hormone production in the thyroid. Only one trial of TRH in a patient with HE has been carried out that demonstrated that TRH infusion effectively produced myoclonus and tremor that were similar to the patient’s symptoms during an exacerbation (26).
HE can manifest in many different ways, but it usually manifested by cognitive impairment, seizures, focal neurologic deficits, myoclonus, neuropsychiatric features and stroke-like episodes (27). The course of the disease may be recurrence–remission or gradual progression. Seizure disorders were seen in about 60– 70% patients, and many of them showed as the first manifestation of the disease. Seizure disorders are more common in children (about 80%) compared to adults (10,28). The mechanisms of seizure disorders in HE are not yet fully understood: possible autoimmune mechanisms may be involved. Seizure presentations include progressive focal or generalized onset seizures and new-onset status epilepticus (SE) (29). SE includes epilepsia partialis continua (EPC) and non-convulsive SE (NCSE), reported in 12% of HE patients (29,30). The most common seizure pattern was focal onset seizures with secondary generalization (7). Patients with HE can also manifest itself with unusual symptoms such as pseudobulbar palsy, sensori-motor polyneuropathy, catatonic symptoms, vertigo, muscle weakness, chorea, opsoclonus and a trigeminal neuralgia type headache (31-36).
The variety of clinical manifestations makes it difficult to differentiate HE from other diseases caused by vascular, infectious, toxic-metabolic, and autoimmune factors, metastasis/neoplasia, iatrogenic/inborn errors of metabolism, neurodegenerative diseases, and systemic diseases/seizures (37). Sometimes the presence of autoimmune inner ear disease is possible, characterized by an immune-mediated vestibule-cochlear dysfunction, probably secondary to the autoimmune reaction of ATAs (38).
In our experience of about seven cases of HE, we had several clinical manifestations both at onset and during follow-up. The most particular symptoms referred were: severe insomnia or hypersomnia, impairment in gross and/or fine motor movements, impairments in memory or learning and exhibits mutism or aphasia, disability, unable to attend school/community activities.
As regards thyroid function, it is reported in literature that 25%-30% of HE cases had subclinical hypothyroidism, 17%-30% had hypothyroidism, 7% had hyperthyroidism and 18%-45% were euthyroid (17).
In more than one third of observations there were comorbidities of HE with other systemic and/or organ specific autoimmune diseases, e.g. lupus, Sjogren’s syndrome, atrophic gastritis/pernicious anemia and myasthenia gravis, sarcoidosis, rheumatoid arthritis, Guillain-Barre syndrome and autoimmune hypophysitis (39).The most remarkable co-morbidity of HE is an association with peripheral poly-neuropathies, involving sensory and autonomic nerves, observed in an adolescent girl (40).
HE is a diagnosis of exclusion and toxins, infections, metabolic, neoplastic and other neuronal antibody syndromes should be excluded. It is suspected in patients with highly variable neurological and neuropsychiatric conditions accompanied by normal or nonspecific MRI and CSF findings and the detection of an elevated concentration of circulating serum ATAs. A clinical response to corticosteroid therapy is supportive of the diagnosis. The first cluster of criteria for the diagnosis of HE was proposed by Peschen-Rosin et al. in 1999 (41). Subsequently in 2016, Graus et al. modified diagnostic criteria for HE (42) (Table 1).
Table 1.
Diagnostic criteria for Hashimoto’s encephalopathy (From: Ref. 42)
| All six criteria are needed for the diagnosis |
| 1. Encephalopathy with seizures, myoclonus, hallucinations, or stroke-like episodes |
| 2 Thyroid disease (Subclinical or mild overt usually hypothyroidism) |
| 3. Brain MRI normal or with non-specific abnormalities |
| 4. Presence of serum thyroid (thyroid peroxidase, thyroglobulin) antibodies |
| 5. Absence of other neuronal antibodies in serum and CSF |
| 6. Reasonable exclusion of alternative causes |
Neuroimaging studies, EEG, and cerebrospinal fluid analysis can be supportive, although they are not diagnostic. Most cases demonstrate a narrow spectrum of EEG findings. Diffusely abnormal electroencephalogram without specific signs has been described in a few pediatric cases. Muhle et al. described a widespread slowing, with an occurrence of generalized delta activity in two recordings (43). The most CSF is the presence of elevated protein and less frequently leukocyte elevation (44). CSF thyroid antibodies have been reported to be elevated in cases of HE (20).
