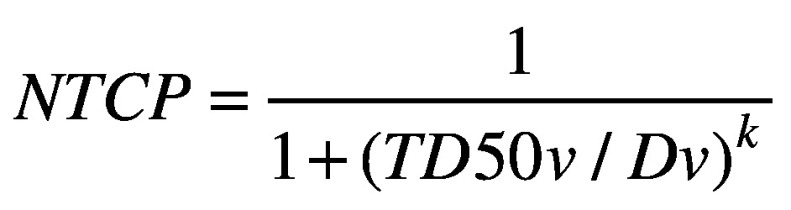Abstract
We sought to evaluate the association between larynx dose and risk of severe late laryngeal toxicity in patients undergoing re-irradiation SBRT for recurrent HNC. Fifty-five patients with an intact larynx underwent re-irradiation SBRT to a median dose of 44 Gy in 5 fractions. Five (41.7%) patients treated for a laryngeal/hypopharyngeal recurrence experienced late grade ≥3 laryngeal toxicity, compared to 0.0-7.1% for other sites. Logistic dose-response models were created to predict risk of severe late laryngeal toxicity, including dysphagia and airway compromise. According to the model, the risk of severe laryngeal toxicity with a larynx D5cc of 5 Gy is 5.8% (95% CI 2.9-9.9%) and rises to 11.4% with a D5cc of 20 Gy and 25.3% with a D5cc of 40 Gy. In patients with a laryngeal/hypopharyngeal recurrence, SBRT planning should carefully assess the dose to laryngeal structures given these dose findings, and SBRT should be approached with significant caution in such patients.
Keywords: SBRT, re-irradiation, head and neck cancer, dose-response, late toxicity
Background
Laryngeal cancer is one of the few major cancers for which survival has decreased nationally (1, 2). The “Olsen hypothesis” states that this trend is a result of a shift in standard of care from laryngectomy to an organ preservation approach for locally-advanced laryngeal cancer, with the inability to salvage some failures (3). This shift occurred in the 1990’s following publication of the VA Laryngeal Cancer Study Group and RTOG 91-11 trials showing comparable oncologic and survival outcomes between surgical and non-surgical approaches (4, 5), with nationwide population-based studies showing a corresponding decrease in survival for larynx cancer patients (1, 2). In RTOG 91-11, long-term follow-up revealed an increased rate of non-cancer related deaths in the concurrent chemoradiation arm, a finding which has been hypothesized to be due to uncaptured late treatment-related toxicities stemming from treatment intensification with the concurrent regimen (4).
Theoretically, any increased risk of late toxicity when treating intact larynx would be magnified in the re-irradiation setting. Currently, salvage laryngectomy is recognized as the standard of care for recurrent head and neck cancer (rHNC) involving the larynx. For patients who are not surgical candidates, interest in salvage re-irradiation with stereotactic body radiation therapy (SBRT) as an alternative to conventional re-irradiation with potentially fewer toxicities has increased in recent years, although severe toxicities have been reported in some patients. We have previously demonstrated a late grade ≥3 toxicity rate of less than 20% overall after re-irradiation SBRT for rHNC, though this rate increased to 50% in patients treated for laryngeal or hypopharyngeal recurrences (6). Laryngeal toxicity, which can manifest as dysphagia, aspiration, laryngeal edema, or laryngeal stenosis, is one of the most commonly reported toxicities after SBRT for rHNC. At this time, no validated dose-volume constraints for the larynx exist to guide SBRT treatment planning. We therefore sought to model the relationship between larynx dose and risk of severe late laryngeal toxicity in patients undergoing re-irradiation SBRT for rHNC.
Methods and Materials
We retrospectively reviewed 186 patients with recurrent, previously-irradiated head and neck cancer of any histology treated between January 2008 and March 2013. Patients treated early in our experience with incomplete dosimetry data or treated with <5 fractions to doses <40 Gy were excluded. Out of 186 patients reviewed, 75 had complete dosimetric data available. Of these 75, 12 were excluded due to prior laryngectomy and 8 were excluded due to death occurring <3 months after SBRT, as the focus of this report is on late laryngeal toxicity in patients with an intact organ, thus leaving a total of 55 patients that were included in the dose modeling analysis. All patients were treated with conventional linear accelerator-based SBRT to a dose of 40-50 Gy in 5 fractions, modulated based on tumor size, delivered every other day. The majority of patients (81.8%, n=45) received concurrent cetuximab (administered at a loading dose of 400 mg/m2 on day −7, followed by 250 mg/m2 on days 0 and +8) with SBRT. Our treatment planning and delivery process has been previously described (7). At our institution, no specific dosimetric constraints for the larynx were used in treatment planning.
