Abstract
Western corn rootworm, Diabrotica virgifera virgifera LeConte, is a serious pest of corn and is often managed with transgenic corn producing insecticidal toxins from the bacterium Bacillus thuringiensis (Bt). This pest has developed field-evolved resistance to all commercially available Bt traits, beginning with Cry3Bb1 in 2009. Fitness costs may accompany Bt resistance, where individuals with alleles for Bt resistance have reduced fitness on non-Bt corn compared to Bt-susceptible individuals. In conjunction with non-Bt refuges, fitness costs can delay the evolution of Bt resistance. Importantly, ecological factors may affect the presence and magnitude of fitness costs. For western corn rootworm, available data suggest that fitness costs of Bt resistance may be present in some cases. Using two Cry3Bb1-resistant western corn rootworm strains (Hopkinton and Cresco), a fitness-cost experiment was performed by rearing rootworm in the absence of Bt for six generations to test for fitness costs of Cry3Bb1 resistance and the effect of larval rearing density on fitness costs. Fitness costs were detected for both strains; however, strains were still resistant to Cry3Bb1 corn at the end of the experiment. Cresco experienced a greater loss of resistance at low versus high density, but no effect of density was detected in Hopkinton. Our study shows that fitness costs can accompany Bt resistance in western corn rootworm and may be more pronounced under low larval density. Even though fitness costs were present, it appears that rootworm populations may remain resistant to Cry3Bb1 corn for years after resistance has evolved.
Keywords: corn, Bacillus thuringiensis, fitness cost, insect resistance management, rootworm
The planting of transgenic crops producing insecticidal proteins derived from the bacterium Bacillus thuringiensis (Bt) has become an important approach for managing insect pests in agriculture. In 2017, Bt crops were planted on over 100 million hectares worldwide (James 2017). Benefits of Bt include the protection of crops from feeding injury by insects, reduced use of conventional insecticides, and reduced harm to beneficial arthropods in agricultural systems (Carpenter 2010, Edgerton et al. 2012). However, the evolution of Bt resistance by agricultural pests can diminish these benefits (Tabashnik and Carrière 2017). The refuge strategy is used in the United States and elsewhere to delay Bt resistance (Gassmann 2016, U.S. EPA 2020). In the United States, the Environmental Protection Agency requires a refuge be planted as a part of an insect resistance management strategy for some Bt crops, and in other cases, alternate host plants serve as a refuge (U.S. EPA 2020). Under this strategy, non-Bt host plants are grown in association with a Bt crop in order to enable the survival of Bt-susceptible individuals, which can then mate with resistant individuals to produce heterozygous offspring (Gould 1998). Refuges will serve to effectively delay resistance if heterozygous individuals have lower fitness on Bt than homozygous-resistant individuals (Gould 1998).
Over time, however, Bt-resistant individuals completing development on refuge plants will lead to an increase in homozygous-resistant insects, and thus the development of Bt resistance at the population level (Gassmann et al. 2009). Importantly, fitness costs associated with Bt resistance will act to remove resistance alleles from refuge populations, thereby maintaining the effectiveness of non-Bt refuges to delay Bt resistance (Carrière and Tabashnik 2001, Gassmann et al. 2009). Fitness costs, or fitness trade-offs, of Bt resistance occur in the absence of Bt (e.g., in non-Bt refuges) when individuals that possess alleles for Bt resistance have lower fitness than Bt-susceptible individuals (Gassmann et al. 2009). The presence and severity of fitness costs affecting Bt resistance may be influenced by ecological factors, such as competition, larval host plants, and entomopathogens (Higginson et al. 2005, Raymond et al. 2005, Gassmann et al. 2006, Hannon et al. 2010, Raymond et al. 2011, Wang et al. 2016). To the extent that ecological factors increase fitness costs, this is expected to further delay or reverse pest adaptation to Bt crops (Carrière and Tabashnik 2001, Gassmann et al. 2009, Pittendrigh et al. 2014).
The western corn rootworm (Diabrotica virgifera virgifera LeConte) is a serious pest of corn in the U.S. Corn Belt and is often managed with transgenic Bt corn (Gray et al. 2009). Furthermore, this pest has evolved resistance to multiple Bt toxins produced by transgenic corn (Gassmann et al. 2011, Gassmann et al. 2014, Jakka et al. 2016, Gassmann et al. 2020). Experiments looking at life-history traits have found evidence of fitness costs affecting Bt resistance by western corn rootworm in some cases, but in other instances, such costs appear to be absent (Ingber and Gassmann 2015, Gassmann 2016, Paolino and Gassmann 2017). Additionally, several life-history traits in western corn rootworm have been found to be affected by high population density (i.e., high density of larvae), which may occur in fields with a history of continuous corn cultivation. These traits include survival to adulthood, development time, and adult size (Branson and Sutter 1985, Weiss et al. 1985, Onstad et al. 2006). Taken together, these studies point to the potential importance of testing how larval density may affect fitness costs of Bt resistance by western corn rootworm.
Because larval density has been shown to influence life-history traits of western corn rootworm, we hypothesized that larval density might also influence fitness costs affecting resistance to Bt corn. In this experiment, we examined two strains of western corn rootworm (Cresco and Hopkinton) with field-evolved resistance to Cry3Bb1, the first Bt trait for which this pest developed resistance (Gassmann et al. 2011). We reared each strain at high and low larval rearing densities and measured resistance to Cry3Bb1 over six generations in the absence of selection. We hypothesized that 1) resistance to Bt would decrease over time in the absence of selection, 2) larval density would affect fitness costs associated with Bt resistance, and 3) populations would retain some level of Bt resistance compared to susceptible controls.
