Abstract
Introduction: Two-staged stereotactic radiosurgery (SRS) has been shown as an effective treatment for brain metastases that are too large for single fraction SRS.
Methods: Patients with large brain metastases (>4 cm3) treated with two-staged SRS from January 2017 to December 2019 at our institution were retrospectively identified.
Results: There were 23 brain metastases treated. The normal brain volume receiving equivalent 12Gy-in-single-fraction was defined as V12E. The V12E for original single-fraction GKS plan (mean of 41.4 cm3, range 5.6-146.1 cm3) was significantly higher compared to that of the second stage (mean of 23.7 cm3, range 2.8-92.7 cm3). The median tumor volume measured at the second stage (4.30 cm3) was reduced by an average of 52.2% compared to the first stage (9.58 cm3). Three patients (27.3%) showed local tumor progression in 4 tumors (20%). The median time to progression was 152 days.
Conclusions: Two-staged SRS is an effective treatment technique for large brain metastasis that results in significant reduction of tumor volume at the second stage SRS. Optimal treatment dose has not yet been defined.
Keywords: Brain tumor, Gamma Knife radiosurgery, oncology, radiation oncology, stereotactic radiosurgery
Introduction
Stereotactic radiosurgery (SRS) is a widely used therapy for patients with brain metastases.[1-6] However, its success is strongly correlated with the size of the target, with smaller and moderate sized lesions having superior outcomes.[4, 6] Large brain metastases have previously been classified as those measuring ≥4-15 cm3 in volume or ≥2-4 cm in maximum diameter. Large tumors are challenging to treat in a single fraction of SRS due to the difficulty in safely delivering an adequate dose to the target while minimizing dose to healthy brain tissue.[4, 7, 8] As such, a radiation dose reduction is necessary to prevent radiation-induced toxicity.[6] However, treatment with a single fraction with such a dose-reduction has been found to result in poor local control rates, ranging from 37-62%.[4, 5, 9] For these reasons, hypofractionated SRS for the management of large brain metastases is increasingly being used as there are higher rates of local tumor control and decreased rates of neurotoxicity compared to single-fraction SRS.[10-12] In 2009, Higuchi et al. described a novel treatment method consisting of a “staged” SRS protocol, which showed encouraging results.[13] It involved using three separate radiation treatments, or “stages,” each delivered with a much longer interval between treatments than classic hypofractionated treatment schedules. This method provides a period of time between stages that is thought to allow for tumor response resulting in a smaller treatment area in subsequent stages due to tumor shrinkage. Thus, staged SRS may allow for both a highly accurate target of the tumor and a period of repair for normal tissue, resulting in reduced neurotoxicity. Following this initial report of three-staged SRS, several other studies have since described alternative treatment paradigms consisting of two-staged SRS.[14-19]
Determining the optimal dose-fractionation treatment paradigm requires a balance between response rate and risk of adverse outcomes. This ideal balance remains unclear, and staged SRS treatment regimens are controversial.[13, 16] Variability of treatment schedules and patient populations between studies also makes comparisons within the body of literature challenging. Nevertheless, several different dose-fractionation regimens have been found to provide good local tumor control with a low risk for radiation necrosis in the management of large brain metastases.[13-20]
Our objectives were to (1) characterize the tumor response between the first and second fraction of two-staged Gamma Knife radiosurgery (GKS) and at follow-up imaging, (2) conduct a dosimetric comparison of V12E in normal brain between two-staged treatment and single fraction GKS, and (3) identify patients with evidence of local tumor progression following two-stage GKS.
Materials and MEthods
We conducted an institutional review board-approved retrospective study of patients treated at a single institution (Rutgers-Robert Wood Johnson University Hospital). Patients with at least one large brain metastatic lesion (defined as those >4 cm3 in volume) managed with two-stage GKS between January 2017 and December 2019 were included in the study. Patient clinical data, tumor volumes, follow-up intervals, and adverse effects were evaluated for each patient. As the normal brain receiving over 12Gy (V12) is a well-established radiation toxicity index for single-fraction SRS,[6] this index was also collected by converting using biological effective dose (BED) concept for two-staged treatment. By assuming ratio of 3Gy for normal brain tissue,[5] the BED for 12Gy in one fraction is equivalent to 8.1Gy in two fractions. Thus, the V12E (defined as the normal brain volume receiving over 12Gy-equivalent in a single fraction) was used to compare the original single dose plan and staged treatment plan for each patient.