The larynx was retrospectively contoured for each patient. For patients treated to a laryngeal site of recurrence, the entire organ was contoured irrespective of the target area. Larynx dosimetric parameters were collected from review of dose-volume histograms. The t-test was used to compare larynx dosimetric parameters between patients who did and did not develop late grade ≥3 laryngeal toxicity. The Chi-square test was used to compare rates of late grade ≥3 laryngeal toxicity among different SBRT treatment sites.
Next, using the DVH Evaluator software (DiversiLabs, LLC, Huntingdon Valley, Pa), logistic dose-response models were created to predict the risk of severe late (occurring >3 months after SBRT) laryngeal toxicity, defined as grade ≥3 dysphagia, laryngeal edema, or laryngeal stenosis based on Common Terminology Criteria for Adverse Events v4.0. The following logistic model was utilized:
where TD50v is the 50% risk level for dose-descriptor Dv, and the slope at Dv = TD50v is k/(4*TD50v). The Dv parameters used included dose corresponding to 0.1 cc of larynx (D0.1cc), 1 cc (D1cc), 2 cc (D2cc), and 5 cc (D5cc) from re-irradiation SBRT. For patients who received more than one course of re-irradiation SBRT, cumulative larynx doses from fused summary plans of all SBRT treatments were recorded. Due to inconsistent availability of prior records over variable re-irradiation intervals, dose from prior external beam radiation was not included.
Results
Median follow-up was 8.8 months (interquartile range, IQR: 5.7-16.4) for all 55 patients included for analysis. Among the 8 patients that were excluded due to death <3 months after SBRT, 3 patients died of progressive head and neck cancer, 1 died of myocardial infarction, 1 died of metastatic esophageal cancer, 1 did not finish SBRT because he was admitted for pneumonia and subsequently died of septic shock and renal failure, and 2 patients were lost to follow-up and died of an unknown cause. Patient and treatment characteristics are summarized in Table 1. The median re-irradiation interval was 21.5 months (IQR: 9.0-62.3 months), and the median prior external beam radiation dose was 68.6 Gy (IQR: 64.3-70.0 Gy). Most patients (94.5%) had squamous cell carcinoma histology, and the most common sites of recurrence treated with SBRT were oropharynx (25.5%), neck (25.5%), larynx (21.8%), and oral cavity (18.2%). Five patients (9.1%) received more than one course of SBRT. Median SBRT prescription dose was 44.0 Gy (IQR: 44.0-44.0) in 5 fractions.
Table 1.
Baseline patient and treatment characteristics
| Characteristic | Number (% or IQR) |
| Median age (years) | 64 (59-72) |
| Male | 29 (52.7%) |
| Prior surgery | 34 (61.8%) |
| Histology | |
| Squamous cell carcinoma | 52 (94.5%) |
| Thyroid carcinoma | 2 (3.6%) |
| Adenocarcinoma | 1 (1.8%) |
| Concurrent cetuximab | 45 (81.8%) |
| Median prior EBRT dose (Gy) | 68.6 (64.3-70.0) |
| Median re-irradiation interval (months) | 21.5 (9.0-62.3) |
| Number of SBRT courses | |
| 1 | 50 (90.9%) |
| 2 | 3 (5.4%) |
| 3 | 2 (3.6%) |
| Site of recurrence | |
| Oropharynx | 14 (25.5%) |
| Neck | 14 (25.5%) |
| Larynx | 12 (21.8%) |
| Oral cavity | 10 (18.2%) |
| Base of skull | 3 (5.5%) |
| Nasopharynx | 2 (3.6%) |
| Median SBRT dose (Gy) | 44.0 (44.0-44.0) |
| Median SBRT PTV (cc) | 35.0 (16.8-59.7) |
IQR = interquartile range. EBRT = external beam radiation therapy. SBRT = stereotactic radiation therapy. PTV = planning target volume.
