Abstract
Objective:
Computed Tomography (CT) is considered part of the routine diagnostic workup for pleural malignancy. The definitive diagnosis of pleural malignancy depends upon histological confirmation by pleural biopsy. The aim of this study is to assess the sensitivity and specificity of CT, in view of the latest imaging technologies, in detecting pleural malignancy compared to definitive histology achieved via thoracoscopy (VATS).
Materials and methods:
We included in this retrospective study 90 patients (36 F, 54 M) with suspected pleural malignancy evaluated in our Institution with CT scan who received a definitive diagnosis after VATS biopsy. Unaware of histopathologic diagnoses CT scans were evaluated by a junior and two experts thoracic radiologist. Conclusions were reached by consensus.
Results:
We evaluated all CT signs suggestive for malignant pleural diseases: pleural thickening > 10 mm (Se 0,41 , Sp 0,79); nodular thickening (Se 0,86, Sp 0,75); circumferential thickening (Se 0,79, Sp 0,69); irregular pleural thickening (Se 0,77, Sp 0,91); mediastinal involvement (Se 0,88, Sp 0,64); costal involvement (Se 0,89, Sp 0,60); diaphragmatic involvement (Se 0,88, Sp 0,53). Furthermore, the diagnostic performance of additional CT features was evaluated: concomitant costal, mediastinal and diaphragmatic pleura lesions (Se 0,84, Sp 0,69); nodular/irregular thickening with mediastinal pleural involvement (Se 0,83, Sp 0,90); nodular/irregular thickening with diaphragmatic pleural involvement (Se 0,81, Sp 0,90).
Conclusions:
CT confirms its central role in the pleura malignancy. The high sensibility, respect to previous studies, especially in the presence of nodular pleural thickening, may lead to reconsider at least partly the diagnostic pathway of diffuse pleural disease, avoiding the use of VATS in patients not eligible for surgery, in favor of US or CT guided core biopsy. (www.actabiomedica.com)
Keywords: Chest CT, Malignant Pleural Mesothelioma, VATS
Introduction
The pleura may be involved by several neoplastic conditions, ranging from benign lipoma to rare aggressive malignancies, such as synovial sarcoma. Benign pleural tumours are relatively uncommon. The majority are lipomas and solitary fibrous tumours. Metastatic adenocarcinoma is the commonest cause of malignant pleural disease, while mesothelioma is the most common malignant primary pleural tumour [1,2].
For the radiologist, as well as for the clinician or the pathologist, it can be a challenge to distinguish among pleural tumours, overlapping radiological findings, clinical manifestations and pathological features.
For these reasons, to achieve a correct diagnosis in order to offer a tailored therapy with better prognosis, it is essential to correlate clinical, radiological, histopathological and immunohistochemical findings [1,2].
The multidisciplinary approach can improve diagnosis and outcomes.
MPM is associated with asbestos exposure in approximately 80% of patients [1,2]. The patient prognosis is poor, with a median survival of 9–17 months after diagnosis. However, improved survival has been demonstrated when the diagnosis is made in the early stages of disease and proper treatment strategies are implemented. [3-6]
Computed tomography (CT) is the mainstay imaging technique for primary assessment of pleural disease and affords improved sensitivity for identification of a malignant pleural process. Magnetic resonance imaging (MRI) and positron-emission tomography (PET) are complementary techniques for the assessment of pleural disease that can provide additional staging and prognostic information [2, 4-7].
Benign pleural disease is commonly characterized by presence of pleural effusion, smooth and thin pleural thickening with sparing of mediastinum pleural layer [5].
CT Signs that are relatively specific for a diagnosis of malignancy include circumferential pleural thickening, nodular pleural thickening, pleural thickening greater than 1 cm, and involvement of the mediastinal pleural surface [1,2,4].
However, the absence of these signs does not exclude malignant pleural disease. As malignant pleural processes often have overlapping imaging features (see diffuse benign pleural thickening ), and can be difficult to differentiate one from another based only on imaging [7-11].
But what has happened with the advent of last generation multi-slice CT scanner?
The aim of this study is to assess the sensitivity and specificity of CT, in the light of the latest imaging technologies, in detecting pleural malignancy compared to definitive histology achieved via thoracoscopy (VATS).
Materials and Methods
We included in this retrospective study all patients with undiagnosed pleural effusion or thickening evaluated in our Institution who had a definitive diagnosis after VATS biopsy and in whom CT scans were available for review. The local Ethics Committee approved the study protocol, and all subjects provided written informed consent.
Out of 434 consecutive patients undergoing VATS between 01/01/2010 and 31/08/2015 in our Institution, we identified 90 patients that satisfied our selection criteria, 36 females and 54 males, with a mean age of 68 years.
The selection criteria were:
No previous pleural disease diagnosis (via VATS plus biopsy)
CT study with contrast media within 30 days preceding VATS
Pathologic diagnosis following VATS
We excluded those patients who did not meet our requirements, in particular when missing the optimal venous phase contrast CT or volumetric acquisition.
CT scans were evaluated retrospectively by a junior and two expert thoracic radiologists. Both were unaware of histopathologic diagnoses. Conclusions were reached by consensus.
All studies were performed in our Unit with a Brilliance CT 64-channels Philips. All patients underwent volumetric CT study (obtained at end-inspiratory lung volumes) with intravenous iodinated contrast (scan delay 60”). The acquired data had 2mm x1 or 4mm x 2 overlapping with 512x512 matrix.
The CT images were visualized in axial, coronal and sagittal section analyzing lung and mediastinal window.