Materials and Methods
Strain History
Susceptible Strain
The Susceptible strain was a Bt-susceptible non-diapausing strain of western corn rootworm, which was brought into laboratory culture in the mid-1970s and has never been exposed to Bt corn (Branson 1976). The Susceptible strain was received in October 2009 from the United States Department of Agriculture, Agricultural Research Service, North Central Agricultural Research Laboratory in Brookings, South Dakota. At the time of this study, it had been maintained in continuous culture at Iowa State University for 35 generations.
Hopkinton Strain
The Hopkinton strain was a non-diapausing strain of western corn rootworm with field-evolved resistance to Cry3Bb1 corn (Ingber and Gassmann 2015). Adult western corn rootworm were sampled from a field where Cry3Bb1 corn had been grown seven consecutive years, and Cry3Bb1 resistance had been confirmed (Gassmann et al. 2011). These adults were crossed with the Susceptible strain, as described in Ingber and Gassmann (2015), to introgress field-evolved Cry3Bb1 resistance into a non-diapausing genetic background. Prior to the start of the experiment, the strain was selected on Cry3Bb1 corn for five generations (F1, F2, F5, F17, and F18) and backcrossed to the Susceptible strain two additional times (F1 and F4).
Cresco Strain
The Cresco strain was also a non-diapausing strain with field-evolved resistance to Cry3Bb1 corn (Ingber and Gassmann 2015). Adult western corn rootworm were sampled from a field that had been planted to 9 yr of continuous corn, with Cry3Bb1 corn grown during the year the field was sampled and during the previous 4 yr (Gassmann et al. 2014). Additionally, Cry3Bb1 resistance was confirmed with laboratory bioassays (Gassmann et al. 2014). These adults were crossed with the Susceptible strain, as described in Ingber and Gassmann (2015). Prior to the start of the experiment, the Cresco strain had been selected on Cry3Bb1 corn for six generations (F3, F5, F8, F9, F15, and F16), and backcrossed to the Susceptible strain two additional times (F3 and F5).
Multiple strains were used in this experiment to provide a more complete understanding of how larval density may affect the fitness costs of Bt resistance. Using multiple strains captures more of the genetic variation that may be present in the mechanisms of resistance and in other traits. In particular, Cresco and Hopkinton have been found to differ in both the fitness costs of Cry3Bb1 resistance and the level of Cry3Bb1 resistance (Ingber and Gassmann 2015). In a previous study, Hopkinton was found to have a higher LC50 for Cry3Bb1 than Cresco, showed complete resistance to Cry3Bb1 corn, non-recessive inheritance of resistance, and an apparent absence of fitness costs, while Cresco showed incomplete resistance to Cry3Bb1, recessive inheritance of resistance, and fitness costs of resistance (Ingber and Gassmann 2015). Thus, using both strain backgrounds may reveal if increased larval density can affect rootworm populations with different characteristics.
Generation of Strains for Experiments
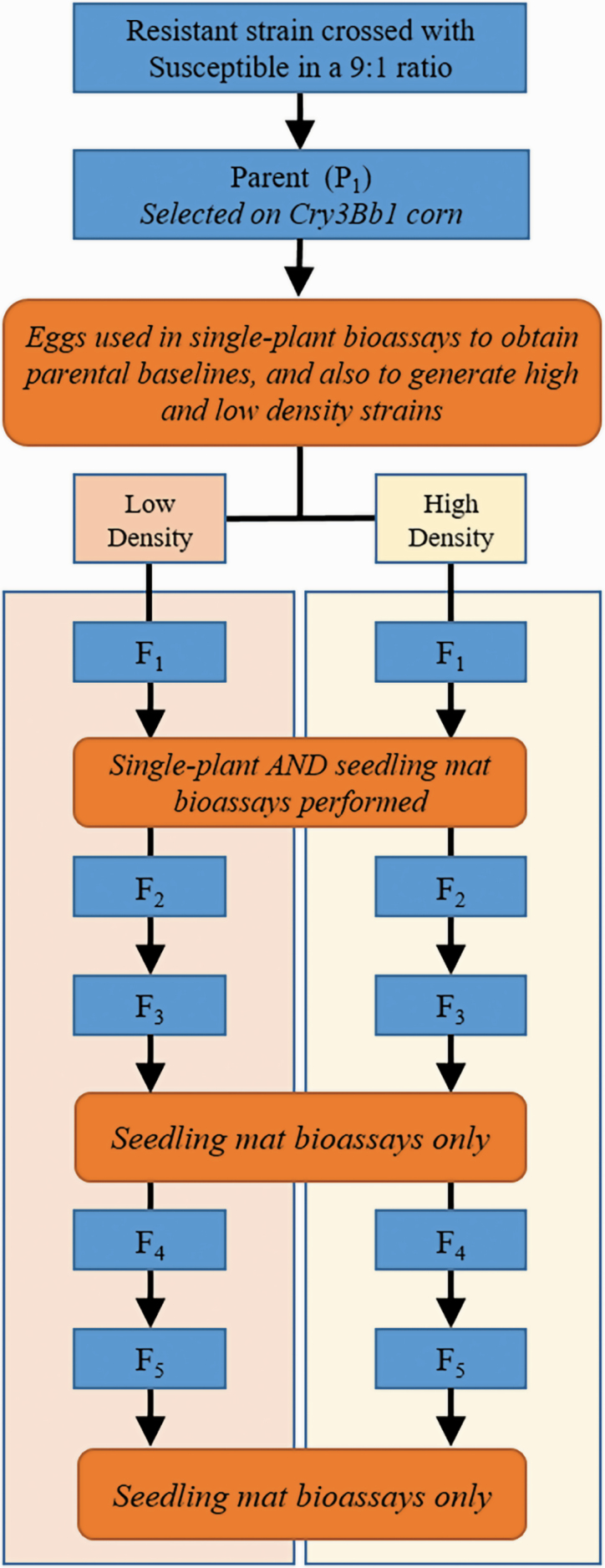
In total, four strains were generated for this study (Cresco High, Cresco Low, Hopkinton High, Hopkinton Low; Fig. 1). First, parental strains derived from Hopkinton and Cresco were generated in September 2015 by crossing adults from either Hopkinton or Cresco with adults from the Susceptible strain in a 9 to 1 ratio (i.e., nine resistant adults for every Susceptible adult). For Hopkinton, F29 was used; for Cresco, the F24 was used; and for Susceptible, the F35 and F36 were used. A cross of 9:1 was used to ensure that there was genetic variation for resistance in the resulting strains.
