Abstract
Estrogens and their receptors play key roles in regulating body weight, energy expenditure, and metabolic homeostasis. It is known that lack of estrogens promotes increased food intake and induces the expansion of adipose tissues, for which much is known. An area of estrogenic research that has received less attention is the role of estrogens and their receptors in influencing intermediary lipid metabolism in organs such as the brain. In this review, we highlight the actions of estrogens and their receptors in regulating their impact on modulating fatty acid content, utilization, and oxidation through their direct impact on intracellular signaling cascades within the central nervous system.
Keywords: Alzheimer’s disease, estrogens, fatty acid oxidation, fatty acids, sexual dimorphism
INTRODUCTION: A BRIEF SUMMARY OF THE IMPACT OF ESTROGENS AND THEIR RECEPTORS ON ENERGY HOMEOSTASIS
Estrogens are a “family” of compounds that exist in three naturally occurring forms, estrone, 17α- (17α-E2) and 17β-estradiol (17β-E2), and estriol, among which 17β-E2 represents the major physiological estrogen (17). Animal studies demonstrate that 17β-E2 suppresses feeding, enhances energy expenditure, and limits energy storage, thus promoting negative energy balance (43, 46, 65). In female rodents, absence of ovarian estrogens, following the removal of the ovaries (OVX), results in increased adiposity, reductions in energy expenditure, dysregulation of lipid homeostasis, and body weight gain (15, 74). Attenuation of these metabolic pathways results from replacement of 17β-E2 (15), implicating 17β-E2 as a critical estrogen in regulating these processes.
Estrogens control metabolic functions primarily through activation of estrogen receptors (ERs). Disruptive mutations in the ER genes, as demonstrated using rodent models, recapitulate alterations in metabolic homeostasis such as glucose intolerance, hyperinsulinemia, and dyslipidemia (19, 52, 56, 73). Two “classical” ERs, ESR1 and ESR2, have been well characterized with respect to their influence on whole body energy homeostasis (4), and current findings indicate a more pronounced role for the action of ESR1 in conveying estrogen’s effects on energy homeostasis compared with ESR2. Mice null for ESR1−/− are obese, insulin resistant, and dyslipidemic and have impaired glucose homeostasis (19, 47, 66); (73). In contrast, the role of ESR2 in energy homeostasis and metabolism has been highly controversial (13, 23, 27, 30, 47, 53, 56). It has been reported that the deletion of ESR2 does not promote obesity or any of the diseases associated with obesity (56). In the hypothalamus, ESR2 was found in the same hypothalamic nuclei as ESR1 but with a lower expression, and its deletion either did not promote food intake and obesity (23) or antagonized the anorectic effect of estrogen (30). Interestingly, a sexual dimorphic response to ESR2 deletion was observed in mice, in which ERβKO male mice presented with a similar body weight and fat distribution as well as lipid and insulin levels than wild-type (WT) controls, whereas ERβKO female mice fed a high fat-diet gained more weight than their WT counterparts (23). In a study by Ohlsson et al. (56), ERβKO adult male mice had a tendency of reduced weight of the retroperitoneal fat pads; however, the mechanisms by which this occurred were not revealed. In humans, investigators found that polymorphisms in the ESR2 gene have been associated with lower BMI or no correlation (47). Therefore, in light of these conflicting reports on the role of ESR2, we have focused our discussion on ESR1 and its impact on lipid metabolism within the central nervous system.
ESTROGEN RECEPTOR-α
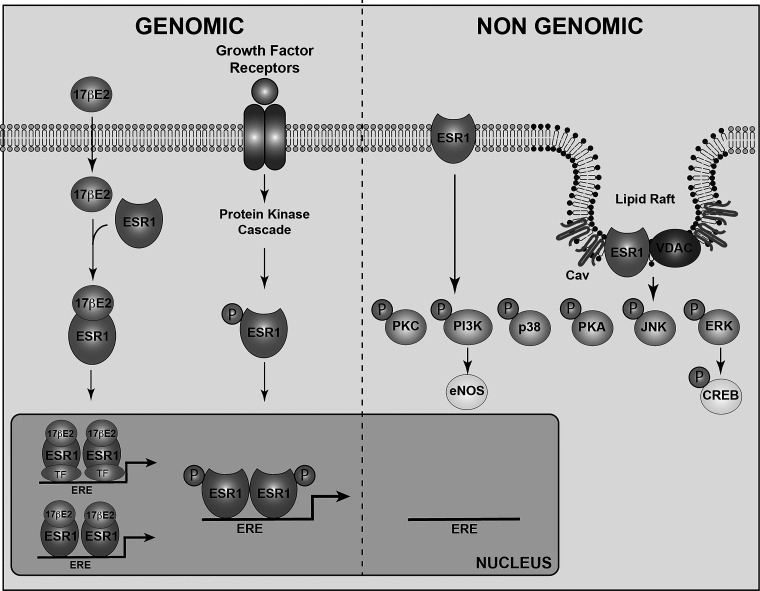
Estrogen receptor-α (ESR1) is located in different subcellular compartments, including the nucleus, cytoplasm, and cell membrane, where it mediates genomic and nongenomic signaling (Fig. 1) (41). In the genomic signaling pathway, estrogens bind to nuclear ESR1, inducing receptor dimerization and activation. Once activated, ESR1 can directly bind to estrogen responsive elements (EREs) on the regulatory regions of target genes (20). Alternatively, ESR1 can be recruited to specific promoter regions through interactions with other DNA-binding transcription factors (29, 54). Through the ERE-dependent (or classical) genomic signaling pathways, ESR1 mediates most of estrogen’s reproductive effects and some of the estrogen-related metabolic actions (58).