All patients underwent GKS in two fractions using the Gamma Knife Perfexion/IconTM radiosurgery unit (Elekta AB, Sweden). This approach was selected for three main reasons. First, was concern for normal brain toxicity with a single, high-dose SRS treatment for large brain metastasis. In such tumors, fractionated or staged treatment is more favorable, allowing for sub-lethal radiation injury repair in healthy tissue between doses.[13] Second, compared to single fraction dosing, two-staged treatment would allow for dose escalation via an increase in BED. Third, if the metastatic tumor is close to critical structures and the primary disease type is radiosensitive, then staged treatment may be considered to shrink the tumor before the second dose delivery. Patients were immobilized with head-frame fixation. Target volumes of brain metastases were delineated as the radiographic enhancements on Gadolinium contrast-enhanced T1-weighted magnetic resonance imaging (MRI) with 1.5 mm slice thickness. Two-staged GKS plans were generated to deliver the prescription dose of 13Gy to the 40%-70% isodose lines with 100% dose coverage to the brain metastases using the Leksell Gamma Plan software (Elekta). The change in tumor volume between the first and second sessions of GKS was measured. For comparison, a single fraction GKS plan was also generated for each patient with a prescription dose of 20Gy, which was the estimated dose delivered in both two-staged GKS plans. Patients with concurrent small brain metastases were treated with a single fraction dose of 20-21Gy at the time of the first stage GKS. Tumor volume of the small brain metastases at subsequent follow-up imaging were measured. The change in tumor volume of the small and large brain metastases were compared.
Follow-up MRI imaging was obtained at approximately 6 weeks after the second stage and then again when clinically indicated. The volumetric measurements and change in volume at each stage of GKS and follow-up were calculated. Similar to response criteria used in the RECIST (response evaluation criteria in solid tumors) guidelines for solid tumors (version 1.1)[21], local tumor progression was defined as an increase in tumor volume of at least 20% compared with the smallest documented tumor volume on MRI, tumor response was defined as a decrease of at least 30% from baseline tumor volume, and stable if the tumor was not classified as progression or response. Time to local progression was determined from date of second stage GKS to date of follow-up showing progression. Treatment failure, defined as local tumor progression following GKS, was treated according to the institution’s clinical care algorithm. Any adverse events attributable to GKS were classified according to the National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE v5.0).
For patient and treatment characteristics, summary statistics were calculated via frequencies for categorical data and medians for continuous variables. For the analysis of change in tumor volume and difference of V12E, two-tailed paired T-tests were used. P values less than 0.01 were considered to be statistically significant. All data analysis was performed using Microsoft Excel version 16.33.
Results
Patient characteristics
Between January 2017 and December 2019, 12 patients were identified and included in this study. Table 1 summarizes the characteristics of the patients in this series. The majority (n = 10, 83.3%) were female, and the median age was 59 years (range: 29-83 years). There were 23 large brain metastases treated with two-staged GKS. The majority of patients (n = 8, 67%) were treated for a single brain metastasis. One (8%) patient had three metastases and 3 (25%) patients had four brain metastases managed with two-staged GKS. The median number of large brain metastases treated was 1 tumor (range: 1-4 tumors). The median number of total brain metastases was 4 tumors (range: 1-8 tumors). The primary tumors were breast (50%), non-small cell lung cancer (25%), lung adenocarcinoma (16.7%), and melanoma (8.3%). Metastases were most commonly located in the frontal lobe (47.8%), cerebellum (26.1%), and parietal lobe (13.0%). There was only one patient (8.3%) that had prior whole brain radiation. One patient (8.3%) had prior surgical resection of one tumor.
Table 1.
Patient characteristics
| Factor | No. (%) or median (range) |
| Sex | |
| Female | 10 (83.3) |
| Male | 2 (16.7) |
| Age at 1st stage GKS (years) | 59 (29-83) |
| Primary Cancer | |
| Breast | 6 (50) |
| Non-small cell lung | 3 (25) |
| Lung adenocarcinoma | 2 (16.7) |
| Melanoma | 1 (8.3) |
| KPS at 1st stage GKS | |
| 70-100 | 7 (58.5) |
| 50-60 | 3 (25) |
| ≤40 | 2 (16.7) |
| RPA Class | |
| I | 3 (25) |
| II | 4 (33.3) |
| III | 5 (41.7) |
| Total number of brain metastases | |
| 1-3 | 5 (41.7) |
| 4-6 | 5 (41.7) |
| ≥7 | 2 (16.7) |
| Extracranial metastases | |
| No | 7 (58.3) |
| Yes | 5 (41.7) |
| Prior WBRT | |
| No | 1 (8.3) |
| Yes | 11 (91.7) |
| Prior surgery | |
| No | 1 (8.3) |
| Yes | 11 (91.7) |
| Controlled primary disease | |
| No | 3 (25) |
| Yes | 9 (75) |
| Large brain metastases location | |
| Frontal | 11 (47.8) |
| Parietal | 3 (13.0) |
| Temporal | 1 (4.3) |
| Occipital | 1 (4.3) |
| Cerebellum | 6 (26.1) |
| Brainstem | 1 (4.3) |
The majority (58.3%) of patients had good performance status, defined as a Karnofsky Performance Scale (KPS) of ≥80 and Eastern Cooperative Oncology Group (ECOG) Performance Status ≤1. In terms of Recursive Partitioning Analysis (RPA) classification, 3 (25%) were RPA Class I, 4 (33%) were RPA Class II, and 5 (41.7%) were RPA Class III. Nearly half of patients had extracranial metastases (41.7%). The majority of patients had controlled primary disease (75%) and presented with neurological symptoms at the time of diagnosis of brain metastases (75%). At the time of this study, to the best of our knowledge, six patients (50%) died at a median of 4.9 months following two-staged GKS. Among the deceased cases, deaths were systemic in one case, neurologic in one case, and unknown in four cases.
