SUMMARY
Objective:
It has been postulated that treatment outcomes are similar between transoral robotic surgery (TORS) and definitive chemoradiation (CRT) for oropharyngeal squamous cell carcinomas (OPSCC). We compared oncologic and quality of life (QOL) outcomes between definitive CRT and definitive TORS.
Materials and methods:
An observational comparison study was performed on 92 patients treated with TORS ± adjuvant therapy and 46 patients treated with definitive CRT between July 2005 and January 2016. The Kaplan Meier method was used for survival analyses, and the Mann-Whitney test was used to compare QOL scores between groups.
Results:
All patients had T0-T2 and N0-N2 disease, although CRT patients had higher clinical staging (p < 0.001). HPV+ disease was present in 79% (n = 73) of TORS patients and 91% (n = 19) of tested CRT patients. Median follow-up was 22.1 months (range: 0.33–83.4). There were no significant differences in locoregional control or overall survival between CRT and TORS groups. Definitive TORS resulted in better saliva-related QOL than definitive CRT at 1, 6, 12, and 24 months (p < 0.001, p = 0.025, p = 0.017, p = 0.011). Among TORS patients, adjuvant therapy was associated with worse QOL in the saliva domain at 6, 12, and 24 months (p < 0.001, p < 0.001, p = 0.007), and taste domain at 6 and 12 months (p = 0.067, p = 0.008).
Conclusion:
Definitive CRT and definitive TORS offer similar rates of locoregional control, overall survival, and disease-free survival in patients with early stage OPSCC. TORS resulted in significantly better short and long-term saliva-related QOL, whereas adjuvant therapy was associated with worse saliva and taste-related QOL compared to TORS alone.
Keywords: Chemoradiotherapy, Head and neck cancer, Robotic surgical procedures, Oropharyngeal cancer, Squamous cell carcinoma, Quality of life, Treatment outcome
Background
Treatment regimens for oropharyngeal squamous cell carcinoma (OPSCC) have transformed over recent decades due to advancements in all treatment modalities [1–3]. Definitive chemoradiation is now the treatment of choice in most institutions, although this regimen is associated with severe acute and late toxicities which are further exacerbated by chemotherapy [4]. Common toxicities include mucositis, dysgeusia, and xerostomia resulting in increased risk of mucosal trauma and oral infections, all of which limit treatment success and detract from quality of life [5]. Given the increasing incidence of cases in younger patients with oral human papillomavirus (HPV) infection who have much improved long-term prognosis, the optimization of long-term post-treatment quality of life is of paramount significance.
Transoral robotic surgery (TORS) has been increasingly used for OPSCC over the last decade. Prior studies have suggested that TORS offers long-term oncologic and functional outcomes equivalent or superior to those of other surgical and non-surgical options [6–8] with decreased length of hospitalization and requirement for tracheostomy or permanent gastrostomy tube [3]. Patients managed by TORS alone score higher on patient-reported quality of life (PR-QOL) indices in multiple domains compared to patients managed by TORS with adjuvant therapy [9–11]. However, few studies to date have directly compared oncologic outcomes and PR-QOL scores between TORS and definitive chemoradiation (CRT). Herein, we compare oncologic outcomes and PR-QOL scores between early stage OPSCC patients treated with definitive CRT and those treated with TORS ± adjuvant therapy at a single institution. We hypothesize that for select patients, definitive TORS offers non-inferior oncologic outcomes while potentially sparing short and long-term adverse effects on quality of life.
Materials and methods
Patient selection
A retrospective observational comparison cohort study was performed on 92 patients treated with definitive TORS with or without adjuvant therapy and 46 patients treated with definitive CRT between July 2005 and January 2016 at the University of Pittsburgh. Definitive TORS is defined in this paper as curative intent TORS with or without adjuvant therapy. All TORS patients had histology-confirmed OPSCC and T0-T2, N0-N2 disease. Definitive CRT patients were selected on the basis of those who had T0-T2, N0-N2 disease and who had also completed at least one post-treatment QOL survey.
Definitive TORS
Patients underwent definitive TORS with unilateral or bilateral neck dissection, depending on the extent of disease. Patients with N0 or N1 disease without extracapsular extension were managed with TORS alone. Patients were recommended adjuvant radiation therapy for adverse prognostic features such as ⩾N2 disease, angiolymphatic or perineural invasion, and close (<3 mm) margins. Adjuvant CRT was offered for extracapsular extension or positive margins. Our institution defines close margins as <3 mm, consistent with the definition used in ECOG 3311 and supported by a recent study which reported an average closest margin of 2.82 mm among patients treated with transoral laser microsurgery [12].