CT is usually normal in patients of all ages but could reveal cerebral atrophy or ventricular dilatation (45,46).
Brain MRI in HE could show various degrees of nonspecific abnormality including white matter abnormalities, cortical irregularities, ischemic lesions and focal vasogenic edema. MRI studies in children have mostly shown prolonged T2-weighted signals of the subcortical white matter, suggesting inflammation or demyelination (44, 47).
All patients should have a brain MRI with and without gadolinium. Over half of patients with AE will have a normal brain and spine MRI at diagnosis. Inflammatory lesions (high signal on T2 and fluid-attenuated inversion recovery sequences) may develop over time, and cerebral atrophy may occur months later.
The current standard treatment of HE is the use of corticosteroids in addition to the treatment of any concurrent dysthyroidism. Generally, the symptoms improve or completely resolve over a few months. The major part of cases, currently over 90% of them, are prone to glucocorticoid treatment with initial high dose (500-1000 mg of methylprednisolone per day for adults or 30 mg/kg of body mass for children) administered for 3-7 days, followed by a prednisone (1–2 mg/kg/days, max 60 mg/days) for 6 to 8 weeks (48). The dose should be gradually reduced. Initially prednisone doses ranging from 50 mg to 150 mg daily have also been used. No greater benefit of one treatment has been demonstrated versus another (49).
In resistant cases of disease other immunosuppression therapies include plasma exchange, IVIG, methotrexate, azathioprine, cyclophosphamide, and mycophenolate mofetil. The first case report of IVIG therapy in HE child has been described by Berger et al. with significant benefit (9). Some authors reported the achievement of a 7-year lasting remission after IVIG treatment in a HE patient poorly responded to glucocorticoids (50). Plasmapheresis was successfully used in steroid non-responders and in case worsened after corticosteroids, presumably because of removal from blood of certain autoantibodies or inflammatory mediators (51).
HE with clinical response to therapeutic plasma exchange (TPE) has been described in literature with reliable reduction in anti-thyroid peroxidase (anti-TPO) antibodies and corresponding improvement in clinical status (52). It can also be used alone or in combination with steroids, immunoglobulins, or other immunosuppressive agents. In 2016, the American Society for Apheresis published treatment guidelines for plasma exchange and proposed that HE is a category II indication for plasma exchange with 2C level of recommendation.
The American Society for Apheresis recommends a total of 3–9 sessions of plasma exchange performed once every other day for HE. The volume of plasma exchanged per treatment should be 1–1.5 times the estimated plasma volume, and albumin should be used as the replacement solution (53,54).
Treatment responses were retrospectively reviewed in six consecutive children diagnosed with HE, between August 2008 and July 2016. Corticosteroid (intravenous methylprednisolone 10–15 mg/kg/day for 3–5 days or prednisolone 2 mg/kg/day for 4 weeks) was administered as initial therapy in all patients. Three did not respond and were treated with i.v. immunglobulin (IVIG: 2 g/kg total, administered over 5 days), resulting in two responders. After the failure of corticosteroid and IVIG, one patient underwent plasmapheresis, and her neurologic manifestations began to remit (55). The prognosis of HE when treated is good. However, the rate of sequela is greater in children and adolescents (56).