Treatment characteristics
The median prescription dose at the first stage of GKS was 13Gy (range 13-15Gy) at the 50% isodose line (range 40%-55%). The median prescription dose at the second stage of GKS was 13Gy (range 12-13Gy) at the 50% isodose line (range 40%-56%). The median interval between the two sessions was 33 days (range 21-66 days). The median interval between the second stage to the first follow-up and to the second follow-up was 44 days and 152 days, respectively. The median total follow-up duration was 6.4 months.
V12E
The median V12E of a single fraction plan was 15.8 cm3 (mean ± standard deviation, 41.4 ± 45.9; range 5.6-146.1 cm3). The V12E for first-stage treatment was identical to that of the single-dose plan due to equivalent dose conversion. However, the median V12E of the second-stage was significantly reduced to 10.4 cm3 (mean ± standard deviation, 23.7 ± 27.4; range 2.8-92.7 cm3). All cases demonstrated a considerable amount of V12E reduction, with an average volume reduction of 48% (range 4-66%).
Adverse effects
According to the National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE v5.0), three patients demonstrated grade 1 toxicity and one patient demonstrated grade 3 toxicity following GKS. The patient with grade 3 toxicity was admitted to the hospital due to symptoms of nausea, vomiting, and severe headache immediately following GKS. Imaging showed vasogenic edema and worsening hydrocephalus which required management with an external ventricular device and subsequently improved.
Tumor response
Median tumor volumes at first and second GKS was 9.58 cm3 (range 4.02-20.92 cm3) and 4.30 cm3 (range 0.78-17.80 cm3), respectively. This difference was statistically significant (p < 0.01). The median change in volume at the second stage compared with baseline was a decrease of 52.2%. Table 2 summarizes the characteristics of the large brain metastases in this series.
Table 2.
Tumor metastases characteristics
| Factor | No. (%) or median (range) |
| First stage GKS dose (Gy) | 13 (13 to 15) |
| Second stage GKS dose (Gy) | 13 (12 to 13) |
| V12E at first stage GKS (cm3) | 15.8 (5.6 to 146.1) |
| V12E at second stage GKS (cm3) | 10.4 (2.8 to 92.7) |
| Tumor volume at first stage GKS (cm3) | 9.6 (4.0 to 20.9) |
| Tumor volume at second stage GKS (cm3) | 4.3 (0.8 to 17.8) |
| Volume change at second stage GKS (%) | −52.2 (−81.8 to +4.3) |
| Tumor volume at first follow-up (cm3) | 2.5 (0.38 to 14.2) |
| Volume change from first stage to first follow-up (%) | −72.9 (−92.1 to −13.9) |
| Volume change from second stage to first follow-up (%) | −46.7 (−59.6 to +26.5) |
| Local failure (%) | 5 (25) |
Eleven patients (92%) returned for follow-up imaging following the second stage GKS. At the first follow-up, the median tumor volume was 2.46 cm3 (range 0.38-14.2 cm3). The median change in volume from the first stage GKS to the first follow-up was a decrease of 72.9%, which was statistically significant (p < 0.01). The median change in volume from the second stage GKS to the first follow-up was a decrease of 46.7%, which was statistically significant (p < 0.01). Eight patients (66.7%) returned for a second follow-up where imaging was obtained. The median tumor volume was 2.37 cm3 (range 0.0-16.5 cm3) at the second follow-up, corresponding to a median decrease of 85.8% from the first stage GKS (p < 0.01), a decrease of 67.9% from the second stage GKS (p = 0.32), and an decrease of 13.0% from the first follow-up (p = 0.41).