Of the 55 included patients, 7 (12.7%) experienced late grade ≥3 laryngeal toxicity, including 4 patients with grade 3 dysphagia, 1 patient with both grade 3 laryngeal edema and dysphagia, 1 patient with grade 4 laryngeal stenosis, and 1 patient with grade 5 dysphagia. Five of the 7 patients with late grade ≥3 laryngeal toxicity had been treated with SBRT to a laryngeal or hypopharyngeal site of recurrence, 1 was treated for an oropharyngeal recurrence, and 1 was treated for a nodal recurrence. Overall, 41.7% (n=5) of patients treated for a laryngeal/hypopharyngeal recurrence experienced late grade ≥3 laryngeal toxicity, compared to 7.1% (n=1) for oropharynx, 7.1% (n=1) for neck, and 0.0% (n=0) for oral cavity, nasopharynx, and base of skull (p=0.034). The median D0.1cc, D1cc, D2cc, D5cc, and mean larynx doses were 17.6 Gy (IQR 0.6-46.9 Gy), 13.0 Gy (IQR 0.5-43.0 Gy), 11.1 Gy (IQR 0.5-36.5 Gy), 4.8 Gy (IQR 0.4-28.3 Gy), and 4.1 Gy (0.3-15.4 Gy), respectively. Table 2 shows a comparison of these larynx dosimetric parameters for patients with and without grade ≥3 late laryngeal toxicity. All larynx dosimetric parameters were significantly higher for patients who developed severe late laryngeal toxicity.
Table 2.
Larynx parameters for patients with and without severe late larynx toxicity
| All Patients (n=55) | No Grade ≥3 Larynx Toxicity (n=48) | Grade ≥3 Larynx Toxicity (n=7) | p-value | |
| Median D0.1cc | 17.6 (IQR 0.6-46.9) | 12.9 (IQR 0.4-44.0) | 49.2 (IQR 46.8-49.9) | 0.028 |
| Median D1cc | 13.0 (IQR 0.5-43.0) | 6.7 (IQR 0.3-34.1) | 47.9 (IQR 46.3-48.9) | 0.011 |
| Median D2cc | 11.1 (IQR 0.5-36.5) | 3.8 (IQR 0.3-26.8) | 47.7 (IQR 45.8-48.4) | 0.004 |
| Median D5cc | 4.8 (IQR 0.4-28.3) | 1.5 (IQR 0.3-13.9) | 46.0 (IQR 32.5-47.4) | 0.001 |
| Mean Larynx Dose | 4.1 (IQR 0.3-15.4) | 1.9 (IQR 0.3-10.0) | 21.6 (IQR 20.1-35.6) | 0.029 |
IQR = interquartile range. All doses given in units of Gy. p-values based on t-test comparison between patients with and without severe larynx toxicity.
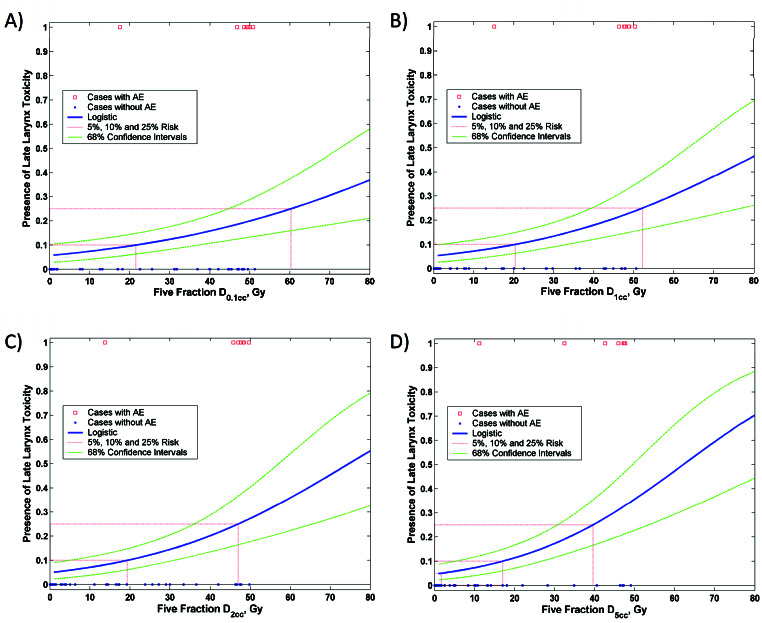
The risk model based on D5cc appeared to result in the strongest dose-response curve (Figure 1). According to the model, the risk of severe laryngeal toxicity with a D5cc of 5 Gy is 5.8% (95% CI 2.9-9.9%). The risk is reduced only minimally to 4.8% with a D5cc of 1 Gy, but rises to 11.4% with a D5cc of 20 Gy, 25.3% with a D5cc of 40 Gy, and 30.2% with a D5cc of 45 Gy. Table 3 shows the risk of grade ≥3 late laryngeal toxicity according to D0.1cc, D1cc, D2cc, and D5cc based on the logistic model.