Conclusion
Autoimmune encephalopathy, as a complication of HT, should be considered also in pediatric patients with potential or known autoimmune thyroiditis with atypical neuropsychiatric manifestations not responding to conventional treatment. Garg et al. (57) reviewed the literature for pediatric patients with HE. Forty-seven articles detailing 74 pediatric patients and the present patient were included. Average age of presentation was 13.1 years (range: 2 years 10 months–18 years) and a majority are female (F:M = 5:1). Duration of symptoms before diagnosis ranged from few days to 9 years. Around 20% presented with acute onset. The most common clinical features awere seizures (69%, status epilepticus: 25%, refractory epilepsy: 3%), alteration in sensorium (60%), psychosis (37%), behavioral alterations (33%), headache (25%), and cognitive decline (21%). Other presenting symptoms included academic issues, sleep disturbances, movement disorders, mood changes, and emotional liability. Recurrent strokes, myoclonus, ataxia, and tremors were less common compared to adults (18).
The diagnostic process of this pathology is not simple and requires a careful differential diagnosis with other numerous pathologies that can induce a state of encephalopathy. In the last decades there has been increased knowledge of thyroid/brain/immunity interactions indicating the importance of multidisciplinary approach of endocrinologists, neurologists, psychiatrists and pediatricians for an early recognition and treatment. Early diagnosis and treatment is very important considering that residual cognitive impairment could occur in patients not treated in time.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Brain L, Jellinek EH, Ball K. Hashimoto’s disease and encephalopathy. Lancet. 1966;2:512–514. doi: 10.1016/s0140-6736(66)92876-5. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto Encephalopathy Case Reports. Turk J Anaesthesiol Reanim. 2018;46:402–405. doi: 10.5152/TJAR.2018.90698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma PM, Javali M, Mahale R, Madhusudhan BK, Majeed AA, Srinivasa R. Hashimoto encephalopathy: a study of the clinical profile, radiological and electrophysiological correlation in a tertiary care center in South India. J Neurosci Rural Pract. 2015;6:309–314. doi: 10.4103/0976-3147.158753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattozzi S, Sabater L, Escudero D, et al. Hashimoto encephalopathy in the 21st century. Neurology. 2020;94:e217–e224. doi: 10.1212/WNL.0000000000008785. [DOI] [PubMed] [Google Scholar]
- 5.Kutluk MG, Haznedar P, Bektas O, et al. Hashimoto’s Encephalopathy in Children: Different Manifestations of Five Case. Acta Neurol Belg. 2019;119:595–599. doi: 10.1007/s13760-019-01191-7. [DOI] [PubMed] [Google Scholar]
- 6.Ferracci F, Bertiato G, Moretto G. Hashimoto’s encephalopathy: epidemiologic data and pathogenetic considerations. J Neurol Sci. 2004;217:165–168. doi: 10.1016/j.jns.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Castillo P, Woodruff B, Caselli R, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63:197–202. doi: 10.1001/archneur.63.2.197. [DOI] [PubMed] [Google Scholar]
- 8.Mahad DJ, Staugaitis S, Ruggieri P, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis and primary CNS demyelination. J Neurol Sci. 2005;228:3–5. doi: 10.1016/j.jns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Caselli RJ, Boeve BF, Scheithauer BW, O’Duffy JD, Hunder GG. Non-vasculitic autoimmune inflammatory meningoencephalitis (NAIM): a reversible form of encephalopathy. Neurology. 1999;53:1579–1581. doi: 10.1212/wnl.53.7.1579. [DOI] [PubMed] [Google Scholar]