Radiation therapy
All patients who underwent radiation therapy were treated with intensity-modulated radiotherapy (IMRT). Patients underwent a computed tomography (CT)-based planning scan on either a combined positron emission tomography-computed tomography (PET-CT) or helical CT scanner (GE Medical Systems, Milwaukee, WI, USA) with intravenous contrast. A custom relocatable thermo-plastic mask was fabricated for each patient on the CT simulation table. All radiation treatments were delivered via 6 MV photons. Treatments for all patients were planned using either ADAC™ (Siemens Medical Solutions, Milpitas, CA, USA) or Eclipse™ (Varian Medical Systems, Palo Alto, CA, USA).
Follow-up
Patients were typically seen 2–4 weeks following radiation therapy or TORS depending on the severity of toxicities during treatment. Beyond 1 month, patients underwent follow-up by members of the multidisciplinary team at regular intervals. Selected low-risk TORS patients who had negative margins, N0 disease, no extracapsular extension, and no perineural or angiolymphatic invasion were managed without adjuvant therapy and did not receive follow-up imaging. For all others, treatment response was assessed using contrast-enhanced CT scans or PET-CT obtained 2–3 months after treatment completion. When local progression was suspected but questionable based on available imaging or clinical examination, biopsy was performed. University of Washington Quality of Life-Revised, Version 4 (UW-QOL V4) questionnaire scores were obtained at 1, 6, 12, and 24 months from the date of TORS or completion of definitive CRT. Surveys were pooled by time point into 4 categories (1 month, 6 months, 12 months, and 24 months after TORS or definitive CRT) for analysis.
Statistics
The chi-square test was used to assess for differences in baseline characteristics. Our primary oncologic endpoints were local and regional control. Local failure was defined as failure within the primary site, and regional failure was defined as failure within the cervical nodes. Secondary endpoints were distant control, disease-free survival, disease-specific survival, and overall survival. The log rank test was used to evaluate for significant differences in these endpoints between the definitive TORS and definitive CRT groups. For the survival analyses, patients were censured at death or last follow-up, whichever was latest. Our PR-QOL endpoints were the mean UW-QOL V4 scores at 1 month, 6 months, 12 months, and 24 months after treatment. The Mann-Whitney test was used to compare scores between groups. Patients who did not complete UW-QOL V4 forms at any given time point were excluded from this analysis. No adjustments were made for multiple testing. Because the most consistent finding was a difference in saliva-related QOL between CRT and TORS groups, we conducted a univariate linear regression analysis to investigate whether other factors such as age, gender, smoking history, HPV status, T classification, or N classification were potentially associated with this difference in QOL. We then performed a multivariate analysis using the factors identified as significant on univariate analysis. A p-value of <0.05 was considered statistically significant.
Results
Patient and treatment characteristics
A flow chart demonstrating our selection of definitive TORS patients for inclusion in this study is outlined in Fig. 1. See Table 1 for patient and tumor characteristics. Median age was 57 (range: 31–85) years, median Karnofsky Performance Status was 90 (range: 80–100), and 83% of patients were male, which was similar between groups. However, definitive CRT patients had significantly higher N classification and clinical staging (p < 0.001, p < 0.001) than definitive TORS patients. HPV+ disease was present in 79% (n = 73) of TORS patients and 91% (n = 19) of tested CRT patients, although HPV status was not tested in 54% of the CRT patients.
Fig. 1.