Fig. 1.
Diagram of experimental design. Note that F6 is not shown in the figure because it was not reared to the adult stage after eggs were collected. High and low density refer to larval rearing density and are described in the Methods. An arrow represents the end of a generation, with each generation ending at the adult stage and the subsequent generation beginning with eggs. Rectangles with an alphanumeric code represent rearing of strains from egg to adulthood (i.e., one generation).
Throughout the experiment, adults were housed in 18 × 18 × 18 cm plastic insect cages (MegaView Science Co. Ltd., Taichung, Taiwan) and were provisioned with a corn leaf and complete adult diet (Frontier Agricultural Sciences, 601 Interchange Blvd., Newark, DE) as food sources, and 1.5% agar solid as a water source (Thermo Fisher Scientific Inc., 81 Wyman St, Waltham, MA). Eggs (P1) from both parental strains were collected in an oviposition substrate of sieved soil (< 180 µm) twice weekly from September to November 2015. One week after collection, eggs were washed using a sieve (250 µm) and placed into small seedling mats, which consisted of 40 ml of Cry3Bb1 corn (VT3 DKC 61–88), without any pesticidal seed treatment, with 60 ml water and 200 g soil in 946 ml containers (Dart Container Corporation, Mason, MI). The soil was a 1:1 mixture of sieved field soil (particle size < 1 cm) and potting medium (Sunshine Mix #1, SunGro Horticulture, Vancouver, British Columbia, Canada). Seedling mats were then covered with mesh fabric (poly chiffon, Hobby Lobby Stores, Inc., Oklahoma City, OK) and secured with corresponding lids to prevent larvae from escaping. Seedling mats were housed in a biological incubator (I41-LL, Percival Scientific, Perry, IA) at 25°C, 16:8 (L:D) h, and 65% RH.
After 2 wk of incubation, two small seedling mats were transferred onto one larger seedling mat to ensure the presence of sufficient root tissue for larvae to complete development. Larger seedling mats contained 150 ml of Cry3Bb1 corn seed in a 5.7-liter storage box (Sterlite Corporation, Townsend, MA). Four-hundred milliliters of water and 2,000 g of soil (1:1 field soil: potting medium mixture) were added to the container. Larger seedling mats were allowed to grow in an incubator for 1 wk prior to receiving the contents of two small seedling mats.
Adults were collected from the larger seedling mats three times per week using an aspirator (BioQuip Products Inc., Rancho Dominguez, CA) attached to a vacuum pump (Gast Manufacturing Inc., Benton Harbor, MI), and eggs were collected two times per week as described previously. Cry3Bb1 corn was used for the first generation of each strain (P1) to increase the level of resistance at the start of the experiment, and all generations thereafter (F1 through F6) were reared on non-Bt corn.
For both parent strains (Hopkinton and Cresco), the eggs obtained from P1 adults were used to generate the high- and low-density strains (F1; Cresco Low, Cresco High, Hopkinton Low, and Hopkinton High). At low densities, 300 eggs were added to each small seedling mat of non-Bt corn. At high densities, 1,800 eggs were added to each small seedling mat of non-Bt corn. These egg densities were chosen based on preliminary experiments in our laboratory, which indicated that the high-density strains would experience greater density-dependent mortality than the low-density strains. All strains were reared from F1 onward using the same methods described for the parental strains except that insects were reared on non-Bt seed corn (Pioneer 33T54, DuPont Pioneer, Johnston, IA), without any pesticidal seed treatment, for the remainder of the experiment, with the final generation being F6 (Fig. 1). Eggs from F6 were used in bioassays but were not reared to the adult stage.
Bioassays
Bioassays to measure resistance to Cry3Bb1 were performed at the following time points: F1 (representing a baseline level one generation after selection on Cry3Bb1, prior to splitting into high and low densities), F2, F4, and F6. These assays were conducted using a subset of eggs produced at each relevant generation. Insects used in bioassays were killed as part of the data collection for the bioassays and did not contribute to subsequent generations of the rootworm strains. Eggs from the Susceptible strain were assayed alongside the Bt-resistant strains, with Susceptible serving as a known susceptible control. The generations of Susceptible used were F37 and F38 (corresponding to F1 of the resistant strains), F39 (F2), F40 (F4), and F42 (F6).
Two bioassay methods were used in this experiment: a single-plant bioassay and a seedling-mat assay. The single-plant bioassay was used for F1 and F2, and the seedling-mat bioassay was used for F2, F4, and F6, with F2 evaluated with both the single-plant and seedling-mat bioassays. The seedling-mat method replaced the single-plant method after F2 because other experiments being conducted at the same time found that the seedling-mat assay was more efficient at measuring differences in resistance with non-diapausing strains than the single-plant assay (Paolino and Gassmann 2017).