- 10.Alink J, de Vries TW. Unexplained seizures, confusion or hallucinations: think Hashimoto encephalopathy. Acta Paediatr. 2008;97:451–453. doi: 10.1111/j.1651-2227.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 11.Montagna G, Imperiali M, Agazzi P, et al. Hashimoto’s encephalopathy: a rare proteiform disorder. Autoimmun Rev. 2016;15:466–476. doi: 10.1016/j.autrev.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Arrojo M, Perez-Rodriguez MM, Mota M, et al. Psychiatric presentation of Hashimoto’s encephalopathy. Psychosom Med. 2007;69:200–201. doi: 10.1097/PSY.0b013e31803174c0. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch M, Koskinas J, Tzannos K, et al. Hashimoto’s encephalopathy with pegylated interferon alfa-2b and ribavirin. Ann Pharmacother. 2005;39:1745–1748. doi: 10.1345/aph.1G144. [DOI] [PubMed] [Google Scholar]
- 14.Hori T, Oike F, Hata K, et al. Hashimoto’s encephalopathy after interferon therapy for hepatitis C virus in adult liver transplant recipient accompanied by post-transplant lymphoproliferative disorder related to Epsteine-Barr virus infection. Transpl Infect Dis. 2010;12:347–352. doi: 10.1111/j.1399-3062.2010.00508.x. [DOI] [PubMed] [Google Scholar]
- 15.Sellal F, Berton C, Andriantseheno M, Clerc C. Hashimoto’s encephalopathy: Exacerbations associated with menstrual cycle. Neurology. 2002;59:1633–1635. doi: 10.1212/01.wnl.0000034178.22733.75. [DOI] [PubMed] [Google Scholar]
- 16.Nagamine M, Yoshino A, Ishii M, et al. Lithium-induced Hashimoto’s encephalopathy: a case report. Bipolar Disord. 2008;10:846–848. doi: 10.1111/j.1399-5618.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferracci F, Carnevale A. The neurological disorder associated with thyroid autoimmunity. J Neurol. 2006;253:975–984. doi: 10.1007/s00415-006-0170-7. [DOI] [PubMed] [Google Scholar]
- 18.Graham BR, Shiff N, Nour M, Hasal S, Huntsman R, Almubarak S. Hashimoto encephalopathy presenting with stroke-like episodes in an adolescent female: a case report and literature review. Pediatr Neurol. 2016;59:62–70. doi: 10.1016/j.pediatrneurol.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Watemberg N, Greenstein D, Levine A. Encephalopathy associated with Hashimoto thyroiditis: pediatric perspective. J Child Neurol. 2006;21:1–5. doi: 10.1177/08830738060210010201. [DOI] [PubMed] [Google Scholar]
- 20.Ferracci F, Moretto G, Candeago RM, et al. Antithyroid antibodies in the CSF: their role in the pathogenesis of Hashimoto’s encephalopathy. Neurology. 2003;60:712–714. doi: 10.1212/01.wnl.0000048660.71390.c6. [DOI] [PubMed] [Google Scholar]
- 21.Bertoni M, Falcini M, Sestini S, Niccoli L, Nannini C, Cantini F. Encephalopathy associated with Hashimoto’s thyroiditis :anadditional case. Eur J Intern Med. 2003;14:434–437. doi: 10.1016/j.ejim.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Chun JK, Lee TJ, Choi KM, Lee KH, Kim DS. Elevated anti-alpha-enolase antibody levels in Kawasaki disease. Scand J Rheumatol. 2008;37:48–52. doi: 10.1080/03009740701607075. [DOI] [PubMed] [Google Scholar]
- 23.Yoneda M. Hashimoto’s Encephalopathy and Autoantibodies. Brain Nerve. 2018;70:305–314. doi: 10.11477/mf.1416201004. [DOI] [PubMed] [Google Scholar]