CT study
Using a fixed protocol, the following CT features were evaluated:
pleural effusion (mono-bilateral)
pleural thickening (< or > 1cm)
pleural morphological characteristics (smooth, nodular, irregular, circumferential thickening)
pleural disease location (mediastinal, diaphragmatic or costal).
Pleural effusion was classified according to the involved side right, left or bilateral [7,8,9].
Pleural soft tissue abnormalities were subdivided based on the morphology in three categories: smooth, irregular thickening and nodular thickening; the last one due to multiple nodules involving pleura with solution of continuity; further growth in number and size of pleural nodules leads to a continuous serosal thickening with an irregular morphology.
According to the maximum diameter of pleural nodules or thickening, pleural abnormalities were divided into smaller than 10 mm and larger than/equal to 10 mm.
Rind-like thickening was reported when pleural thickening involved the entire hemithorax.
Furthermore, findings were classified according to the anatomical location of pleural abnormalities: costal pleura, mediastinal pleura and diaphragmatic pleura.
In costal pleura involvement, abnormalities originated from the pleural surface extending from the sternum to the paravertebral gutters.
In mediastinal pleura abnormalities originated from the mediastinal sierosa including the vertebral column was involved while in diaphragmatic pleura involvement, abnormalities were located on the diaphragmatic dome [9].
We identified CT signs as pleural thickening > 10 mm, nodular, irregular or circumferential thickening, mediastinal, diaphragmatic or costal involvement, such as suggestive for malignant pleural diseases.
Finally, in order to improve diagnostic accuracy, according to the literature [1-4], we identified in addition to the signs already considered, the following features:
- Involvement of costal pleura + involvement of mediastinal pleura + Involvement of diaphragmatic pleura with or without interruption
- nodular thickening + diaphragmatic involvement
- nodular thickening + mediastinal involvement
CT scans were reported as malignant or benign. We classified as indeterminate, those cases where no absolutely clear pleural involvement or evident malignant CT findings were found.
VATS classification
Operative notes of VATS procedures were individually reviewed and the macroscopic appearance and location of pleural abnormalities (thickening and/or nodules involving the costal, diaphragmatic, mediastinal, and visceral pleura surfaces) were evaluated and compared with CT findings.
Histological classification
Pathology reports were reviewed and compared with thoracoscopic and CT findings.
Histological findings were divided into benign and malignant disease. Benign disease included nonspecific acute pleuritis, chronic pleuritis and mesothelial hyperplasia.
Regarding the mesothelial hyperplasia, should be carefully evaluated those atypical border-line cases, which assumes pre-neoplastic significance with possible changes in malignant mesothelioma; differential diagnosis relies mostly on the evidence of cellular atypia and cell proliferation (for example stromal invasion is a sign of malignancy) as well as on immunohistochemical markers (such as calretinin, GLUT-1, Vimentin and cytokeratins), particularly useful for the diagnosis of MPM. [12]. Malignant disease included malignant pleural mesothelioma, pleural metastasis, and lymphomas.
Statistical analysis
The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of CT in the assessment of malignant pleural disease were determined by using histological results as reference standard. Statistical analyses were performed using Microsoft Office Excel (Microsoft Corp., Seattle, WA, USA).
Results
A final histological diagnosis of malignancy was established in 70 patients while a benign disease was diagnosed in the 20 remaining patients.
As shown in Table 1, out of the 70 patients found to have pleural malignancy, 33 had pleural metastases (Fig. 1), 35 had malignant pleural mesothelioma (MPM) (Fig. 2, Fig. 3), 2 had pleural Lymphoma (Fig. 4).
Table 1.
Histological diagnosis
| MPM | Pleural metastases | Lymphoma | Pleurisy | Mesothelial Hyperplasia | |
| Histological Diagnosis | 35 | 33 | 2 | 18 | 2 |
Fig. 1.
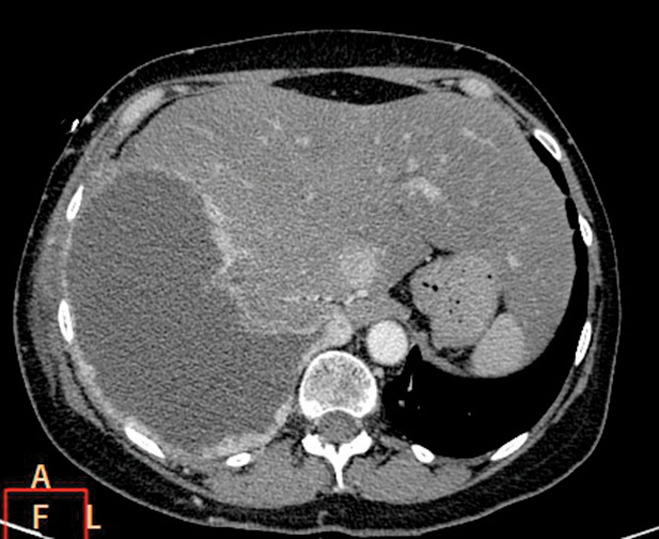
Pleural metastases in 54 year old woman. Axial contrast-enhanced CT image at the level of the right hemidiaphragm shows a moderate-sized right pleural effusion. There is complete involvement of the right hemithorax with pleural thickening and irregularity of the surface contour of the hemidiaphragm.
Fig. 2.