The single-plant bioassay procedure followed Paolino and Gassmann (2017). Two corn hybrids, Cry3Bb1 corn (DKC 61-69) and its non-Bt genetic isoline (DKC 61-72), both without any pesticidal seed treatment, were grown in greenhouses as individual plants in 0.95-liter clear plastic cups (Placon, Madison, WI). Plants were grown to the V5 to V6 stage (Abendroth et al. 2011). Twelve neonate larvae (<24 h old) were placed on the exposed root tissue of each plant using a paintbrush. The stalks of the plants were cut to approximately 20 cm, with leaves trimmed to 10 cm, to allow the plant to fit in an environmental chamber. Plants were placed in an environmental chamber for 14 d at 24°C, 16:8 (L:D) h, 65% RH. After 14 d, larvae were extracted using a Berlese funnel and surviving larvae were counted using a stereo microscope (MZ6, Leica, Microsystems, Wetzlar, Germany). The sample size was 18 Cry3Bb1 and 18 non-Bt corn plants for each rootworm strain at F1 and F2.
For seedling-mat bioassays, seeds of Cry3Bb1 corn (either DKC 62-63 or DKC 43-48) and its non-Bt isoline (either DKC 62-61 or DKC 43-46), without any pesticidal seed treatment, were used. Corn seeds were germinated with moistened paper towels for 3–4 d. Three germinated seeds, with approximately 3–5 cm of root tissue per seed, were transferred to a 44 ml cup (Solo, Souffles, P150N, DART, Mason, MI) and then 12 neonate larvae were placed on these germinated seeds using a paintbrush (<24 h old). The larvae and root tissue were then covered with 30 g moistened sieved field soil (particle size < 600 µm) and 4.5 ml deionized water. The larval cups were covered with poly chiffon fabric and secured with corresponding lids with an open center (4 cm diameter), then incubated in a dark growth chamber at 25°C. Cups were remoistened with deionized water every other day. Larger seedling mats were prepared on the same day as the 44 ml cups. These consisted of 25 ml of corn seed in 200 ml soil, with 60 ml of deionized water, in a 500 ml container (TD40016, DeliPRO, TRIPAC, White Plains, NY). The contents of the 44 ml containers were transferred to these larger seedling mats after 1 wk in order to provide adequate root tissue for the larvae to survive to adulthood. The containers (larger seedling mats that contained the contents of the 44 ml cups) were then placed in an environmental chamber at 25°C, 16:8 (L:D) h, 65% RH for an additional 3 wk.
Four weeks after larvae were added to the germinated seeds, containers were checked for adult emergence three times per week until no adults were collected for two consecutive weeks. Adult rootworm were preserved in 1.5 ml microcentrifuge tubes in 85% ethanol and counted at a later time. Six larval bioassays with Bt corn and six with non-Bt corn were conducted every week for 3 wk for each strain of rootworm at each generation tested. Total sample sizes were 18 Cry3Bb1 seedling mats and 18 non-Bt seedling mates for each strain at F2, F4, and F6.
Data Analysis
All statistical tests were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). To confirm that the density treatment imposed density-dependent mortality, the total number of adults collected during rearing in each generation per strain was divided by the total number of eggs used to propagate that strain in that generation, yielding proportion survival per strain per generation. Proportion survival was calculated for F1 through F5 for each strain. A paired T-test (PROC TTEST) was conducted to compare proportion survival between high-density and low-density treatments within a parental background (i.e., Hopkinton High vs Hopkinton Low and Cresco High vs Cresco Low).
Analysis of all bioassay results (i.e., single-plant and seedling-mat assays) used corrected survival based on Abbott (1925), which was calculated as the proportion of surviving larvae on Cry3Bb1 corn in a replicate divided by the mean proportion of larvae surviving on non-Bt corn per strain per generation, such that a population in which an equal proportion of larvae survived on both non-Bt corn and Cry3Bb1 corn would have a corrected survival value of 1.
A one-tailed T-test (PROC TTEST) was performed to test whether the Hopkinton and Cresco strains were resistant to Cry3Bb1 corn compared to the susceptible control at the beginning of the experiment, comparing corrected survival for both parental strains (at F1 but before the strains were split into high and low density) to the Susceptible strain. The null hypothesis was that survival on Cry3Bb1 corn did not differ between Cresco or Hopkinton versus Susceptible, and the alternative hypothesis was that the survival of Cresco or Hopkinton was significantly greater than Susceptible.
A two-way analysis of variance (ANOVA) (PROC GLM) was used to determine whether there was a difference in corrected survival between assay types (i.e., single plant vs seedling mat). This was done by comparing the corrected survival between these assays at F2 (i.e., the generation during which both bioassays were conducted). The ANOVA included the factors of strain (Hopkinton High, Hopkinton Low, Cresco High, Cresco Low, and Susceptible) assay type, and their interaction.
To test our first hypothesis, that resistance would decrease over time, a one-tailed T-test (PROC TTEST) was conducted to test if corrected survival was lower in the final generation of the experiment (F6) compared to the beginning of the experiment (F1). A one-tailed test was used because corrected survival was expected to decrease over time the absence of selection, but not increase. The null hypothesis was that corrected survival did not differ between F6 and F1 for each strain, with the alternative hypothesis that corrected survival was significantly lower at F6 compared to F1. To test our second hypothesis, that density would affect the fitness trade-off associated with resistance, we conducted two-tailed T-tests (PROC TTEST) on corrected survival between the high-density and low-density strains, within a parental background, at each generation (e.g., Cresco High versus Cresco Low at generation F2, F4, and F6). The null hypothesis was that corrected survival would not differ between high and low densities at the same generation, and the alternative hypothesis was that corrected survival would be significantly different. To test our third hypothesis, that strains would retain some level of resistance over time, we conducted one-tailed T-tests (PROC TTEST) to test if corrected survival was significantly higher in each resistant strain at F6 compared to the susceptible control (i.e., Susceptible strain). A one-tailed test was used because corrected survival would be expected to be higher for the known Cry3Bb1-resistant strains than the Susceptible strains, but not lower. The null hypothesis was that corrected survival would not differ between Bt-resistant strains versus Susceptible at F6, with the alternative hypothesis that corrected survival would be greater for Bt-resistant strain than Susceptible strain at F6.