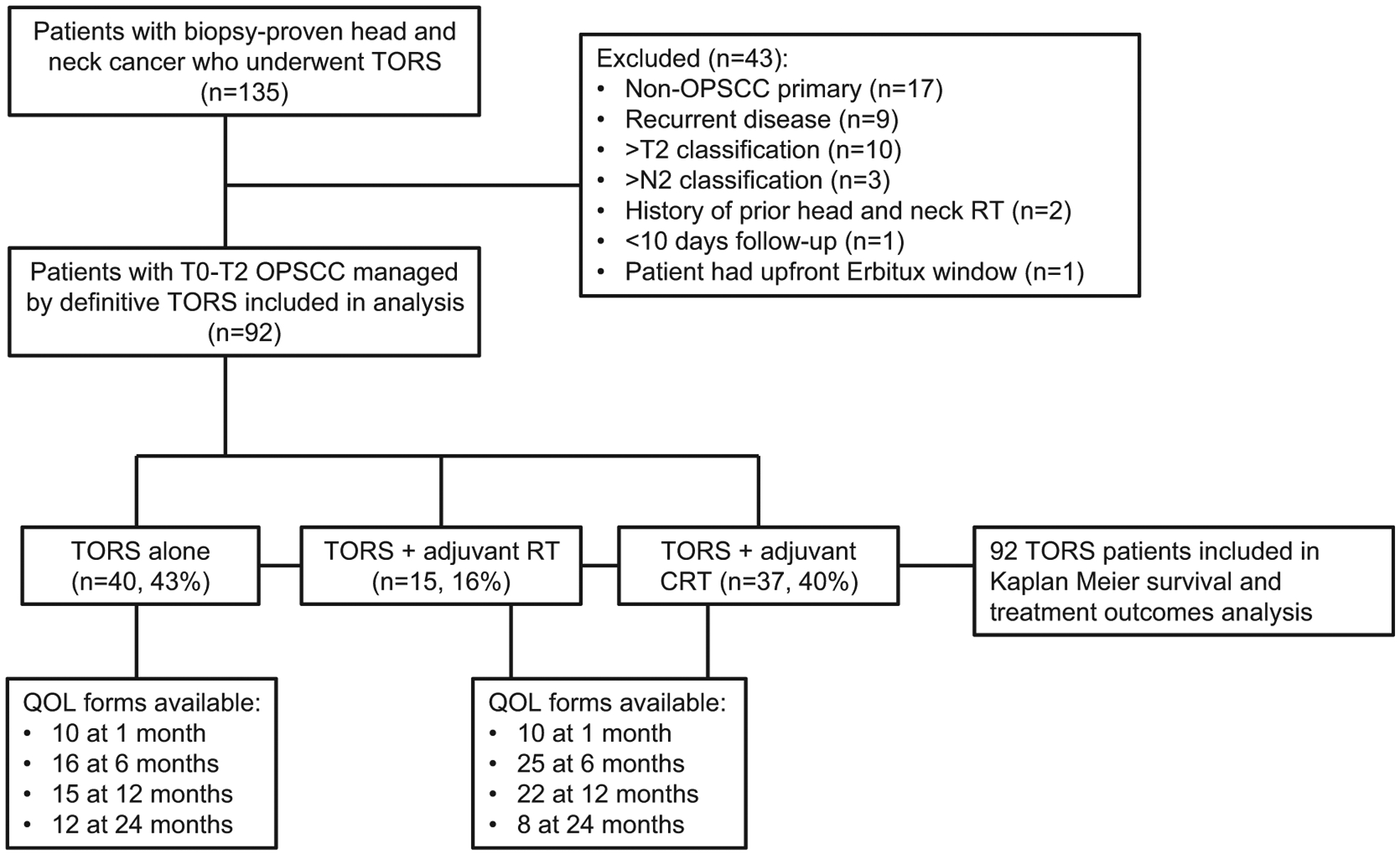
Flow diagram outlining our patient selection process. TORS = transoral robotic surgery; OPSCC = oropharyngeal squamous cell carcinoma; RT = radiation therapy; CRT = chemoradiation therapy; QOL = quality of life.
Table 1.
Patient and tumor characteristics.
| TORS alone | TORS + adjuvant RT or CRT | Definitive CRT | ||
|---|---|---|---|---|
| N (% or range) | N (% or range) | p value | ||
| Median age | 57 (41–73) | 56 (31–85) | 58 (36–82) | 0.762 |
| Male | 32 (80) | 45 (87) | 38 (83) | 0.697 |
| History of smoking | 27 (68) | 31 (60) | 33 (72) | 0.514 |
| Primary site | 0.443 | |||
| Tongue base | 16 (40) | 31 (60) | 20 (43) | |
| Tonsil | 20 (50) | 18 (35) | 20 (43) | |
| Pharyngeal wall | 2 (5) | 0 (0) | 2 (4) | |
| Soft palate | 1 (3) | 0 (0) | 1 (2) | |
| Unknown | 1 (3) | 3 (6) | 3 (7) | |
| HPV status | ||||
| Positive | 31 (78) | 42 (81) | 19 (41) | |
| Not evaluated | 1 (3) | 4 (8) | 23 (50) | |
| T stage | 0.083 | |||
| T0 | 1 (3) | 3 (6) | 1 (2) | |
| T1 | 21 (53) | 32 (62) | 17 (37) | |
| T2 | 18 (45) | 17 (33) | 28 (61) | |
| N stage | <0.001 | |||
| N0 | 18 (45) | 1 (2) | 1 (2) | |
| N1 | 15 (38) | 12 (23) | 10 (22) | |
| N2 | 7 (18) | 39 (75) | 35 (76) | |
| Clinical stage | <0.001 | |||
| I | 10 (25) | 1 (2) | 0 (0) | |
| II | 9 (23) | 0 (0) | 0 (0) | |
| III | 14 (35) | 12 (23) | 10 (22) | |
| IV | 7 (18) | 39 (75) | 36 (78) | |
| Margins | ||||
| Positive | 1 (3) | 13 (25) | N/A | |
| Close (<3 mm) | 5 (13) | 10 (19) | N/A | |
| Extracapsular extension | 4 (10) | 22 (42) | N/A | |
| Angiolymphatic invasion | 9 (23) | 28 (54) | N/A | |
| Perineural invasion | 4 (10) | 9 (17) | N/A |
TORS = transoral robotic surgery; RT = radiation therapy; CRT = chemoradiation; HPV = human papilloma virus.