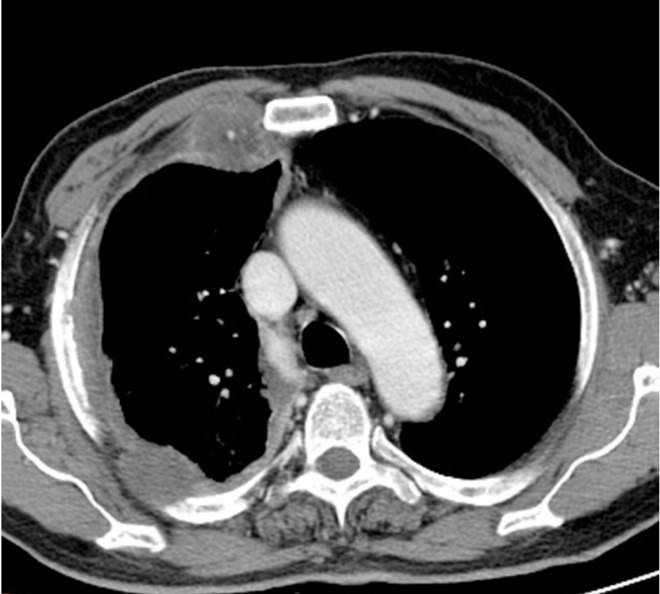
Malignant pleural mesothelioma in a 75 year old man. Axial contrast-enhanced CT image just inferior to the THORACIC aorta shows circumferential nodular pleural thickening in the right hemithorax. Note the pleural thickening in the anterior and posterior hemithorax with focal nodular chest invasion.
Fig. 3.
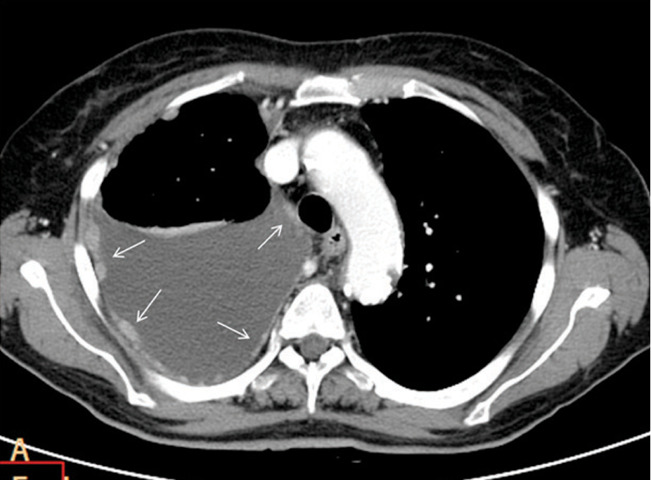
Malignant pleural mesothelioma in a 61 year old man. Axial contrast-enhanced CT image at the level of transverse thoracic aorta shows enhancing nodular pleural thickening involves the costal and mediastinal pleural (white arrows). A moderate-sized right pleural effusion is also seen.
Fig. 4.
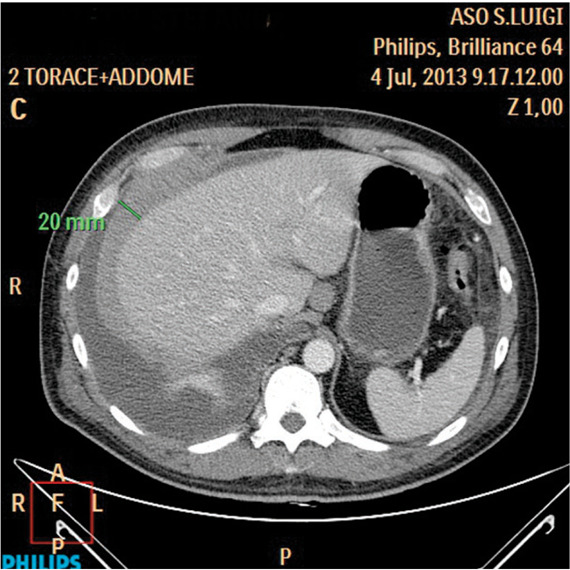
Pleural lymphoma in a 45 year old man. Axial contrast-enhanced CT image shows pleural thickening at the level of the right hemidiaphragm. There is complete encasement of the right hemidiaphragm and loss of the fat plane between the diaphragm and liver.
Out of the 20 benign patients, 18 were found to have pleurisy (Fig. 5), while 2 cases were diagnosed as mesothelial hyperplasia (superficial mesothelial proliferation).
Fig. 5.
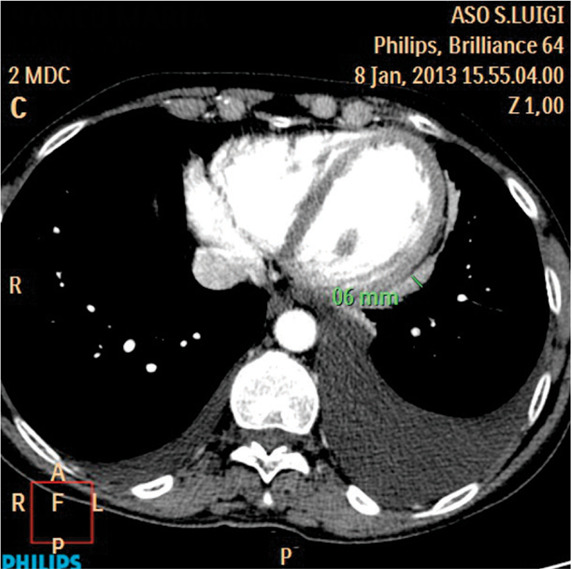
Pleurisy in 59-year-old woman. Axial contrast-enhanced CT image at the level of the heart shows pleural thickening involving the mediastinal pleural (>6 mm). A moderate-sized right pleural effusion is also seen.