Results
In strain rearing, proportion survival to adulthood was significantly greater at low larval density compared to high density. For F1 to F5, during which time all strains were reared on non-Bt corn, proportion survival for Cresco High was 0.12 ± 0.03 (mean ± SE), while proportion survival for Cresco Low was 0.43 ± 0.07 (T4 = 6.0, P = 0.004). Similarly, Hopkinton High had significantly lower survival (0.11 ± 0.3) than Hopkinton Low (0.40 ± 0.05) for F1 to F5 (T4 = 8.4, P = 0.001). These results indicate that density affected both rootworm strains.
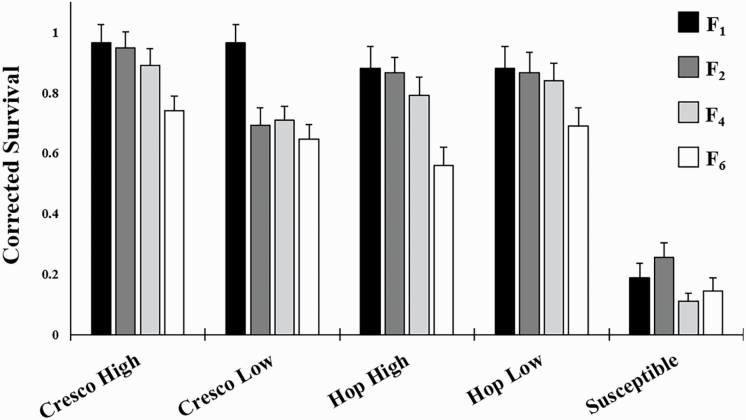
Corrected survival of Cresco and Hopkinton at F1 were significantly higher than Susceptible (Cresco: T34 = 10.13, P < 0.0001; Hopkinton: T34 = 7.85, P < 0.0001), indicating that both parental strains were resistant to Cry3Bb1 corn at the beginning of the experiment (Fig. 2).
Fig. 2.
Corrected survival of western corn rootworm larvae on Bt corn in plant-based bioassays. Bar heights represent sample means and error bars are the standard error of the mean. Note that the black bars (F1 parental baselines) represent Cresco and Hopkinton strains before being split into high-density and low-density treatments, and thus are represented twice in the graph for clarity. The F2, F4, and F6 represent corrected survival at one, three, and five generations in the absence of selection, respectively. Statistical tests among means, as they relate to the hypotheses addressed in this study, are presented in Table 1.
ANOVA revealed no difference in corrected survival between the two assay types used at generation F2, although the effect of strain was significant (F4, 168 = 25.46, P < 0.0001). Neither assay type nor the interaction term was significant (F1, 168 = 0.01, P = 0.92; F4, 168 = 1.88, P = 0.12, respectively). This indicated that there was no difference in the measurement of corrected survival between assay types used in the experiment, and thus, the data for the two assay types were combined in all subsequent analyses.
For our first hypothesis, that resistance would decrease over time, we found that all four resistant strains had lower corrected survival at F6 compared to F1 (Fig. 2; Table 1). However, corrected survival for the Susceptible strain did not differ for F1 versus F6. Thus, because resistance decreased in the absence of selection on Cry3Bb1, a cumulative fitness cost of Bt resistance was detected for all strains.
Table 1.
Results for statistical tests of experimental hypothesesa,b
| df | T | P | |
|---|---|---|---|
| Hypothesis 1c | |||
| Cresco high | 34 | 2.89 | 0.003 |
| Cresco low | 34 | 4.09 | <0.001 |
| Hopkinton high | 33 | 3.12 | 0.002 |
| Hopkinton low | 33 | 1.75 | 0.044 |
| Susceptible | 33 | 0.53 | 0.301 |
| Hypothesis 2d | |||
| Cresco high/low | |||
| F2 | 70 | 3.34 | 0.001 |
| F4 | 34 | 2.53 | 0.016 |
| F6 | 34 | 1.38 | 0.177 |
| Hopkinton high/low | |||
| F2 | 68 | 0.06 | 0.950 |
| F4 | 34 | 0.59 | 0.561 |
| F6 | 34 | 1.51 | 0.139 |
| Hypothesis 3e | |||
| Cresco high | 34 | 9.14 | <0.001 |
| Cresco low | 34 | 7.57 | <0.001 |
| Hopkinton high | 34 | 5.60 | <0.001 |
| Hopkinton low | 34 | 7.22 | <0.001 |
aAnalyses presented in this table were conducted on correct survival in plant-based bioassays. Correct survival was calculated by dividing survival on Bt corn by survival on non-Bt corn. Additional details are provided in the Data Analysis section of the Materials and Methods.
bMeans tested in Table 1 correspond to the means presented in Fig. 2.
cHypothesis 1: One-tailed T-tests of corrected survival between F1 and F6 for western corn rootworm strains. H0: corrected survival is equal at beginning of the experiment compared to the end of the experiment. HA: corrected survival is significantly lower at the end of the experiment compared to the beginning of the experiment.
dHypothesis 2: Two-tailed T-tests between high-density and low-density treatments within the same parental background (i.e., Hopkinton or Cresco) at each of three generations (i.e., F2, F4 and F6). H0: corrected survival is equal between strains reared at high density vs low density. HA: corrected survival is significantly different between strains reared at high density vs low density.
eHypothesis 3: One-tailed T-tests comparing corrected survival at F6 for each strain to corrected survival for Susceptible at F6. H0: corrected survival is equal between each strain and the Susceptible strain at the end of the experiment. HA: corrected survival is significantly lower in the Susceptible strain compared to each strain at the end of the experiment.