Definitive CRT patients received IMRT to a median dose of 72 Gy (range: 66–75.6 Gy). Of 92 patients who underwent TORS, 29 (32%) were not recommended to have adjuvant therapy because of low-risk features. Of the 63 (70%) who were recommended adjuvant therapy, 11 patients refused for personal reasons. Of the 52 (57%) TORS patients who underwent adjuvant therapy, 37 (71%) received CRT and 15 (29%) underwent RT alone. Eighty-two out of 92 TORS patients (89%) underwent selective neck dissections.
Oncologic outcomes
At a median follow-up of 22.1 months (range: 0.33–83.4), the local failure rate for the entire cohort was 4.3% (6.5% for definitive CRT, 3.3% for definitive TORS, p = 0.48). Overall regional failure rate was 4.3% (2.2% for definitive CRT, 5.4% for definitive TORS, p = 0.32). Of the 40 patients managed by definitive TORS alone, 4 had local and/or regional failure; two of these patients had been recommended but refused adjuvant therapy, and all 4 subsequently underwent salvage chemoradiation. Of 3 definitive CRT patients who had local failure, salvage therapy consisted of none for one patient, transoral wide resection of a pharyngeal recurrence for one patient performed at 9 months, and left neck dissection for one patient with persistent disease at 2 weeks post-treatment. Overall distant failure rate was 4.3% (8.7% for definitive CRT, 2.2% for definitive TORS, p = 0.21). Two-year actuarial overall survival, disease-free survival, and disease-specific survival were 92.3%/90.3%/97.6% and 97.1%/91.6%/98.2% for definitive CRT patients and TORS patients, respectively (p = 0.22, p = 0.90, and p = 0.38).
Patient-reported quality of life outcomes
Among the CRT group, 30 QOL surveys were completed at 1 month, 21 at 6 months, 20 at 12 months, and 14 at 24 months. Among the TORS group, 20 QOL surveys were completed at 1 month, 41 at 6 months, 37 at 12 months, and 20 at 24 months. Within the TORS alone group, 10 surveys were completed at 1 month, 16 at 6 months, 15 at 12 months, and 12 at 24 months.
Table 2 contains a summary of the mean QOL outcomes for definitive CRT and definitive TORS groups. Patients managed by definitive TORS scored significantly better than patients treated with definitive CRT on QOL in the saliva domain at 1, 6, 12, and 24 months (p < 0.001, p = 0.025, p = 0.017, p = 0.011) (Fig. 2A). Definitive CRT patients had a mean saliva-related QOL ranging between 43 and 53 over the study period, compared to 65–82 for definitive TORS patients. TORS patients had higher scores in the chewing domain at 12 months (mean 95 vs. 83, p = 0.029). Definitive CRT patients had better shoulder domain scores at 1 month (mean 92 vs. 81, p = 0.027). There appeared to be a slight trend toward higher scores among TORS patients in the swallowing domain at 6 and 12 months (mean 79 vs. 79, p = 0.049; 84 vs. 83, p = 0.094). Of note, because the comparisons in QOL between groups were performed using the Mann-Whitney test as opposed to a direct comparison of the means, the differences in QOL values suggested by the above p-values may not be fully reflected in the mean scores recorded in Table 2 given the relatively small sample sizes, as appears to be the case for swallowing-related QOL.
Table 2.