Of the patients with follow-up imaging, tumor response was seen in 15 (75%) lesions and a stable response was seen in one (5%) lesion. The median change in volume in those with tumor response was a decrease of 78.5% from baseline tumor volume. Local progression was seen in 4 tumors (20%) distributed among 3 patients (27.3%). All three patients were deceased at the time of this study. Patient and tumor characteristics in cases with tumor progression are described in Table 3. Comparison of factors in cases of tumor response and tumor progression is shown in Table 4. The median change in volume in the lesions that progressed was an increase of 427% from the smallest documented volume. The median time to progression was 152 days (range 106-157 days). Of the tumor volumes that increased based on imaging, all four tumors were found to have progressed at the second follow-up. The volumes at first stage GKS of the tumors that progressed were 4.02, 7.92, 8.43, and 12.83 cm3. One patient underwent laser interstitial thermotherapy (LITT) for two recurrences following two-stage GKS treatment failure.
Table 3.
Specific patient and tumor characteristics in which tumor progression was identified
| Patient 1 | Patient 2 | Patient 3 | ||
| Tumor 1 | Tumor 2 | Tumor 3 | Tumor 4 | |
| Primary cancer | Breast | Breast | Melanoma | Lung adenocarcinoma |
| Tumor volume at 1st stage GKS (cm3) | 8.4 | 12.8 | 4.0 | 7.9 |
| Tumor volume at 2nd stage GKS (cm3) | 4.3 | 4.1 | 2.5 | 3.6 |
| Tumor volume at last follow-up (cm3) | 13.9 | 11.9 | 4.5 | 16.5 |
| Time to progression (days) | 157 | 157 | 106 | 147 |
| Increase in volume from smallest tumor volume (%) | 500.6 | 353.9 | 88.4 | 730.6 |
Table 4.
Patient and tumor characteristics in cases of tumor response and tumor progression
| Factor |
Tumor response No. (%) or median (range) |
Tumor progression No. (%) or median (range) |
| Age (years) | 55 (29 to 79) | 57 (29 to 67) |
| KPS score ≤70 (%) | 2 (25) | 1 (33.3) |
| Extracranial metastasis present (%) | 2 (25) | 2 (66.7) |
| Uncontrolled primary disease (%) | 1 (12.5) | 2 (66.7) |
| Primary cancer (%) | ||
| Breast | 5 (62.5) | 1 (33.3) |
| Non-small cell lung | 3 (37.5) | 0 (0) |
| Lung adenocarcinoma | 0 (0) | 1 (33.3) |
| Melanoma | 0 (0) | 1 (33.3) |
| Tumor volume at first stage GKS (cm3) | 9.9 (4.3 to 20.9) |
8.2 (4.0 to 12.8) |
| Tumor volume at second stage GKS (cm3) | 4.5 (0.8 to 17.8) |
3.8 (0.9 to 4.3) |
| Volume change at second stage GKS (%) | −53.1 (−81.8 to 4.3) |
−51.7 (−68.2 to −37.2) |
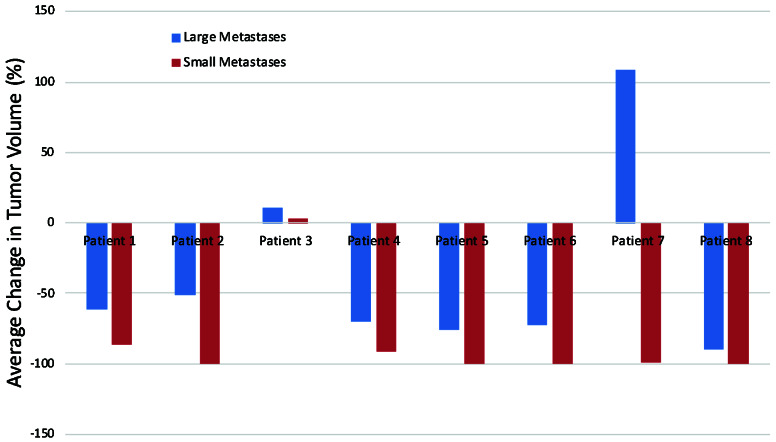
Eight patients with large brain metastases were simultaneously treated for small brain metastases at the first stage GKS. There was no significant difference in the average change in tumor volume of each patient’s small metastases compared to the large metastases (p = 0.087). Figure 1 demonstrates the average change in tumor volume of large and small brain metastases for each of these patients.
Figure 1.
Average change in tumor volume of large and small brain metastases for eight patients.
Discussion
Staged SRS is a novel approach to the management of large brain metastases. We found that two-staged GKS demonstrated a significant reduction in tumor volume at the second stage GKS and first follow-up with an acceptable toxicity rate. However, there was local tumor progression in 20% of tumors at follow-up imaging with a median time to progression of 152 days.