For our second hypothesis, that density would affect the fitness cost associated with resistance, we found that Cresco High was more resistant to Cry3Bb1 than Cresco Low at F2 and F4, but not at F6. By contrast, levels of resistance in Hopkinton High and Hopkinton Low did not differ at any of the generations (Fig. 2; Table 1). Thus, the fitness cost of Bt resistance appeared to be greater at low density for the Cresco parental background, but we did not detect an effect of density on the fitness trade-off for the Hopkinton background.
For our third hypothesis, that strains would retain some level of resistance over time, we found that all strains had significantly higher levels of survival on Cry3Bb1 at F6 compared to the Susceptible strain, indicating that all strains retained some level of Cry3Bb1 resistance through six generations in the absence of selection on Cry3Bb1 (Fig. 2; Table 1).
Discussion
We found evidence that fitness costs of Cry3Bb1 resistance were present in both the Hopkinton and Cresco strains of western corn rootworm, at both larval densities (Table 1; Fig. 2). Ingber and Gassmann (2015), evaluated numerous life-history characteristics and found evidence of fitness costs for Cresco but not for Hopkinton. In general, using selection studies, as was done in the experiments reported here, represents a more sensitive method for detecting fitness costs compared to measurements of individual life-history characteristics (Gassmann et al. 2009, Fry 2003). While fitness costs were present, all strains still exhibited significantly higher survival on Cry3Bb1 corn, compared to the Susceptible strain, at the end of the experiment, suggesting that Cry3Bb1 resistance may persist within field populations for years after Cry3Bb1 corn is no longer used, a pattern that has been observed for some other insecticide-resistance traits in western corn rootworm (Parimi et al. 2003). However, the significantly greater fitness costs for the Cresco strain at the lower larval density suggests that maintaining rootworm populations at lower abundance in the field may accelerate the loss of resistance, in the absence of Cry3Bb1 corn, in some cases.
In the case of Bt crops and the refuge strategy, fitness costs are important because they will prevent the accumulation of resistance alleles within refuge populations, and maintain the capacity of refuges to produce Bt-susceptible individuals (Gassmann et al. 2009, Pittendrigh et al. 2014). To the extent that ecological factors in refuges can be manipulated to increase fitness costs, this should increase the extent to which Bt resistance can be delayed (Pittendrigh 2004, Gassmann et al. 2008). For example, introducing entomopathogenic nematodes to refuges or selecting certain host-plant varieties for refuges has the potential to increase fitness costs of Bt resistance (Carrière et al. 2004, Bird and Akhurst 2007, Raymond et al. 2007, Gassmann et al. 2008). In this study, the Cresco strain exhibited higher fitness costs under low density compared to high density. This implies that using integrated pest management techniques such as crop rotation to maintain rootworm populations at a lower abundance may enhance fitness costs of Bt resistance in some cases and delay the development of Bt resistance by western corn rootworm.
Our experiment examined fitness in the context of overall selection as opposed to the measurement of individual life-history components, with the former likely providing a more compressive measurement of fitness (Fry 2003, Gassmann et al. 2009). Previous studies with the Cresco strain have revealed trade-offs affecting development rate, survival to adulthood, and fecundity (Ingber and Gassmann 2015). Interestingly, the same study did not detect trade-offs in the Hopkinton strain when examining the same life-history metrics (Ingber and Gassmann 2015). Thus, the present study illustrates the utility of selection experimentation relative to comparing individual life-history components when testing for the presence of fitness trade-offs (Gassmann et al. 2009). Because resistance declined over time in both strains derived from Hopkinton, a cumulative cost was evident even though no reduction in any single life-history component was found in previous work.
Despite the apparent presence of some fitness costs affecting Cry3Bb1 resistance, field-evolved Cry3Bb1 resistance was still detected in Iowa 6 yr after the commercialization of this Bt trait, and is now widely distributed in Iowa, and found in other Midwest states (Gassmann et al. 2011, Schrader et al. 2016, Zukoff et al. 2016, Reinders 2018, Shrestha et al. 2018). While planting of non-Bt refuges coupled with fitness costs likely acted to delay resistance, these effects were counterbalanced by non-recessive inheritance of resistance, a lack of compliance in planting of refuges, and limited mating between insects from refuges and Bt corn, factors that are expected increase the rate of resistance evolution (Gassmann et al. 2011, Spencer et al. 2013, Ingber and Gassmann et al. 2015, Paolino and Gassmann 2017). Furthermore, the finding in this study that all western corn rootworm strains remained resistant to Cry3Bb1 corn throughout the experiment, a timeframe that would equate to 6 yr in the field for this univoltine species, indicates that Bt resistance, once it evolves, many persist within a field for many years, even in the absence of selection for resistance.
In several studies, fitness costs have been found to increase under more stressful ecological conditions, for example in the presence of host-plant secondary metabolites, entomopathogens, or competition (Carriere et al. 2004, Higginson et al. 2005, Raymond et al. 2005, Gassmann et al. 2006, Hannon et al. 2010, Raymond et al. 2011). In this study, we found that costs were increased by a less stressful ecological condition, specifically lower compared to higher larval density. This may have arisen because when stressors become too great, the effect of the stress on fitness may eclipse any fitness costs (Rosenheim et al. 2010). In other words, fitness costs of a particular trait may not manifest within a population if ecological conditions do not permit (e.g., resource limitation defines the upper boundary of fitness for all genotypes). We saw in this experiment that the Cresco population experienced a more rapid reduction in resistance in conditions of lower intraspecific competition, suggesting that susceptible individuals were more capable of exploiting abundant resources. At high density, resistance declined at a lower rate. That this was not seen in the Hopkinton strain may indicate that Cresco responded more readily to the effects of density. Ingber and Gassmann (2015) found differences between Hopkinton and Cresco in the life-history traits displaying fitness costs, the inheritance of resistance, and the magnitude of resistance, and it also appears that these strains differ in the extent to which ecological conditions may affect the manifestation of fitness costs. Furthermore, a recent study examining gene expression showed that Cry3Bb1 does not elicit a stress response in Hopkinton, further demonstrating that resistance in this strain is robust (Rault et al. 2018). This may also partially explain why Cresco was affected by differing larval densities, while Hopkinton was not.