Descriptive quality of life data.
| Months | Response rate | Pain | Appearance | Activity | Recreation | Swallowing | Chewing | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Definitive CRT | 1 | 61% | 64 | 24 | 81 | 16 | 59 | 15 | 67 | 19 | 72 | 24 | 71 | 34 | |
| 6 | 43% | 83 | 21 | 87 | 15 | 80 | 15 | 86 | 17 | 79 | 16 | 86 | 23 | ||
| 12 | 39% | 78 | 27 | 88 | 17 | 79 | 20 | 88 | 17 | 83 | 17 | 83 | 24 | ||
| 24 | 26% | 82 | 25 | 96 | 13 | 91 | 12 | 89 | 16 | 84 | 17 | 86 | 23 | ||
| Definitive TORS | 1 | 22% | 52 | 27 | 85 | 15 | 66 | 28 | 65 | 27 | 63 | 26 | 67 | 29 | |
| 6 | 45% | 81 | 22 | 84 | 14 | 77 | 20 | 82 | 19 | 79 | 23 | 82 | 27 | ||
| 12 | 40% | 89 | 23 | 86 | 15 | 87 | 18 | 92 | 17 | 84 | 18 | 95 | 16 | ||
| Months | Speech | Shoulder | Taste | Saliva | Mood | Anxiety | Overall | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Definitive CRT | 1 | 90 | 15 | 92 | 21 | 35 | 23 | 51 | 26 | 68 | 26 | 73 | 27 | 59 | 19 |
| 6 | 91 | 15 | 91 | 19 | 62 | 24 | 44 | 31 | 86 | 13 | 81 | 17 | 71 | 19 | |
| 12 | 95 | 12 | 88 | 23 | 74 | 17 | 53 | 23 | 78 | 23 | 82 | 25 | 73 | 18 | |
| 24 | 98 | 9 | 95 | 18 | 74 | 23 | 43 | 24 | 88 | 21 | 85 | 22 | 80 | 19 | |
| Definitive TORS | 1 | 85 | 17 | 81 | 26 | 61 | 34 | 82 | 23 | 70 | 32 | 88 | 18 | 65 | 21 |
| 6 | 89 | 15 | 84 | 22 | 60 | 29 | 66 | 34 | 81 | 23 | 81 | 15 | 71 | 21 | |
| 12 | 89 | 19 | 91 | 21 | 73 | 31 | 65 | 28 | 85 | 17 | 84 | 15 | 76 | 19 | |
| 24 | 91 | 14 | 85 | 30 | 75 | 23 | 66 | 27 | 80 | 31 | 84 | 22 | 78 | 24 | |
CRT = chemoradiation; TORS = transoral robotic surgery; SD = standard deviation.
Fig. 2.
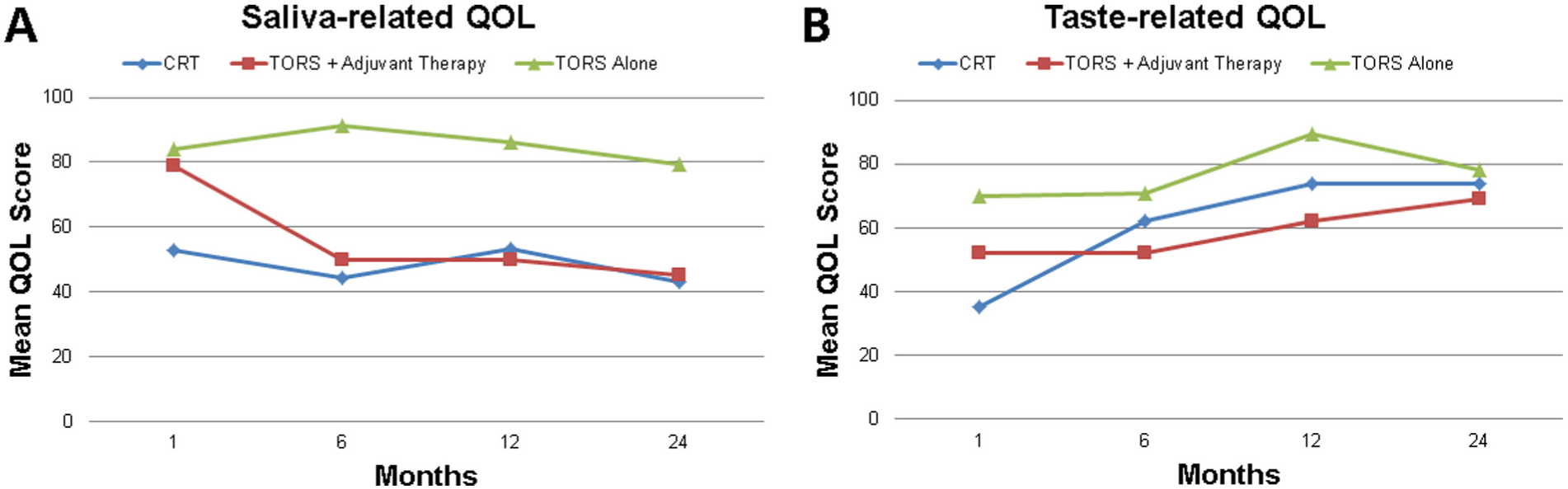
Quality of Life Outcomes. (A) Definitive TORS as a whole resulted in significantly better saliva-related QOL than definitive CRT at 1, 6, 12, and 24 months (p < 0.001, p = 0.025, p = 0.017, p = 0.011). However, among TORS patients, adjuvant therapy was associated with worse QOL in the saliva domain at 6, 12, and 24 months (p < 0.001, p < 0.001, p = 0.007). (B) Among TORS patients, adjuvant therapy was associated with worse QOL in the taste domain at 6 and 12 months (p = 0.067, p = 0.008).