- 24.Blanchin S, Coffin C, Viader F, et al. Anti-thyroperoxidase antibodies from patients with Hashimoto’s encephalopathy bind to cerebellar astrocytes. J Neuroimmunol. 2007;192:13–20. doi: 10.1016/j.jneuroim.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Mamoudjy N, Korff C, Maurey H, et al. Hashimoto’s encephalopathy: identification and long-term outcome in children. Eur J Paediatr Neurol. 2013;17:280–287. doi: 10.1016/j.ejpn.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Ishii K A, Hayashi A, Tamaoka A, Usuki S, Mizusawa H, Shoji S. Case report: thyrotropin-releasing hormone-induced myoclonus and tremor in a patient with Hashimoto’s encephalopathy. Am J Med Sci. 1995;310:202–205. doi: 10.1097/00000441-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Lee HS, Hwang JS, Jung DE. A case of Hashimoto’s encephalopaty presenting with seizures and psychosis. Korean J Pediatr. 2012;55:111–113. doi: 10.3345/kjp.2012.55.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasconcellos E, Pina-Garza JE, Fakhoury T, Fenichel GM. Pediatric manifestations of Hashimoto’s encephalopathy. Pediatr Neurol. 1999;20:394–398. doi: 10.1016/s0887-8994(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 29.Chaigne B, Mercier E, Garot D, Legras A, Dequin PF, Perrotin D. Hashimoto’s encephalopathy in the intensive care unit. Neurocrit Care. 2013;18:386–390. doi: 10.1007/s12028-013-9834-1. [DOI] [PubMed] [Google Scholar]
- 30.Gul Mert G, Horoz OO, Herguner MO, et al. Hashimoto’s encephalopathy: four cases and review of literature. Int J Neurosci. 2014;124:302–306. doi: 10.3109/00207454.2013.836706. [DOI] [PubMed] [Google Scholar]
- 31.Tuncer GO, Teber S, Kutluk MG, Albayrak P, Deda G. Hashimoto’s Encephalopathy Presenting as Pseudobulbar Palsy. Case Reports Childs Nerv Syst. 2018;34:1251–1254. doi: 10.1007/s00381-018-3720-2. [DOI] [PubMed] [Google Scholar]
- 32.Beckmann YY, Top D, Yigit T. Unusual presentations of Hashimoto’s encephalopathy: trigeminal neuralgiaform headache, skew deviation, hypomania. Endocrine. 2011;40:495–496. doi: 10.1007/s12020-011-9506-x. [DOI] [PubMed] [Google Scholar]
- 33.Salazar R, Mehta C, Zaher N, Miller D. Opsoclonus as a manifestation of Hashimoto’s encephalopathy. J. Clin. Neurosci. 2013;19:1465–1466. doi: 10.1016/j.jocn.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Sharan A, Sengupta S, Mukhopadhyay S, Ghosh B. Hashimoto’s Encephalopathy Presenting with Chorea. J Assoc Physicians India. 2015;63:83–84. [PubMed] [Google Scholar]
- 35.Ueno H, Nishizato C, Shimazu T, et al. Hashimoto’s encephalopathy presenting with vertigo and muscle weakness in a male pediatric patient. No Hattatsu. 2016;48:45–47. [PubMed] [Google Scholar]
- 36.Emeksiz S, Kutlu NO, Alacakir N, Caksen H. A case of steroid-resistance Hashimoto’s encephalopathy presenting with sensorimotor polyneuropathy. Turk. J. Pediatr. 2018;60:310–314. doi: 10.24953/turkjped.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Paterson RW, Takada LT, Geschwind MD. Diagnosis and treatment of rapidly progressive dementias. Neurol Clin Pract. 2012;2:187–200. doi: 10.1212/CPJ.0b013e31826b2ae8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fayyaz B, Upreti S. Autoimmune inner ear disease secondary to Hashimoto’s thyroiditis: a case report. J Community Hosp Intern Med Perspect. 2018;8:227–229. doi: 10.1080/20009666.2018.1503917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Yang H, Xiao F. Hashimoto’s encephalopathy: a report of three cases and relevant literature reviews. Int J Clin Exp Med. 2015;8:16817–16826. [PMC free article] [PubMed] [Google Scholar]
- 40.Salpietro V, Mankad K, Polizzi A, et al. Pediatric Hashimoto’s encephalopathy with peripheral nervous system involvement. Pediatr Int. 2014;56:413–416. doi: 10.1111/ped.12262. [DOI] [PubMed] [Google Scholar]