Out of the 70 patients found to have pleural malignancy, 50 had CT scans (prior to thoracoscopy) reported as malignant. Of the 20 patients with benign disease, 13 had CT scans reported as benign. 27 patients were reported as indeterminate, when no clear cause of malignancy has been found.
We calculated the overall sensitivity and specificity of CT scan reporting malignancy or benign diseases, as shown in Table 2, compared to Hallifax study [13].
Table 2.
Sensitivity and specificity of CT scan reporting malignancy or benign disease, compared to Hallifax [13]
|
Our Study (n=90) [95% CI) |
Hallifax (n=370) | |
| Overall CT sensitivity | 0,71 [0,60 to 0,82] | 0,68 |
| Overall CT specificity | 0,68 [0,48 to 0,88] | 0,78 |
We evaluated all CT signs (see Table 3) suggestive for malignant pleural diseases (by calculating sensitivity, specificity, positive predictive value, negative predictive value and accuracy) and selected those comparable with literature [7-8] (see table 4) such as pleural thickening greater than 10 mm (Fig. 6), mediastinal involvement (Fig. 7), nodular thickening (Fig. 8) and circumferential thickening (Fig. 9).
Table 3.
Sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and accuracy (Acc) of CT signs examined
| Se (95% CI) | Sp (95% CI) | PPV | NPV | Acc | |
| Pleural thickening >10 mm | 0,41 (0,29 to 0,53) |
0,79 (0,61 to 0,97) |
0,87 | 0,26 | 0,79 |
| Nodular thickening | 0,86 (0,76 to 0,96) |
0,75 (0,51 to 0,99) |
0,93 | 0,60 | 0,84 |
| Circumferential thickening | 0,79 (0,64 to 0,94) |
0,69 (0,44 to 0,94) |
0,85 | 0,60 | 0,76 |
| Irregular thickening | 0,77 (0,61 to 0,93) |
0,91 (NS) |
0,95 | 0,60 | 0,81 |
| Mediastinal involvement | 0,88 (0,79 to 0,97) |
0,64 (0,39 to 0,89) |
0,90 | 0,60 | 0,83 |
| Diaphragmatic involvement | 0,88 (0,79 to 0,97) |
0,53 (0,29 to 0,77) |
0,85 | 0,60 | 0,79 |
| Costal involvement | 0,89 (0,81 to 0,97) |
0,60 (0,35 to 0,85) |
0,89 | 0,60 | 0,83 |
Table 4.
|
Our Study (n=90) |
Metintas et al. 2002 (n=215) |
Leung et al. 1990 (n=73) |
||||
| Se | Sp | Se | Sp | Se | Sp | |
| Pleural thickening >10mm | 0,41 | 0,79 | 0,47 | 0,64 | 0,36 | 0,94 |
| Circumferential thickening | 0,79 | 0,69 | 0,54 | 0,95 | 0,41 | 1 |
| Nodular thickening | 0,86 | 0,75 | 0,38 | 0,96 | 0,51 | 0,94 |
| Mediastinal involvement | 0,88 | 0,64 | 0,70 | 0,83 | 0,56 | 0,88 |
Fig. 6.
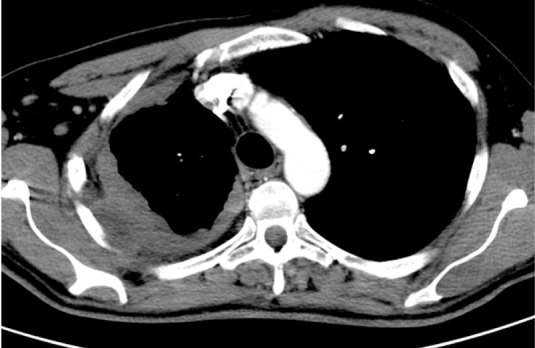
Malignant pleural mesothelioma in 76 year old man. Axial contrast-enhanced CT image shows irregular costal pleural thickening > 1 cm partly involving mediastinal pleura. Note also rib erosion and chest wall invasion.
Fig. 7.
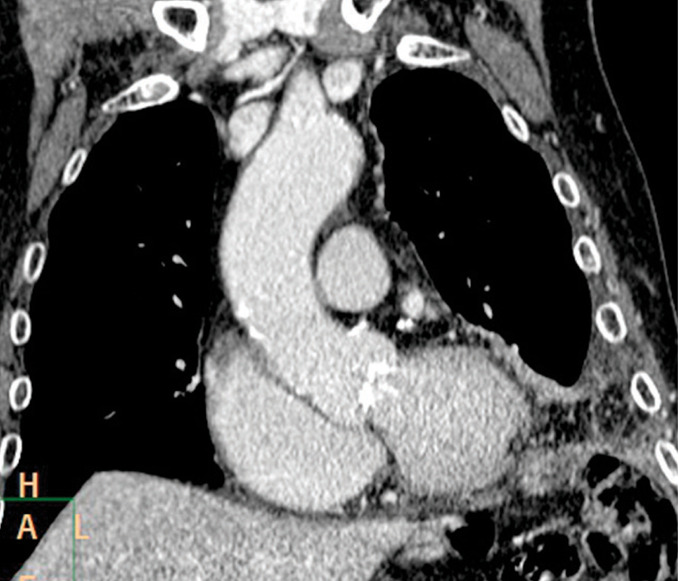
Pleural metastasis in a 83 year old man. Coronal reformatted contrast-enhanced CT image shows mediastinal involvement with diffuse small nodules and lower chest pleural thickening.