Among TORS patients, adjuvant therapy was associated with worse QOL in the saliva domain at 6, 12, and 24 months (mean 50 vs. 91, p < 0.001; mean 50 vs. 86, p < 0.001; mean 45 vs. 79, p = 0.007) and taste domain at 6 and 12 months (mean 52 vs. 71, p = 0.067; mean 62 vs. 89, p = 0.008) (Fig. 2A and B). Adjuvant therapy was also associated with worse scores in appearance and recreation at 6 months (p = 0.042, p = 0.016), speech at 12 and 24 months (p = 0.066, p = 0.008), and chewing and swallowing at 24 months (p = 0.008, p = 0.041). Compared to TORS with adjuvant therapy, definitive CRT resulted in worse saliva-related QOL at 1 month (p = 0.006) and worse chewing-related QOL at 12 months (p = 0.034).
On univariate analysis, treatment strategy (TORS vs. CRT) (p = 0.019) and N classification (p = 0.012) were significant predictors of saliva-related QOL at 6 months. The time point of 6 months was chosen for this analysis because the highest number of surveys were available from this time. On multivariate analyses, both factors remained significant (p = 0.043, p = 0.027, respectively).
Discussion
We found that definitive CRT and definitive TORS offer similar rates of locoregional control, overall survival, and disease-free survival in patients with early stage OPSCC. TORS resulted in significantly higher short and long-term saliva-related QOL, whereas adjuvant therapy was associated with worse saliva and taste-related QOL compared to TORS alone (Fig. 2).
In an era of increasing incidence of HPV-related disease among younger patients with improved prognosis and long-term survival, the importance of maximizing QOL has been increasingly recognized. However, head and neck cancer patients continue to experience significant treatment-related toxicities with current standard-of-care chemoradiation regimens [13–15]. While definitive TORS is a rising alternative treatment algorithm for early stage OPSCC, no randomized controlled trials have compared outcomes to definitive CRT. To our knowledge, the current study is the largest retrospective study to date comparing oncologic and functional outcomes between TORS and CRT patients.
In a prospective non-randomized study comparing functional swallowing outcomes between 20 patients with advanced stage oropharyngeal and supraglottic cancer managed by TORS with adjuvant therapy and 20 similar patients treated with definitive CRT, More et al. [16] demonstrated that TORS resulted in significantly improved swallowing outcomes at 6 (p = 0.004) and 12 months (p = 0.006), as measured by the M.D. Anderson Dysphagia Inventory. These results are consistent with our findings of decreased saliva-related QOL among CRT patients up to 24 months from treatment, considering that lower saliva production in OPSCC patients treated with CRT has been associated with increased patient perception of swallowing problems [17]. This may in part be due to the important role saliva plays in bolus formation and the oral phase of swallowing, in addition to providing protection of oro-esophageal mucosa [18]. Our study identified a trend toward improved swallowing-related QOL in TORS patients, although it may have been underpowered to detect a true difference.