Figure 1.
Logistic models of larynx dose-tolerance: A) D0.1cc, B) D1cc, C) D2cc, D) D5cc. AE = adverse event (defined as grade ≥3 late laryngeal toxicity).
Table 3.
Estimated risk of grade ≥3 late laryngeal toxicity as a function of cumulative SBRT dose and volume in 5 fractions
| Dose (Gy) | Volume (cc) | Risk (%) |
| 5 | 0.1 | 6.70 |
| 5 | 1 | 6.10 |
| 5 | 5 | 5.80 |
| 20 | 0.1 | 9.60 |
| 20 | 1 | 9.90 |
| 20 | 5 | 11.40 |
| 45 | 0.1 | 17.80 |
| 45 | 1 | 20.60 |
| 45 | 5 | 30.20 |
Discussion
Salvage SBRT for rHNC appears to offer comparable local control and overall survival with shorter treatment times, and potentially decreased toxicity compared with conventional techniques (8, 9). However, concerns over severe toxicities persist. Acute and late severe toxicities after re-irradiation SBRT have been reported in up to 11.3% and 18.9% of patients, respectively (6). Patients with an intact larynx who undergo re-irradiation with SBRT remain at long-term risk of severe toxicities related to organ dysfunction. Laryngeal toxicity can manifest as dysphagia, laryngeal edema, or laryngeal stenosis, all of which were combined into our composite endpoint of severe grade ≥3 laryngeal toxicity. Herein, we have used logistic modeling to quantify the relationship between larynx dose and risk of grade ≥3 late laryngeal toxicity following re-irradiation SBRT for recurrent head and neck cancer in patients with an intact larynx. We identified D5cc as the most efficient predictor of risk of severe late laryngeal toxicity, with a predicted risk of 5.8% when D5cc is 5 Gy (95% CI 2.9-9.9%). However, the model estimates a rise in risk to >30% with a D5cc of 45 Gy, a dose which is often unavoidable when treating laryngeal/hypopharyngeal recurrences.
We have previously reported that in a cohort of 227 patients with long-term follow-up following re-irradiation SBRT for rHNC, the grade ≥3 late toxicity rate was <20% overall but varied according to treatment site, with a risk of 50% among patients treated for a laryngeal/hypopharyngeal recurrence (6). In comparison, the risk was 20%, 19%, 7%, and 7% for lymph node, oropharynx, oral tongue, and base of skull/nasal cavity/paranasal sinus sites of recurrence, respectively. The most common late toxicities observed were dysphagia, osteonecrosis, laryngeal edema, trachea-esophageal fistula, and trismus. Consistent with these numbers, in our current study’s cohort of 55 patients, the rate of severe late laryngeal toxicity was 12.7% overall but rose dramatically to 41.7% for laryngeal/hypopharyngeal patients, compared to 0.0% to 7.1% for all other sites of recurrence. Five out 7 patients experiencing severe late laryngeal toxicity in our study cohort had been treated with re-irradiation SBRT to a laryngeal/hypopharyngeal site of recurrence. Reflecting this finding, the median larynx D5cc for patients with severe laryngeal toxicity was much higher at 46.0 Gy, compared to 1.5 Gy for all others, since it is impossible to spare the larynx when the target is contained within. While evidence of a dose-volume response relationship with local control has been demonstrated with the steep portion of the curve across 35-45 Gy (10-12), our results suggest a high risk of complications with doses in this range to the intact larynx.