Fig. 8.
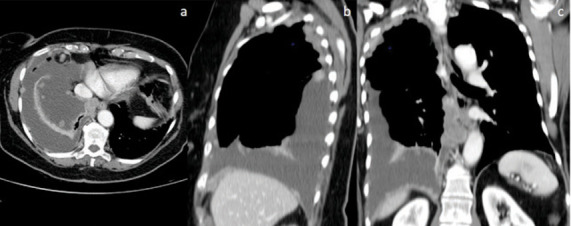
Malignant pleural mesothelioma in a 72 year old woman. Axial (a), Sagittal (b) and coronal (c) contrast-enhanced CT demonstrates multiple nodules in costal and diaphragmatic. Note also a large-sized pleural effusion in the right emithorax.
Fig. 9.
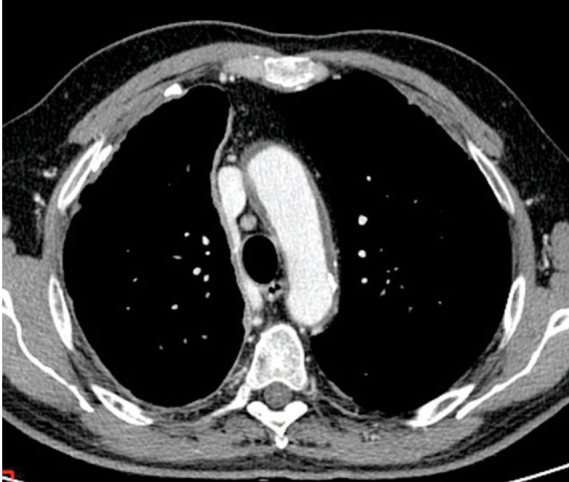
Malignant pleural mesothelioma in a 68 year old man. Axial contrast-enhanced CT image shows subtle rind-like pleural thickening.
Furthermore, the diagnostic performance of additional CT features (table 5) was evaluated:
Table 5.
Sensitivity, specificity, PPV, NPV and accuracy of further CT signs examined
| Se (95% CI) | Sp (95% CI) | PPV | NPV | Acc | |
| Costal + diaphragmatic + mediastinal involvement | 0,84 (0,72 to 0,96) |
0,69 (0,44 to 0,94) |
0,89 | 0,60 | 0,80 |
| Nodular thickening + diaphragmatic involvement | 0,81 (0,67 to 0,95) |
0,90 (NS) |
0,86 | 0,60 | 0,86 |
| Nodular thickening + mediastinal involvement | 0,83 (0,71 to 0,95) |
0,90 (NS) |
0,97 | 0,60 | 0,84 |
concomitant costal, mediastinal and diaphragmatic pleura lesions
nodular/irregular thickening with mediastinal pleural involvement
nodular/irregular thickening with diaphragmatic pleural involvement (Fig. 10)
Fig. 10.
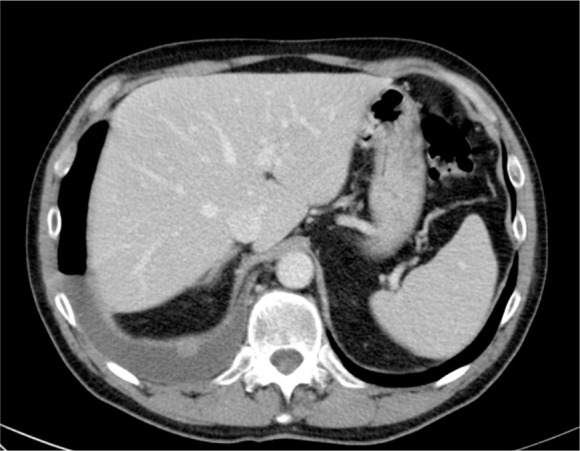
Malignant Pleural mesothelioma in a 74 year old man. Axial contrast-enhanced CT shows smooth and nodular diaphragmatic pleural thickening together. Right pleural effusion is also seen.
Comparison with Vats Findings
We evaluated the detection rate of every findings between CT and VATS (Table 6), adding a Fisher test for better comparison.
Table 6.
Comparison of pleural lesion’s morphology and location detection rate between CT and VATS
| CT | VATS | P-value (Fisher test) | |
| No pleural thickening | 16,66 % | 4,44% | 0,013 |
| Smooth thickening | 40,00 % | 31,11% | 0,276 |
| Pleural nodules | 45,55 % | 44,44% | NS |
| Costal involvement | 58,89 % | 92,22% | 0,0001 |
| Mediastinal involvement | 55,55 % | 34,44% | 0,007 |
| Diaphragmatic involvement | 53,33 % | 61,11% | NS |
In our series, one patient demonstrated non-specific pleuritis on histological analysis following VATS. However, an eventual histological diagnosis of malignant mesothelioma was established on repeat US-guided biopsy.
Discussion
CT
In the literature, there are just few outdated publications, that have assessed the role of CT in the differential diagnosis of diffuse pleural disease [7,8,13].
Nowadays are available more performing CT scanners capable of thin-section volumetric acquisitions, with multiplanar reformatting systems. As a result, these multiplanar reformations have shown to improve the visualization of subtle pleural thickening.
This study echoes the results obtained by Leung and Metintas, published respectively in 1990 in 2002 [7,8]. For the first time ever, Positive and Negative Predictive Values (PPV, NPV) of all CT findings were analyzed, taking into account data about diaphragmatic pleura involvement.