We found that patients managed by TORS with adjuvant therapy experienced significantly worse saliva outcomes at 6, 12, and 24 months and a trend toward worse taste and speech outcomes compared to those managed by TORS alone. These results are consistent with previously published reports suggesting that adjuvant therapy is associated with worsened functional outcomes in multiple domains among TORS patients [9–11]. Dziegielewski et al. recently published a prospective study of 81 patients with OPSCC who underwent definitive TORS with neck dissection and completed the Head and Neck Cancer Inventory (HNCI) at multiple time points following treatment. With a mean follow-up time of 22.7 months, the investigators found that adjuvant radiation therapy or CRT was negatively correlated with speech, eating, and overall QOL (p < 0.05), with no differences by type of adjuvant treatment [11]. Similarly, Hurtuk et al. reported that among 56 patients who underwent TORS for head and neck squamous cell carcinoma, those who underwent adjuvant therapy had significantly lower eating scores, social function, overall function, and overall QOL scores on HNCI surveys completed at 3 months post-TORS [9]. In a study on 38 OPSCC patients treated with TORS, Leonhardt et al. found that patients treated with TORS alone had significantly higher Performance Status Scale eating and diet scores at 6 months compared with those treated with TORS and adjuvant CRT [10]. Our study provides further evidence that adjuvant therapy is associated with worsened QOL in multiple domains, thus highlighting the necessity of careful recommendations for adjuvant therapy.
We found that TORS resulted in worse QOL outcomes in the shoulder domain at 1 month compared to definitive CRT. Shoulder and neck morbidity are known complications of neck dissection [19] and have been implicated as an important factor in the QOL of post-operative head and neck cancer patients [20]. In addition, many of the TORS patients ultimately underwent trimodality therapy with combined adjuvant CRT, and treatment intensification with multiple modalities has been associated with increased acute toxicity [21]. Thus, our findings demonstrate that the selection of patients to undergo definitive TORS as opposed to definitive CRT is in itself an important and challenging decision, as many patients will still ultimately require adjuvant therapy, potentially further detracting from QOL especially in cases requiring trimodality therapy.
While our study strongly suggests that patients who undergo definitive TORS experience superior saliva-related QOL with preserved oncologic outcomes compared to those treated with definitive CRT, patient selection remains an area of controversy. We found that adjuvant therapy, which is required by a high percentage of patients, is associated with worse outcomes in multiple QOL domains, whereas for a subset of patients TORS alone appears to be an effective treatment that may spare the morbidity of adjuvant treatment. However, it is oftentimes unknown at the time of initial surgery whether a patient will demonstrate significant nodal disease or pathologic risk factors that warrant adjuvant treatment, thereby posing a diagnostic and therapeutic challenge.
As with most retrospective studies, there exist some limitations. First, the definitive CRT cohort had significantly higher disease staging, with 98% (n = 45) of CRT patients having stage III/IV disease compared to 78% (n = 72) stage III/IV and 22% (n = 20) stage I/II among TORS patients. This may theoretically have biased QOL outcomes to favor TORS patients due to higher disease burden experienced by CRT patients at baseline, although both N classification and use of TORS were identified as independent predictors of saliva-related QOL on multivariate analysis. Other limitations include the small sample size compounded by the limited number of completed QOL surveys available, as not all patients completed surveys. Our study is subject to selection bias as we only included patients with newly diagnosed OPSCC; patients with recurrent disease remain a challenging situation outside the scope of the current study.
In the setting of increasing HPV-related disease, there is growing interest in treatment de-intensification given the potential gains in QOL [22]. While our study adds to the body of retrospective evidence supporting good oncologic and functional outcomes for TORS, prospective data is needed to further establish the feasibility and outcomes of treatment de-intensification methods. The role of both TORS and dose de-escalation of radiation therapy is currently being explored in a phase II randomized trial of TORS for p16+ patients followed by observation or radiation therapy, with intermediate-risk patients randomized to either a high-dose or low-dose radiation arm (ECOG 3311). Likewise, another prospective randomized trial is evaluating the potential of de-escalating definitive CRT among p16+ patients with advanced OPSCC, with patients being randomized to CRT vs. radiation therapy alone (NRG-HN002).
Conclusion
Definitive CRT and definitive TORS offer similar rates of locoregional control, distant control, and disease-free survival in patients with early stage OPSCC. Definitive TORS resulted in significantly improved long-term saliva-related PR-QOL compared to CRT. Adjuvant therapy is associated with worsened saliva and taste outcomes among patients treated with definitive TORS.
Funding
This work was funded in part by the Department of Veterans Affairs Career Development Award and the PNC Foundation. This manuscript does not represent the views of the US Government or the Department of Veterans Affairs.
Footnotes
Conflict of interest
None declared.