Clearly, our results highlight the importance of surgical salvage laryngectomy whenever feasible. Our findings draw a parallel with those of RTOG 91-11, where long-term follow-up revealed increased non-cancer related deaths in the concurrent chemoradiation arm, highlighting the potential morbidity of aggressive treatment to the intact larynx even in the primary chemoradiation setting (4). While data on site-specific late toxicity for conventional re-irradiation is limited, in RTOG 99-11 there were 22 patients with larynx or hypopharynx sites out of 99 total, and late G3-4 laryngeal/esophageal toxicities were seen in 15 patients (13). While no details are provided on the risk of toxicity by subsite, if it is assumed that most of these toxicities occurred in patients treated to the larynx or hypopharynx, then this data might suggest that toxicity associated with re-irradiating an intact larynx may be high with conventional re-irradiation as well. It remains unclear if toxicity rates are similar between SBRT and conventional fractionation when treating the larynx, as our analysis highlights that the majority of severe toxicities occurred in patients being treated for laryngeal/hypopharyngeal recurrences, where the larynx cannot be spared. Others have noted that hypofractionation even in the primary, non-re-irradiation setting for early-stage glottic larynx cancer results in severe toxicities (14, 15). In a phase I study of SBRT for early-stage glottic larynx cancer by Sher et al, two patients experienced dose-limiting toxicities consisting of grade 4 laryngeal edema and grade 3 dysphagia in one patient treated to 45 Gy in 10 fractions, and grade 3 laryngeal necrosis and dysphagia in one patient treated to 42.5 Gy in 5 fractions (14). Meanwhile, a phase I study by Kang et al was terminated early due to unexpected dose-limiting toxicities with a regimen of 55 Gy and 40.7 Gy in 11 fractions to the gross tumor volume and remaining larynx, respectively (15). Late toxicities in this study included grade 3 laryngeal inflammation in 2 (33.3%) patients, vocal cord ulcer in 1 patient, and arytenoid cartilage necrosis requiring supraglottic laryngectomy in 1 patient.
Our dose-response models are inherently limited by the complexity of patient and treatment factors which can potentially affect the risk of toxicity but may not be captured in the analysis, such as nuances of the dose distribution within the larynx and to surrounding organs, treatment delivery schedule, and patient comorbidities. The majority of laryngeal events in this study consisted of grade ≥3 dysphagia, which is often multifactorial in a heavily treated head and neck cancer population. Due to the small sample size, the model may have a large margin of error. However, our rate of toxicity overall and for patients with larynx/hypopharynx recurrence sites are consistent with that of published rates (6) and appear to confirm the high risk of severe toxicity with re-irradiation SBRT to the larynx, while affirming the feasibility of SBRT to non-laryngeal/hypopharyngeal sites. Given the limited data on dose tolerance of the larynx with re-irradiation SBRT, the risk estimates offered by our models are an important contribution to efforts to delineate clearer dose constraints in the setting of re-irradiation SBRT for rHNC and may be used to guide application of SBRT for future clinical trial design.
Conclusion
Based on our logistic dose-response models, the risk of severe late laryngeal toxicity following SBRT for recurrent, previously-irradiated head and neck cancer is less than 6% with a cumulative D5cc of 5 Gy from SBRT. This larynx dose is feasible in patients being treated for non-laryngeal/hypopharyngeal site of recurrence, and represents a dose constraint that may be incorporated into future clinical trials. Significant caution should be exerted when considering patients with a laryngeal/hypopharyngeal recurrence for re-irradiation SBRT, as the therapeutic-ratio does not appear to favor SBRT in this patient population, highlighting the importance of surgical salvage for these patients if at all feasible.
Acknowledgments
Source of Financial Support/Funding: No funding was provided for this project.