Our study provides further evidence that the fitness costs associated with Cry3Bb1 resistance in western corn rootworm are present and may be influenced by larval density. Understanding the nature of fitness costs in western corn rootworm, and their interaction with ecological stressors, may help to improve resistance management for this pest, by enhancing the capacity of refuges to delay resistance (Pittendrigh et al. 2004, Gassmann et al. 2009). This is especially important as next-generation technologies, such as dsRNA and other transgenic innovations, enter the market in the coming years. Understanding how fitness costs may be manipulated can aid in the development of more effective resistance management strategies for current and future transgenic traits.
Acknowledgments
We thank Siva Jakka, John Doudna, and Eric Yu for assistance with the experiment. This research was supported by the Biotechnology Risk Assessment Grants (BRAG) program, award number 2012-33522-20010, from the U.S. Department of Agriculture.
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Abendroth L. J., Elmore R. W., Boyer M. J., and Marlay S. K.. 2011. Corn growth and development (PMR 1009). Iowa State University, Ames, IL. [Google Scholar]
- Bird L. J., and Akhurst R. J.. 2007. Effects of host plant species on fitness costs of Bt resistance in Helicoverpa armigera (Lepidoptera: Noctuidae). Biol. Control 40: 196–203. [Google Scholar]
- Branson T. F. 1976. The selection of a non-diapause strain of Diabrotica virgifera (Colepotera: Chrysomelidae). Entomol. Exp. Appl. 19: 148–154. [Google Scholar]
- Branson T. F., and Sutter G. R.. 1985. Influence of population density of immatures on size, longevity, and fecundity of adult Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Environ. Entomol. 14: 687–690. [Google Scholar]
- Carpenter J. E. 2010. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat. Biotechnol. 28: 319–321. [DOI] [PubMed] [Google Scholar]
- Carrière Y., and Tabashnik B. E.. 2001. Reversing insect adaptation to transgenic insecticidal plants. Proc. Biol. Sci. 268: 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière Y., Ellers-Kirk C., Biggs R., Higginson D. M., Dennehy T. J., and Tabashnik B. E.. 2004. Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. J. Econ. Entomol. 97: 1710–1718. [DOI] [PubMed] [Google Scholar]
- Edgerton M. D., Fridgen J., Anderson J. R. Jr, Ahlgrim J., Criswell M., Dhungana P., Gocken T., Li Z., Mariappan S., Pilcher C. D., . et al. 2012. Transgenic insect resistance traits increase corn yield and yield stability. Nat. Biotechnol. 30: 493–496. [DOI] [PubMed] [Google Scholar]
- Fry J. D. 2003. Detecting ecological trade-offs using selection experiments. Ecology 84: 1672–1678. [Google Scholar]
- Gassmann A. J. 2016. Resistance to Bt maize by western corn rootworm: insights from the laboratory and the field. Curr. Opin. Insect Sci. 15: 111–115. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Stock S. P., Carrière Y., and Tabashnik B. E.. 2006. Effect of entomopathogenic nematodes on the fitness cost of resistance to Bt toxin crylac in pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 99: 920–926. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Stock S. P., Sisterson M. S., Carrière Y., and Tabashnik B. E.. 2008. Synergism between entomopathogenic nematodes and Bacillus thuringiensis crops: integrating biological control and resistance management. J. Appl. Ecol. 45: 957–966. [Google Scholar]
- Gassmann A. J., Carrière Y., and Tabashnik B. E.. 2009. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 54: 147–163. [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Keweshan R. S., and Dunbar M. W.. 2011. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One 6: e22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Clifton E. H., Dunbar M. W., Hoffmann A. M., Ingber D. A., and Keweshan R. S.. 2014. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. U. S. A. 111: 5141–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann A. J., Shrestha R. B., Kropf A. L., St Clair C. R., and Brenizer B. D.. 2020. Field-evolved resistance by western corn rootworm to Cry34/35Ab1 and other Bacillus thuringiensis traits in transgenic maize. Pest Manag. Sci. 76: 268–276. [DOI] [PubMed] [Google Scholar]
- Gould F. 1998. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43: 701–726. [DOI] [PubMed] [Google Scholar]
- Gray M. E., Sappington T. W., Miller N. J., Moeser J., and Bohn M. O.. 2009. Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annu. Rev. Entomol. 54: 303–321. [DOI] [PubMed] [Google Scholar]
- Hannon E. R., Sisterson M. S., Stock S. P., Carrière Y., Tabashnik B. E., and Gassmann A. J.. 2010. Effects of four nematode species on fitness costs of pink bollworm resistance to Bacillus thuringiensis toxin Cry1Ac. J. Econ. Entomol. 103: 1821–1831. [DOI] [PubMed] [Google Scholar]
- Higginson D. M., Morin S., Nyboer M. E., Biggs R. W., Tabashnik B. E., and Carrière Y.. 2005. Evolutionary trade-offs of insect resistance to Bacillus thuringiensis crops: fitness cost affecting paternity. Evolution. 59: 915–920. [PubMed] [Google Scholar]
- Ingber D. A., and Gassmann A. J.. 2015. Inheritance and fitness costs of resistance to Cry3Bb1 corn by Western Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 108: 2421–2432. [DOI] [PubMed] [Google Scholar]