The median total number of brain metastases was 4 in our study, which exceeds that in other comparable series. For instance, the mean number of brain metastases in Serizawa et al. was 2.59 lesions.[16] Despite the substantial intracranial burden of disease and that the majority of our patients belonged to the poorest prognostic groups, two-stage SRS may still be favorable compared to other approaches for large brain metastases in certain cases, particularly in patients unable or unwilling to undergo surgical resection.
The incidence of local tumor progression in this series (20%) was comparable to others in the literature (ranging from 10.3%-26.9%).[14-19] One patient had a radioresistant primary cancer (melanoma), and the fastest time to progression (3.5 months) among the tumors that progressed. Radiosensitivity of primary cancers may affect tumor progression rates, although this relationship has not yet been studied in the context of two-stage SRS for brain metastases. In our cases of tumor progression, the majority of patients had uncontrolled primary disease and extracranial metastases at the time of two-stage SRS. These factors have been associated with decreased patient survival.[15] At the time of this study, all three patients with tumor progression had deceased. These three patients were in RPA Class II or III, which is associated with a poor prognosis. Using the prognostic groups described in Angelov et al., two of these deceased patients fall into the unfavorable prognostic group (≥2 poor risk features), which have a significantly decreased overall survival compared to the favorable prognostic group.[17]
In eight patients, we compared the response of large brain metastases treated with two-stage GKS to small brain metastases treated with 20-21Gy at the first stage GKS. The mean change in tumor volume was not significantly different between the patient’s large and small brain metastases. This suggests that the two-staged GKS treatment for large metastases may be as effective in tumor control as a single fraction GKS plan. One patient (Patient 3) showed tumor progression in one large brain metastasis and in 2 out of 3 small brain metastases following GKS treatment. This patient had melanoma, which is considered a radioresistant primary cancer and may account for this finding. Another patient (Patient 7) demonstrated tumor progression in one large brain metastasis but tumor response in all four small brain metastases. This patient had a history of multiple primary cancers, including lung adenocarcinoma, melanoma, and ovarian cancer. Additionally, at the second stage GKS, a new small brain metastasis was identified and treated with 20Gy. The patient likely had poor control of their primary cancer.
The V12E at the first stage GKS was equivalent to single-dose plan due to equivalent dose conversion. However, the V12E exposure was significantly reduced (by average of 48% reduction) at the second stage GKS. This is favorable as less volume of normal brain tissue received radiation at the second stage. V12 has been found to be an independent risk factor for radiation necrosis in single fraction SRS.[6, 22, 23] Specifically, Minniti et al. found that lesions with V12 greater than 8.3 cm3 were associated with a radionecrosis risk of over 10%.[6] In this study, we used the well-accepted ratio of 3Gy for normal brain tissue to convert V12 in single fraction into V12E in two-fractionated treatment. Yet, using V12E may not be exactly relevant when attempting to quantitate the risk of radionecrosis since the normal tissue response to a divided dose is markedly different. Prior studies were designed to predict radionecrosis risk for single fraction SRS, and there is yet to be a study to validate V12E data for hypofractionated or staged SRS. As a result, V12E may not be exactly predictive of radionecrosis risk for staged SRS, and one may be able to expose the tissue to more than 8.3 cm3 as was suggested by Minniti et al. for single fraction treatments. Regardless, our findings suggest that staged SRS may minimize normal brain tissue dose at the V12E level, which may possibly translate to a decreased radiation necrosis risk. Overall, this remains a first attempt to quantitate the exposure in an effort to understand dosing constraints in hypofractionated and staged stereotactic radiation therapy schemes.
Limitations in our study exist. This is a single institution, retrospective study of a small number of patients with a mix of primary tumors. Additionally, the follow-up period was short and varied among patients, including one patient who did not complete any follow-up imaging. As a result, the risk of local tumor progression and toxicity rates may be underestimated. Third, follow-up MRI images varied from 1.5 to 6 mm slice thickness. Thus, measured tumor volumes from MRIs with larger slice thickness may be underestimated for some metastases. In light of these limitations, we recommend larger, prospective studies with longer follow-up to further assess the rate of local tumor progression and describe the cases at greatest risk for treatment failure.
Currently, there are few published studies in the literature investigating the efficacy of staged SRS for large brain metastases (Table 5).[13-19] The first study on this topic was published by Higuchi et al. and included 43 patients with 46 large brain metastases treated with a 10Gy x 3 regimen separated by two-week intervals.[13] Patients were followed for a mean of 7.8 months. They found a significant reduction in tumor volume after each stage. The local tumor control rates at 6 and 12 months were 90% and 76%, respectively. They suggested that this treatment scheme could be applied as an alternative for managing large brain metastases.