We included the diaphragm as the elective site for pleural malignancy, because lesions not rarely affect the lower regions of the chest [13-16]. This was not considered by Leung in 1990 because the CT scanners were outdated and not capable to get isotropic voxel for a full assessment of the diaphragm, taking advantage of MPR reconstructions.
Our study demonstrates, compared to the past literature [7-8], as could be expected, an increase in the sensitivity.
If we analyze Table 3 and 4, we note that the three CT’s fundamental parameters examined, like cicumferential thickening , nodular thickening and mediastinal involvement, compared with Leung and Metintas [7-8], respectively, improved from 0,41 to 0,79, from 0,51 to 0,86 and 0,56 to 0,88, confirming a better CT performance in recognition of ever subtle nodules. All features, discussed above, presents conversely a slight reduction in specificity.
The mediastinum is confirmed as a critical area because when a lesion develops from the mediastinal pleura, there is always a malignancy suspect.
In our study, compared to mediastinum, the costal and diaphragmatic pleura involvement, was related to malignant disease diagnosis with a sensitivity and specificity of 0,89 and 0,60 for the first feature and 0,88 and 0,53 for the second one. These data need more explanations.
Our results regarding the presence of diaphragmatic lesions are concordant with literature [4, 8], which describes this feature as a possible suggestive sign for malignant disease, as explained above.
In reference to the costal involvement, data analysis should be made carefully. In the literature [7-9] has been described as a non-specific sign of pleural diseases and although our study showed a good correlation with malignant disease, it was present also in 6 patients with benign disease. Accordingly, we considered this finding not relevant for our purposes.
In our study, also irregular pleural thickening (Sp 0,91; Se 0,77) (Fig. 11) has been considered suggestive for malignant pleural disease, in accordance with Metintas et al. [8].
Fig. 11.
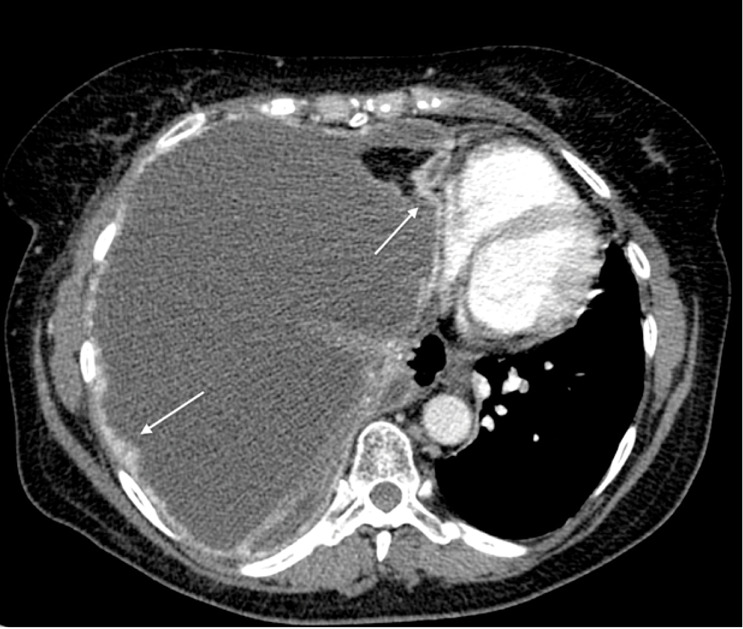
Malignant pleural mesothelioma in a 71-year-old man. Axial contrast-enhanced CT image at the level of descending thoracic aorta shows enhancing irregular pleural thickening involves the costal and mediastinal pleural (white arrows). A massive right pleural effusion with contralateral mediastinal shift is also seen.
Finally, we tried to add some more data such as the association between different signs, like nodular thickening with diaphragmatic (Se 0,81; Sp 0,90) or mediastinal involvement (Se 0,83; Sp 0,90), which have never been evaluated before and we have noticed that this association has led to an increase in specificity (see Table 5).
The majority of the CT signs, according to Hallifax and Tsim [13, 14], analyzed has a high PPV but a low NPV (Table 3); it means that the features suggest, with good accuracy, the presence of malignant disease, but its absence cannot exclude it with the same certainty. Therefore, approximately one third of patients with pleural effusion and a CT scan without evidence of malignant disease of the pleura will in fact have malignancy. For further confirmation, a clinical follow up of patients with benign disease highlighted that a small minority of them (2/20, which were patients with mesothelial Hyperplasia, a border-line but still benign pathology) has progressed to MPM after more than two years. It is not clear whether Mesothelioma in follow-up represents a new diagnosis at that point or secondly a false negative based on the original assessment [16].
This suggests anyway that a benign CT report should not dissuade clinicians from pursuing invasive pleural investigations if the presence of primary or secondary pleural malignancy would alter management.
To summarize, according with literature [4, 7, 8], we ensure that pleural thickening which is nodular, rind-like, and greater than 1 cm in thickness together with mediastinal involvement is highly suggestive of malignant pleural disease, although a distinction between mesothelioma and pleural metastases cannot be done easily.
Moreover, 50 of 70 patients were reported as malignant with an overall CT sensitivity to detect pathology equal to 71%. Instead, 13 of 20 were reported as benign with an overall CT specificity equal to 68%. The results of the current study are quite concordant with those reported by Hallifax [13] (see table 2).
However, the correct and definitive diagnosis of malignant pleural disease requires a histopathologic evaluation of tissue samples.
CT vs VATS
The VATS showed superior results compared to CT (Table 6) and international guidelines can explain and justify his role. [15,16,17,18]
The recommendation of the Guidelines of the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) [15] are supporting a thoracoscopic tissue biopsy in order to get multiple and deep tissue biopsy. A definitive diagnosis can only be made, if the material is representative in terms of biopsy location (normal and abnormal pleura), depth (to assess fat and/or muscle tumor invasion), and quantity (enough material to allow immunohistochemical characterization).