- Jakka S. R., Shrestha R. B., and Gassmann A. J.. 2016. Broad-spectrum resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera virgifera). Sci. Rep. 6: 27860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. 2017. Global status of commercialized biotech/GM crops in 2017. ISAAA, Ithaca. [Google Scholar]
- Onstad D. W., Hibbard B. E., Clark T. L., Crowder D. W., and Carter K. G.. 2006. Analysis of density-dependent survival of Diabrotica (Coleoptera: Chrysomelidae) in cornfields. Environ. Entomol. 35: 1272–1278. [Google Scholar]
- Paolino A. R., and Gassmann A. J.. 2017. Assessment of inheritance and fitness costs associated with field-evolved resistance to Cry3Bb1 maize by western corn rootworm. Toxins 9: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parimi S., Scharf M. E., Meinke L. J., Chandler L. D., and Siegfried B. D.. 2003. Inheritance of methyl-parathion resistance in Nebraska western corn rootworm populations (Coleoptera: Chrysomelidae). J. Econ. Entomol. 96: 131–136. [DOI] [PubMed] [Google Scholar]
- Pittendrigh B. R., Gaffney P. J., Huesing J. E., Onstad D. W., Roush R. T., and Murdock L. L.. 2004. “Active” refuges can inhibit the evolution of resistance in insects towards transgenic insect-resistant plants. J. Theor. Biol. 231: 461–474. [DOI] [PubMed] [Google Scholar]
- Pittendrigh B. R., Huesing J., Walters K., Olds B., Steele L. D., Sun L., Gaffney P., and Gassmann A. J.. 2014. Negative cross-resistance: history, present status, and emerging opportunities. InOnstad D. W. (ed.), Insect resistance management: Biology, economics and predictions. 2nd ed. Elsevier, London, United Kingdom. [Google Scholar]
- Rault L. C., Siegfried B. D., Gassmann A. J., Wang H., Brewer G. J., and Miller N. J.. 2018. Investigation of Cry3Bb1 resistance and intoxication in western corn rootworm by RNA sequencing. J. Appl. Entomol. 142: 921–936. [Google Scholar]
- Raymond B., Sayyed A. H., and Wright D. J.. 2005. Genes and environment interact to determine the fitness costs of resistance to Bacillus thuringiensis. Proc. Biol. Sci. 272: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond B., Sayyed A. H., Hails R. S., and Wright D. J.. 2007. Exploiting pathogens and their impact on fitness costs to manage the evolution of resistance to Bacillus thuringiensis. J. Appl. Ecol. 44: 768–780. [Google Scholar]
- Raymond B., Wright D. J., and Bonsall M. B.. 2011. Effects of host plant and genetic background on the fitness costs of resistance to Bacillus thuringiensis. Heredity (Edinb). 106: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders J. D., Hitt B. D., Stroup W. W., French B. W., and Meinke L. J.. 2018. Spatial variation in western corn rootworm (Coleoptera: Chrysomelidae) susceptibility to Cry3 toxins in Nebraska. PLoS One 13: e0208266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheim J. A., Alon U., and Shinar G.. 2010. Evolutionary balancing of fitness-limiting factors. Am. Nat. 175: 662–674. [DOI] [PubMed] [Google Scholar]
- Schrader P. M., Estes R. E., Tinsley N. A., Gassmann A. J., and Gray M. E.. 2016. Evaluation of adult emergence and larval root injury for Cry3Bb1-resistant populations of the western corn rootworm. J. Appl. Entomol. 141: 41–52. [Google Scholar]
- Shrestha R. B., Dunbar M. W., French B. W., and Gassmann A. J.. 2018. Effects of field history on resistance to Bt maize by western corn rootworm, Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae). PLoS One 13: e0200156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Onstad D., Krupke C., Hughson S., Pan Z., Stanley B., and Flexner L.. 2013. Isolated females and limited males: evolution of insect resistance in structured landscapes. Entomol. Exp. Appl. 146: 38–49. [Google Scholar]
- Tabashnik B. E., and Carrière Y.. 2017. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 35: 926–935. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. 2018. Current and previously registered section 3 plant-incorporated protectant (pip) registrations. http://www.epa.gov/ingredients-used-pesticide-products/current-previously-registered-section-3-plant-incorporated. [Google Scholar]
- U.S. Environmental Protection Agency. 2020. Insect resistance management for Bt plant-incorporated protectants. https://www.epa.gov/regulation-biotechnology-under-tsca-and-fifra/insect-resistance-management-bt-plant-incorporated. [Google Scholar]
- Wang R., Tetreau G., and Wang P.. 2016. Effect of crop plants on fitness costs associated with resistance to Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in cabbage loopers. Sci. Rep. 6: 20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Seevers K. P., and Mayo Z. B.. 1985. Influence of western corn rootworm larval densities and damage on corn rootworm survival, developmental time, size and sex ratio (Coleoptera: Chrysomelidae). J. Kansas Entomol. Soc. 58: 397–402. [Google Scholar]
- Zukoff S. N., Ostlie K. R., Potter B., Meihls L. N., Zukoff A. L., French L., Ellersieck M. R., Wade French B., and Hibbard B. E.. 2016. Multiple assays indicate varying levels of cross resistance in Cry3Bb1-selected field populations of the Western Corn Rootworm to mCry3A, eCry3.1Ab, and Cry34/35Ab1. J. Econ. Entomol. 109: 1387–1398. [DOI] [PubMed] [Google Scholar]