Footnotes
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: John A. Vargo, Diane C. Ling, Dwight E. Heron
Data collection: Diane C. Ling, Brian J. Gebhardt
Data analysis and interpretation: Diane C. Ling, Rachel J. Grimm
Manuscript writing: Diane C. Ling, John A. Vargo
Final approval of manuscript: Diane C. Ling, John A. Vargo, Rachel J. Grimm, Brian J. Gebhardt, David A. Clump, Robert L. Ferris, James P. Ohr, Dwight E. Heron
REFERENCES
- 1. Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A, Robinson RA. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006. September;116(9 Pt 2 Suppl 111):1-13. [DOI] [PubMed] [Google Scholar]
- 2. Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1270–1276. [DOI] [PubMed] [Google Scholar]
- 3. Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. 2010. January;32(1):1-7. [DOI] [PubMed] [Google Scholar]
- 4. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, Thorstad W, Wagner H, Ensley JF, Cooper JS. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013. March 1;31(7):845-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991. June 13;324(24):1685-90. [DOI] [PubMed] [Google Scholar]
- 6. Ling DC, Vargo JA, Ferris RL, Ohr J, Clump DA, Yau WW, Duvvuri U, Kim S, Johnson JT, Bauman JE, Branstetter BF, Heron DE. Risk of severe toxicity according to site of recurrence in patients treated with stereotactic body radiation therapy for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2016;95:973-980. [DOI] [PubMed] [Google Scholar]
- 7. Gebhardt BJ, Vargo JA, Ling DC, Jones B, Mohney M, Clump DA, Ohr JP, Ferris RL, Heron DE. Carotid Dosimetry and the Risk of Carotid Blowout Syndrome After Reirradiation With Head and Neck Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2018. May 1;101(1):195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vargo JA, Heron DE, Ferris RL, Rwigema JC, Kalash R, Wegner RE, Ohr J, Burton S. Examining tumor control and toxicity after stereotactic body radiotherapy in locally recurrent previously irradiated head and neck cancers: implications of treatment duration and tumor volume. Head Neck 2014;36:1349-55. [DOI] [PubMed] [Google Scholar]
- 9. Lartigau EF, Tresch E, Thariat J, Graff P, Coche-Dequeant B, Benezery K, Schiappacasse L, Degardin M, Bondiau PY, Peiffert D, Lefebvre JL, Lacornerie T, Kramar A. Multi institutional phase II study of concomitant stereotactic re-irradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol 2013;109:281-5. [DOI] [PubMed] [Google Scholar]
- 10. Rwigema JC, Heron DE, Ferris RL, Andrade RS, Gibson MK, Yang Y, Ozhasoglu C, Argiris AE, Grandis JR, Burton SA. The impact of tumor volume and radiotherapy dose on outcome in previously irradiated recurrent squamous cell carcinoma of the head and neck treated with stereotactic body radiation therapy. J Am Clin Oncol. 2011. August;34(4):372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yorke E, Clump D, Milano M, Soltys S, Naqa El. Outcomes of hypofractionated treatments – Results of the WGSBRT: Recurrent HNC treated with SBRT TCP-based outcomes estimates. Med Phys 2015;42:3685-6. [Google Scholar]
- 12. Vargo JA, Moiseenko V, Grimm J, Caudell J, Clump DA, Yorke E, Xue J, Vinogradskiy Y, Moros EG, Mavroidis P, Jain S, El Naqa I, Marks LB, Heron DE. Head and Neck Tumor Control Probability: Radiation Dose-Volume Effects in Stereotactic Body Radiation Therapy for Locally Recurrent Previously-Irradiated Head and Neck Cancer: Report of the AAPM Working Group. Int J Radiat Oncol Biol Phys. 2018. January 31. pii: S0360-3016(18)30107-X. doi: 10.1016/j.ijrobp.2018.01.044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langer CJ, Harris J, Horwitz EM, Nicolaou N, Kies M, Curran W, Wong S, Ang K. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Protocol 9911. J Clin Oncol. 2007. October 20;25(30):4800-5. [DOI] [PubMed] [Google Scholar]
- 14. Sher DJ, Timmerman RD, Nedzi L, Ding C, Pham NL, Zhao B, Sumer BD. Phase I fractional dose escalation study of equipotent stereotactic radiotherapy regimens for early-stage glottic larynx cancer. Int J Radiat Oncol Biol Phys. 2019. September 1;105(1):110-118. [DOI] [PubMed] [Google Scholar]
- 15. Kang BH, Yu T, Kim JH, Park JM, Kim JI, Chung EJ, Kwon SK, Kim JH, Wu HG. Early closure of a Phase I clinical trial for SABR in early-stage glottic cancer. Int J Radiat Oncol Biol Phys. 2019. September 1;105(1):104-109. [DOI] [PubMed] [Google Scholar]