Table 5.
Review of studies on staged SRS for large brain metastasis
| Author(s), year | No. of Patients | No. of large brain metastases | Med. SRS dose × No. of stages | Med. or mean tumor volume at first stage (cm3) |
Local control rates, 6 mo./12 mo. (%) |
Tumor progression (%) | Time to progression (mo.) |
| Higuchi et al., 2009 | 43 | 46 | 10Gy × 3 | 17.6 | 90/76 | NA | NA |
| Yomo et al., 2012 | 27 | 28 | 13.3Gy × 2 | 17.8 | 85/61 | 21.4 | 6.2 |
| Yomo and Hayashi, 2014 | 58 | 61 | 13.3Gy × 2 | 16.4 | 85/64 | 26.9 | 6.2 |
| Dohm et al., 2018 | 33 | 39 | 15Gy × 2 | 11.68 | NA/86 | 10.3 | NA |
| Angelov et al., 2018 | 54 | 63 | 15Gy × 2 | 10.54 | 88/NA | 14.3 | 5.2 |
| Serizawa et al., 2019 | 106 | NA | 10Gy × 3 | 18.8 | NA/NA | 21.6 | NA |
| 106 | NA | 13.2Gy × 2 | 18.4 | NA/NA | 16.7 | NA | |
| Ito et al., 2020 | 178 | 182 | 13Gy × 2 | 16.6 | NA/NA | 26.2 | NA |
Following this first study, Yomo et al. conducted a prospective study of 27 patients with 28 large brain metastases managed with a 10-16Gy (median 13.3Gy) x 2 protocol separated by 3- to 4-week intervals.[14] Patients were followed for a median of 8.9 months. The study reported a local control rate at 6 and 12 months of 85% and 61%, respectively, with few cases of treatment-related adverse effects. Failure of local control was seen in 19% of patients and 21.4% of tumors, with a median time to progression of 6.2 months after two-stage SRS. The authors Yomo and Hayashi later published another prospective study of 58 patients with 61 large brain metastases treated with two-stage SRS using the same protocol as the previous study (10-16Gy x 2 with 3-4 weeks between fractions).[15] The mean follow-up period was 9 months. Their results were similar to their first study, with local tumor control rates at 6 and 12 months of 85% and 64%, respectively, and low rates of treatment morbidity. Local failure was diagnosed in 26.9% of lesions at a median of 6.2 months after the initial session. Similar to Higuchi et al., these results together also support that staged SRS is effective and safe in the management of large brain metastases. This is particularly appealing to patients who are poor candidates for surgical resection.
Dohm et al. reported a two-staged SRS protocol with a median dose of 15Gy at first SRS and 14Gy at second SRS, separated by one month.[18] They included 33 patients with 39 lesions. At 6 and 12 months, the local failure rates were 3.2% and 13.3%, respectively. Local progression was seen in 12% of patients and in 10% of total lesions. Other series in the literature described report 12-month local control rates of 61-76%, which are considerably lower than the rate of 86% presented in this study by Dohm et al. With these encouraging results, Dohm et al. published another study comparing staged SRS to surgery with postoperative SRS.[24] The incidence of local failure and median overall survival were not statistically significant between the two treatment groups. Thus, staged SRS is an attractive alternative to surgery with postoperative SRS in the management of large brain metastases given these comparable outcomes.
Another study evaluated the efficacy and toxicity of a two-staged SRS for large brain metastases using a 15Gy x 2 treatment regimen with a median inter-fraction interval of 34 days.[17] In this study, Angelov et al. presented a series of 63 large brain metastases in 54 patients treated with staged SRS. Overall, two-staged SRS resulted in local control rates of 95% at three months and 88% at six months. They reported local progression in 14.3% of lesions with a median time to progression of 5.2 months, and 11% of patients demonstrated adverse effects. They found that shorter time to progression was associated with larger pre-treatment tumor volumes and a less decrease in tumor volume at the second stage.
Serizawa et al. conducted the largest series of large brain metastases treated with staged GKS in the current literature.[16] This retrospective multi-institutional study in Japan included 212 patients either treated with 3-stage (9-11Gy x 3) or 2-stage (11.8-14.2Gy x 2) GKS in a case-matched cohort. There was no significant difference in median survival time, cumulative incidence of tumor progression, or serious radiation-related adverse events. The incidence of tumor progression was 21.6% and 16.7% in the 3-stage group and 2-stage groups, respectively, but this difference was not statistically significant. As such, both protocols seem to be comparable treatment modalities. This study, in addition to the previous ones, further emphasizes that the optimal treatment scheme remains to be elucidated, given that all these studies describe distinct dose-fractionation regimens.