Compared to CT, the VATS showed higher ability to detect any features analyzed (as shown in Table 6) except for the presence of mediastinal involvement, because the vision of the pleura during thoracoscopy is not very easy due to anatomical and technical limitations: trans-thoracic access mode, thoracoscope lens positioning, intraprocedural use of conscious sedation and analgesia.
VATS pointed out costal pleural lesions in a consistently superior percentage compared to CT.
This gap is reduced in the case of nodular thickening (Fig. 12)(equivalence) and inverted for smooth thickenings (Fig. 13), detected in higher percentage by CT, mostly because of VATS has not a multiplanar view, but only bidimensional.
Fig. 12.
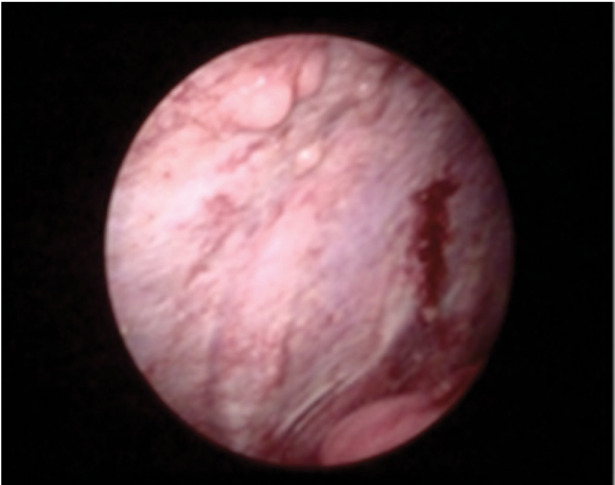
Malignant Pleural mesothelioma in a 74 year old man. Intraoperative (video-assisted thoracic surgery) photograph shows nodular costal pleural thickening.
Fig. 13.
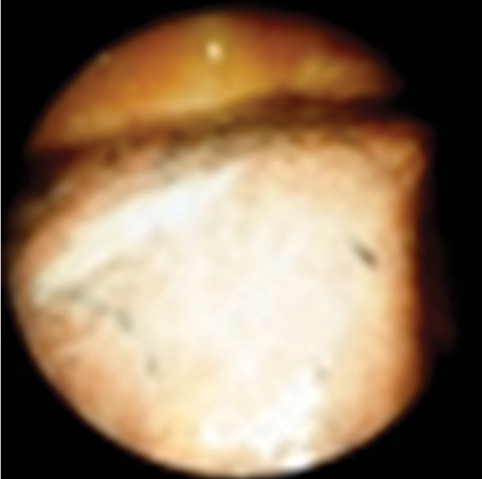
Malignant Pleural mesothelioma in a 71 year old man. Intraoperative (video-assisted thoracic surgery) photograph shows smooth visceral pleural thickening.
It is observed a substantial equivalence between the two techniques for detecting diaphragmatic involvement.
Some of the lower results in identifying lesions by VATS are not attributable to actual technological limits, but rather to the inability to compare some findings [6] detected by surgeons (effusion aspect, VATS macroscopic lesions appearance, sierosa texture and color and its alterations) with the ones present on CT.
It should be noted, moreover, that it was not possible to proceed to the comparison of certain VATS with the CT because of the lack of a structured thoracoscopic report by the Surgeon.
It would be appropriate to arrange a standardized and structured medical reports that show schematically the lesions morphology and localization.
Limitations of the Study
The present study has some limitations that require consideration. First, it is inherently limited by the type of study, retrospective monocentric rather than prospectic multicentric. Second, the sample size (with particular reference to benign cases) may have been too small, and further larger studies are required to confirm these results. Third, the vast majority of cases were evaluated by one of the two study observers hampering the assessment of the interobserver variability analysis.
In conclusion, CT confirms its importance in the pleura evaluation. In light of the results, is there anything new in the diagnostic work up?
Certainly, thanks to the subtle collimation and the possibility to reformat the cross sectional images in multiple planes, it is possible to improve the performance of the CT scan.
The good sensibility such as detection of nodular pleural thickening, it may lead to reconsider the diagnostic pathway of diffuse pleural disease, avoiding the use of VATS in patients not eligible for surgery, in favor of US or CT guided core biopsy [18-21].
In fact, according to Van Zandwijk et al. [22], CT-guided core biopsy is suitable for cases where imaging studies have demonstrated pleural thickening or a nodular/mass lesion, and in such cases this procedure has a high diagnostic yield and few complications [18-19]. On the other hand VAT-guided biopsy remains indicated for patients with a pleural effusion but no mass lesion, or patients in whom surgical pleurodesis is considered.