References
- [1].Lohia S, Rajapurkar M, Nguyen SA, Sharma AK, Gillespie MB, Day TA. A comparison of outcomes using intensity-modulated radiation therapy and 3-dimensional conformal radiation therapy in treatment of oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg 2014;140(4):331–7. [DOI] [PubMed] [Google Scholar]
- [2].de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 2006;64(2):363–73. [DOI] [PubMed] [Google Scholar]
- [3].Moore EJ, Hinni ML. Critical review: transoral laser microsurgery and robotic-assisted surgery for oropharynx cancer including human papillomavirus-related cancer. Int J Radiat Oncol Biol Phys 2013;85(5):1163–7. [DOI] [PubMed] [Google Scholar]
- [4].Al-Mamgani A, van Rooij P, Verduijn GM, Mehilal R, Kerrebijn JD, Levendag PC. The impact of treatment modality and radiation technique on outcomes and toxicity of patients with locally advanced oropharyngeal cancer. Laryngoscope 2013;123(2):386–93. [DOI] [PubMed] [Google Scholar]
- [5].Logan R. Review: advances in understanding of toxicities of treatment for head and neck cancer. Oral Oncol 2009;45:844–8. [DOI] [PubMed] [Google Scholar]
- [6].White HN, Moore EJ, Rosenthal EL, et al. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: one- and 2-year survival analysis. Arch Otolaryngol Head Neck Surg 2010;136(12):1248–52. [DOI] [PubMed] [Google Scholar]
- [7].Weinstein GS, Quon H, Newman HJ, et al. Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch Otolaryngol Head Neck Surg 2012;138(7):628–34. [DOI] [PubMed] [Google Scholar]
- [8].Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc 2012;87(3):219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hurtuk AM, Marcinow A, Agrawal A, Old M, Teknos TN, Ozer E. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg 2012;146 (1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Leonhardt FD, Quon H, Abrahão M, O’Malley BW Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck 2012;34(2):146–54. [DOI] [PubMed] [Google Scholar]
- [11].Dziegielewski PT, Teknos TN, Durmus K, et al. Transoral robotic surgery for oropharyngeal cancer: long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg 2013;139(11):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hinni ML, Zarka MA, Hoxworth JM. Margin mapping in transoral surgery for head and neck cancer. Laryngoscope 2013;123(5):1190–8. [DOI] [PubMed] [Google Scholar]
- [13].Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 2007;68(4):1110–20. [DOI] [PubMed] [Google Scholar]
- [14].Meyer F, Fortin A, Wang CS, Liu G, Bairati I. Predictors of severe acute and late toxicities in patients with localized head-and-neck cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys 2012;82(4):1454–62. [DOI] [PubMed] [Google Scholar]
- [15].Ling DC, Kabolizadeh P, Heron DE, Ohr JP, Wang H, Johnson J, et al. Incidence of hospitalization in patients with head and neck cancer treated with intensity-modulated radiation therapy. Head Neck 2014. 10.1002/hed.23821 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [16].More YI, Tsue TT, Girod DA, Harbison J, Sykes KJ, Williams C, et al. Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. JAMA Otolaryngol Head Neck Surg 2013;139(1):43–8. [DOI] [PubMed] [Google Scholar]
- [17].Logemann JA, Smith CH, Pauloski BR, Rademaker AW, Lazarus CL, Colangelo LA, et al. Effects of xerostomia on perception and performance of swallow function. Head Neck 2001;23(4):317–21. [DOI] [PubMed] [Google Scholar]
- [18].Pedersen AM, Bardow A, Jensen SB, Nauntofte B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis 2002;8 (3):117–29. [DOI] [PubMed] [Google Scholar]
- [19].Dijkstra PU, van Wilgen PC, Buijs RP, et al. Incidence of shoulder pain after neck dissection: a clinical explorative study for risk factors. Head Neck 2001. November;23(11):947–53. [DOI] [PubMed] [Google Scholar]
- [20].van Wilgen CP, Dijkstra PU, van der Laan BF, et al. Shoulder and neck morbidity in quality of life after surgery for head and neck cancer. Head Neck 2004;26 (10):839–44. [DOI] [PubMed] [Google Scholar]
- [21].Palazzi M, Tomatis S, Orlandi E, et al. Effects of treatment intensification on acute local toxicity during radiotherapy for head and neck cancer: prospective observational study validating CTCAE, version 3.0, scoring system. Int J Radiat Oncol Biol Phys 2008;70(2):330–7. [DOI] [PubMed] [Google Scholar]
- [22].Quon H, Richmon JD. Treatment deintensification strategies for HPV-associated head and neck carcinomas. Otolaryngol Clin North Am 2012;45 (4):845–61. [DOI] [PubMed] [Google Scholar]