Most recently, Ito et al. investigated outcomes of 182 large brain metastases across three primary cancer types managed with two-stage SRS.[19] Their protocol involved a median prescribed dose of 13Gy at both stages, which were separated by an average of three weeks. Local tumor progression was reported in 26.2% lesions. They found a median survival time of 6.6 months from the first stage GKS. Patients with primary gastrointestinal tract cancers had decreased overall survival and lower tumor volume reduction rates compared to patients with breast cancer or non-small cell lung cancer. The authors recommend attention to the patient’s primary cancer when considering two-stage SRS for large brain metastases due to these differences in clinical outcomes between gastrointestinal tract, breast, and non-small cell lung cancers.
The studies to date are summarized in Table 5. Of the studies published, not all report local control rates or time to progression for treatment failures. The characteristics and factors reported across the studies are somewhat variable, making broad judgements and conclusions difficult. Relevant to this study is the observation that two studies (Dohm et al. and Angelov et al.) used a 15Gy x 2 strategy, in which the average tumor progression rate was 12%. The remaining studies utilized either a 13Gy x 2 or a 10Gy x3 strategy, where the recurrence rate averaged 23%, almost double. The use of a 13Gy x 2 scheme, as an alternative to a single fraction of 20Gy, may in fact underdose these tumors leading to a greater failure rate. Additionally, larger and more careful studies may be able to identify a maximum 12Gy exposure delivered over two doses that allows for an acceptable radionecrosis risk. Ultimately, a larger study may help to identify patients who should undergo surgical resection and those who may benefit from staged SRS.
Conclusion
This study reports a single institution experience with two-staged SRS for the management of large brain metastases. Our results demonstrated significant tumor shrinkage between the two stages and at the first follow-up. Review of the literature potentially reveals that we are underdosing these treatments by using 13Gy x 2 treatment protocol. The optimal dose-fraction regimen and favorable patient considerations remains to be established.
Symbols and Abbreviations
ECOG: Eastern Cooperative Oncology Group
GKS: Gamma Knife Surgery
KPS: Karnofsky Performance Scale
LITT: Laser Interstitial Thermotherapy
MRI: Magnetic Resonance Imaging
RECIST: Response Evaluation Criteria in Solid Tumors
RPA: Recursive Partitioning Analysis
SRS: Stereotactic Radiosurgery
Acknowledgments
None.
Footnotes
Source of Support: None.
Authors disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Conception and design: Elizabeth E. Ginalis, Taoran Cui, Joseph Weiner, Shabbar Danish
Data collection: Elizabeth E. Ginalis, Taoran Cui
Analysis and interpretation: Elizabeth E. Ginalis, Taoran Cui, Ke Nie, Shabbar Danish
Manuscript writing: Elizabeth E. Ginalis, Taoran Cui, Joseph Weiner, Ke Nie, Shabbar Danish
Approval of manuscript: Elizabeth E. Ginalis, Taoran Cui, Joseph Weiner, Ke Nie, Shabbar Danish
References
- 1. Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, Hudgins WR, Weiner R, Harsh GRt, Sneed PK, Larson DA. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28(4):797-802. doi: 10.1016/0360-3016(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 2. Adler JR, Cox RS, Kaplan I, Martin DP. Stereotactic radiosurgical treatment of brain metastases. J Neurosurg. 1992;76(3):444-9. doi: 10.3171/jns.1992.76.3.0444. [DOI] [PubMed] [Google Scholar]
- 3. Alexander E, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, Kooy HM, Loeffler JS. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87(1):34-40. doi: 10.1093/jnci/87.1.34. [DOI] [PubMed] [Google Scholar]
- 4. Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro Oncol. 2017;19(suppl_2):ii38-ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kondziolka D, Shin SM, Brunswick A, Kim I, Silverman JS. The biology of radiosurgery and its clinical applications for brain tumors. Neuro Oncol. 2015;17(1):29-44. doi: 10.1093/neuonc/nou284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol (London, England). 2011;6:48. doi: 10.1186/1748-717x-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiau CY, Sneed PK, Shu HK, Lamborn KR, McDermott MW, Chang S, Nowak P, Petti PL, Smith V, Verhey LJ, Ho M, Park E, Wara WM, Gutin PH, Larson DA. Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys. 1997;37(2):375-83. doi: 10.1016/s0360-3016(96)00497-x. [DOI] [PubMed] [Google Scholar]