- 41.Peschen-Rosin R, Schabet M, Dichgans J. Manifestation of Hashimoto’s encephalopathy years before onset of thyroid disease. Eur Neurol. 1999;41:79–84. doi: 10.1159/000008007. [DOI] [PubMed] [Google Scholar]
- 42.Graus F, Titulaer MJ, Balu R, et al. A clinicalapproach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhle H, van Baalen A, Riepe FG, Rohr A, Stephani U. Hashimoto Encephalopathy in a 15-year-Old-Girl: EEG Findings and Follow-Up. Pediatr Neurol. 2009;41:301–304. doi: 10.1016/j.pediatrneurol.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy syndrome or myth? Arch Neurol. 2003;60:164–171. doi: 10.1001/archneur.60.2.164. [DOI] [PubMed] [Google Scholar]
- 45.Shaw PJ, Walls TJ, Newman PK, Cleland PG, Cartlidge NEF. Hashimoto’s encephalopathy: A steroid-responsive disorder associated with high anti-thyroid antibody titers—report of 5 cases. Neurology. 1991;41:228–233. doi: 10.1212/wnl.41.2_part_1.228. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi S, Mitamura R, Itoh Y, Suzuki N, Okuno A. Hashimoto encephalopathy: Etiologic considerations. Pediatr Neurol. 1994;11:328–331. doi: 10.1016/0887-8994(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 47.Kelley BP, Patel SC, Marin HL, Corrigan JJ, Mitsias PD, Griffith B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38:1070–1078. doi: 10.3174/ajnr.A5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devon IR. Hashimoto encephalopathy. UpToDate. Jul 10, 2017. [Google Scholar]
- 49.Mahmud FH, Lteif AN, Renaud DL, Reed AM, Brands CK. Steroid-responsive Encephalopathy associated with Hashomoto’s Thyroiditis in an Adolescent With Chronic Hallucinations and Depression: Case Report and Review. Pediatrics. 2003;112:686–90. doi: 10.1542/peds.112.3.686. [DOI] [PubMed] [Google Scholar]
- 50.Drulovic J, Andrejevic S, Bonaci-Nikolic B, et al. Hashimoto’s encephalopathy: a long-lasting remission induced by intravenous immunoglobulins. Vojnosanit Pregl. 2011;68(5):452–4. doi: 10.2298/vsp1105452d. [DOI] [PubMed] [Google Scholar]
- 51.Churilov LP, Sobolevskaia PA, Stroev YI. Thyroid gland and brain: Enigma of Hashimoto’s encephalopathy. Best Pract Res Clin Endocrinol Metab. 2019;33:101364. doi: 10.1016/j.beem.2019.101364. doi: 10.1016/j.beem.2019.101364. [DOI] [PubMed] [Google Scholar]
- 52.Attique HB, Rao A, Haider L, Trivedi R. Successful treatment of Hashimoto encephalopathy with therapeutic plasma exchange. Ther Apher Dial. 2019 doi: 10.1111/1744-9987.13466. 10.1111/1744-9987.13466. doi:10.1111/1744-9987.13466. [DOI] [PubMed] [Google Scholar]
- 53.Cook MK, Malkin M, Karafin MS. The use of plasma exchange in Hashimoto’s encephalopathy: a case report and review of the literature. J Clin Apheresis. 2015;30:188–192. doi: 10.1002/jca.21353. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Y, Tian X, Gu Y, Li F, Wang X. Application of Plasma Exchange in Steroid-Responsive Encephalopathy. Front Immunol. 2019;10:324. doi: 10.3389/fimmu.2019.00324. Published 2019 Feb 27. doi:10.3389/fimmu.2019.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J, Yu HJ, Lee J. Hashimoto encephalopathy in pediatric patients: Homogeneity in clinical presentation and heterogeneity in antibody titers. Brain Dev. 2018;40:42–48. doi: 10.1016/j.braindev.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Erol I, Sayg S, Alehan F. Hashimoto’s encephalopathy in children and adolescents. Pediatr Neurol. 2011;45:420–422. doi: 10.1016/j.pediatrneurol.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Garg M, Sharma SD, Hajela A, Gupta P. Hashimoto Encephalopathy in Children. Ann Indian Acad Neurol. 2019;22:357–359. doi: 10.4103/aian.AIAN_18_19. [DOI] [PMC free article] [PubMed] [Google Scholar]