The two procedures, lastly, are included in a diagnostic workup [18-23] in which CT imaging guides the surgical approach and VATS represents the final step, in order to optimize the biopsy sampling and achieve a definitive diagnosis. Nowadays CT-guided core biopsy however is a trusted and safe alternative to VATS in terms of diagnostic accuracy [18,22] and the final choice is based on the clinical circumstances and the medical expertise available each time in our Hospital.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.De Paoli L, Quaia E, Poillucci G, Gennari A, Cova MA. Imaging characteristics of pleural tumours. Insights Imaging. 2015 Oct 16 (2015);6:729–740. doi: 10.1007/s13244-015-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salahudeen HM, Hoey ETD, Robertson RJ, Darby MJ. CT appearances of pleural tumours. Clinical Radiology. 2009;64:918e930. doi: 10.1016/j.crad.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale L, Ardissone F, Asteggiano F, Laugelli EM, Penna D, Fava C. Diffuse neoplasms of the pleural serosa. Radiol Med. 2013 Apr;118(3):366–78. doi: 10.1007/s11547-012-0877-8. doi: 10.1007/s11547-012-0877-8. Epub 2012 Sep 17. [DOI] [PubMed] [Google Scholar]
- 4.Nickell LT Jr, Lichtenberger JP 3rd, Khorashadi L, Abbott GF, Carter BW. Multimodality imaging for characterization, classification, and staging of malignant pleural mesothelioma. Radiographics. 2014 Oct;34(6):1692–706. doi: 10.1148/rg.346130089. doi: 10.1148/rg.346130089. [DOI] [PubMed] [Google Scholar]
- 5.Jeong YJ, Kim S, Kwak SW, et al. Neoplastic and non-neoplastic conditions of serosal membrane origin: CT Findings. Radiographics. 2008;28:801–818. doi: 10.1148/rg.283075082. [DOI] [PubMed] [Google Scholar]
- 6.Perikleous P, Rathinam S, Waller DA. VATS and open chest surgery in diagnosis and treatment of benign pleural diseases. Journal of Visualized Surgery. 2017;3:84. doi: 10.21037/jovs.2017.05.03. doi:10.21037/jovs.2017.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung AN, Müller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol. 1990 Mar;154(3):487–92. doi: 10.2214/ajr.154.3.2106209. [DOI] [PubMed] [Google Scholar]
- 8.Metintas M, Ucgun I, Elbek O, et al. Computed tomography features in malignant pleural mesothelioma and other commonly seen pleural diseases. EurJ Radiol. 2002;41(1):1–9. doi: 10.1016/s0720-048x(01)00426-0. [DOI] [PubMed] [Google Scholar]
- 9.Dynes MC, White EM, Fry WA, Ghahremani GG. Imaging manifestations of pleural tumors. Radiographics. 1992 Nov;12(6):1191–201. doi: 10.1148/radiographics.12.6.1439021. [DOI] [PubMed] [Google Scholar]
- 10.Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003 Apr 19;361(9366):1326–30. doi: 10.1016/s0140-6736(03)13079-6. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez de Sierra Garcia B, Olmedilla P, Exposito D. Malignant Pleural Mesothelioma. What´s new? 2012 Update of the Consensus Statement from the International Mesothelioma Interest Group. Poster No.: C-1374 Congress: ECR 2013. European Society of Radiology [Google Scholar]
- 12.Husain AN, Colby T, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma. 2012 Update of the Consensus Statement from the International Mesothelioma Interest Group. [Google Scholar]
- 13.Hallifax RJ, Haris M, Corcoran JP, Leyakathalikhan S, Brown E. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax. 2015;70(2):192–3. doi: 10.1136/thoraxjnl-2014-206054. [DOI] [PubMed] [Google Scholar]
- 14.Tsim S, Stobo DB, Alexander L, Kelly C, Blyth KG. The diagnostic performance of routinely acquired and reported computed tomography imaging in patients presenting with suspected pleural malignancy. Lung Cancer (Amsterdam, Netherlands) 2017;103:38–43. doi: 10.1016/j.lungcan.2016.11.010. doi:10.1016/j.lungcan.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010 Mar;35(3):479–95. doi: 10.1183/09031936.00063109. doi: 10.1183/09031936.00063109. Epub 2009 Aug 28. [DOI] [PubMed] [Google Scholar]
- 16.Lang-Lazdunski L. Surgery for malignant pleural mesothelioma: why, when and what? Lung Cancer. 2014 May;84(2):103–9. doi: 10.1016/j.lungcan.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Cao C, Tian D, Park J, Allan J, Pataky KA, Yan TD. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer. 2014 Feb;83(2):240–5. doi: 10.1016/j.lungcan.2013.11.026. doi: 10.1016/j.lungcan.2013.11.026. Epub 2013 Dec 6. [DOI] [PubMed] [Google Scholar]
- 18.Novello S, Pinto C, Torri V, et al. The Third Italian Consensus Conference for Malignant Pleural Mesothelioma: State of the art and recommendations. Crit Rev Oncol Hematol. 2016;104:9–20. doi: 10.1016/j.critrevonc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Armato SG 3rd, Coolen J, Nowak AK, Robinson C, Gill RR, Khanwalkar A. Imaging in pleural mesothelioma: a review of the 12th International Conference of the International Mesothelioma Interest Group. Lung Cancer. 2015 Nov;90(2):148–54. doi: 10.1016/j.lungcan.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Hooper C, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline. Thorax. 2010 doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 21.Stahel RA, Weder W, Lievens Y, Felip E. Malignant pleural mesothelioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl5):v126–v128. doi: 10.1093/annonc/mdq173. [DOI] [PubMed] [Google Scholar]
- 22.Van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. Journal of Thoracic Disease. 2013;5(6):E254–E307. doi: 10.3978/j.issn.2072-1439.2013.11.28. doi:10.3978/j.issn.2072–1439.2013.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardinale L, Ardissone F, Gned D, Sverzellati N, Piacibello E, Veltri A. Diagnostic Imaging and workup of Malignant Pleural Mesothelioma. Acta Biomed. 2017 Aug 23;88(2):134–142. doi: 10.23750/abm.v88i2.5558. doi: 10.23750/abm.v88i2.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]