- 8. Serizawa T, Saeki N, Higuchi Y, Ono J, Iuchi T, Nagano O, Yamaura A. Gamma knife surgery for brain metastases: indications for and limitations of a local treatment protocol. Acta Neurochir (Wien). 2005;147(7):721-6. doi: 10.1007/s00701-005-0540-4. [DOI] [PubMed] [Google Scholar]
- 9. Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 2006;104(6):907-12. doi: 10.3171/jns.2006.104.6.907. [DOI] [PubMed] [Google Scholar]
- 10. Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, Osti M, Enrici RM, Esposito V. Single-fraction versus multifraction (3 × 9 Gy) Stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142-8. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 11. Manning MA, Cardinale RM, Benedict SH, Kavanagh BD, Zwicker RD, Amir C, Broaddus WC. Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(3):603-8. doi: 10.1016/s0360-3016(00)00475-2. [DOI] [PubMed] [Google Scholar]
- 12. Wegner RE, Leeman JE, Kabolizadeh P, Rwigema JC, Mintz AH, Burton SA, Heron DE. Fractionated stereotactic radiosurgery for large brain metastases. J Am Clin Oncol. 2015;38(2):135-9. doi: 10.1097/COC.0b013e31828aadac. [DOI] [PubMed] [Google Scholar]
- 13. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, Iwadate Y, Saeki N. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009;74(5):1543-8. doi: 10.1016/j.ijrobp.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 14. Yomo S, Hayashi M, Nicholson C. A prospective pilot study of two-session Gamma Knife surgery for large metastatic brain tumors. J Neurooncol. 2012;109(1):159-65. doi: 10.1007/s11060-012-0882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yomo S, Hayashi M. A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma Knife stereotactic radiosurgery. Radiat Oncol (London, England). 2014;9:132. doi: 10.1186/1748-717x-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serizawa T, Higuchi Y, Yamamoto M, Matsunaga S, Nagano O, Sato Y, Aoyagi K, Yomo S, Koiso T, Hasegawa T, Nakazaki K, Moriki A, Kondoh T, Nagatomo Y, Okamoto H, Kohda Y, Kawai H, Shidoh S, Shibazaki T, Onoue S, Kenai H, Inoue A, Mori H. Comparison of treatment results between 3- and 2-stage Gamma Knife radiosurgery for large brain metastases: A retrospective multi-institutional study. J Neurosurg. 2018:1-11. doi: 10.3171/2018.4.Jns172596. [DOI] [PubMed] [Google Scholar]
- 17. Angelov L, Mohammadi AM, Bennett EE, Abbassy M, Elson P, Chao ST, Montgomery JS, Habboub G, Vogelbaum MA, Suh JH, Murphy ES, Ahluwalia MS, Nagel SJ, Barnett GH. Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases </= 2 cm. J Neurosurg. 2018;129(2):366-382. doi: 10.3171/2017.3.Jns162532. [DOI] [PubMed] [Google Scholar]
- 18. Dohm A, McTyre ER, Okoukoni C, Henson A, Cramer CK, LeCompte MC, Ruiz J, Munley MT, Qasem S, Lo HW, Xing F, Watabe K, Laxton AW, Tatter SB, Chan MD. Staged stereotactic radiosurgery for large brain metastases: Local control and clinical outcomes of a one-two punch technique. Neurosurgery. 2018;83(1):114-121. doi: 10.1093/neuros/nyx355. [DOI] [PubMed] [Google Scholar]
- 19. Ito D, Aoyagi K, Nagano O, Serizawa T, Iwadate Y, Higuchi Y. Comparison of two-stage Gamma Knife radiosurgery outcomes for large brain metastases among primary cancers. J Neurooncol. 2020;147(1):237-246. doi: 10.1007/s11060-020-03421-y. [DOI] [PubMed] [Google Scholar]
- 20. Higuchi Y, Yamamoto M, Serizawa T, Aiyama H, Sato Y, Barfod BE. Modern management for brain metastasis patients using stereotactic radiosurgery: literature review and the authors’ gamma knife treatment experiences. Cancer Manag Res. 2018;10:1889-1899. doi: 10.2147/cmar.S116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990). 2009;45(2):228-47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996-1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23. Korytko T, Radivoyevitch T, Colussi V, Wessels BW, Pillai K, Maciunas RJ, Einstein DB. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64(2):419-24. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 24. Dohm AE, Hughes R, Wheless W, Lecompte M, Lanier C, Ruiz J, Watabe K, Xing F, Su J, Cramer C, Laxton A, Tatter S, Chan MD. Surgical resection and postoperative radiosurgery versus staged radiosurgery for large brain metastases. J Neurooncol. 2018;140(3):749-756. doi: 10.1007/s11060-018-03008-8. [DOI] [PubMed] [Google Scholar]