Fig. 1.
Estrogen receptor signaling pathways. Estrogen receptor-α (ESR1) signaling occurs through genomic and nongenomic mechanisms. During the genomic signaling pathway, ESR1 is activated by 17β-estradiol (17β-E2), which represents the strongest natural ligand. 17β-E2 binds to ESR1 in the cytoplasm; here, ER-α dimerizes and translocates to the nucleus, where it modulates the transcription of target genes by interacting with the estrogen-responsive elements (ERE) on the DNA. ESR1 can also be activated by growth factors. Growth factors activate their tyrosine kinase receptors, which stimulate a tyrosine kinase cascade leading to the phosphorylation and activation of ESR1. Phosphorylated ESR1 (P) translocates to the nucleus, where it modulates gene transcription. Through the genomic signaling pathway, ESR1 mediates most of estrogen’s reproductive effects and some of the estrogen-related metabolic actions. During the nongenomic signaling pathway, ESR1 located at the cell membrane initiates a rapid cytosolic signaling cascade. ESR1 at the plasma membrane generally localizes in the lipid rafts, where it interacts with the proteins caveolin-1 (Cav) and voltage-dependent anion channel (VDAC), where it activates rapid cytosolic signaling events. Through the nongenomic signaling pathway, ESR1 has been shown to regulate adiposity and control body weight as well as to exert neuroprotective functions. eNOS, endothelial nitric oxide synthase; PI3K, phosphatidylinositol 3-kinase; CREB, cAMP-responsive element binding. TF, transcription factor.
ESR1 located in extranuclear compartments plays key roles in mediating estrogen’s nongenomic signaling pathways, which involve rapid activation of intracellular signaling molecules such as protein kinase A (PKA), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) (Fig. 1) (3, 29). Genetic rescue of ESR1−/− mice using an ESR1 knock-in mouse model that is unable to bind to EREs demonstrates that restoring non-ERE-dependent receptor functions normalizes body weight and adiposity in the ESR1−/− mice to the WT levels (58), indicating that non-ERE-dependent ESR1 signaling is sufficient to mediate some of estrogen’s effects on energy balance (58). In contrast, the mice expressing only the nuclear ESR1 (nuclear only estrogen receptor, or “NOER”, as named by the authors) maintain the increased body weight phenotype of the obese ESR1−/− mice (60). It is important to note that although these mice have been named “nuclear-only ER”, the genetically engineered cells express receptors that lack palmitoylation sites, and therefore, they cannot be tethered to the plasma membrane; however, they can traffic and eventually act in the cytoplasm. These findings indicate that the ERE-dependent genomic ESR1 signaling may not be involved in estrogen’s nongenomic effect on controlling energy balance. Importantly, however, these data do not exclude the possibility that there is a cross-talk between extranuclear (non-ERE dependent) and nuclear (ERE dependent) ESR1 actions underlying estrogen’s intracellular signaling pathways that control body weight homeostasis. The exact subcellular location of extranuclear ESR1 action has remained elusive. For example, a transgenic mouse model expressing only the functional E domain of ESR1, which exclusively localizes ESR1 at the plasma membrane and which is required to stimulate the activation of the kinase cascade starting from the plasma membrane (membrane-only estrogen receptor mice), is characterized as obese and with increased visceral fat depots when compared with wild-type mice, further suggesting that ESR1 tethered at the plasma membrane is not sufficient to revert the increased body weight phenotype characteristic of the ESR1 mice−/− (61). These data provide the possibility that ESR1 in other extranuclear compartments, such as cytoplasm and mitochondrion, may play a role in mediating ESR1’s actions of controlling energy homeostasis and body weight.
DO ESTROGENS INFLUENCE FATTY ACID CONCENTRATIONS IN THE BRAIN?
To begin to understand the impact of estrogens on lipid content/fatty acid (FA) composition of the brain, we recently evaluated the concentration of different classes of FAs in the central nervous system (CNS) of male and female mice. Interestingly, we reported that the FA content of brains from male and female mice differed under both low-fat diet (12% calories from fat) and chronic (16 wk) exposure to a Western-style [saturated FA, 42% high-fat diet (HFD)] feeding condition. Specifically, we found that males have increased levels of saturated FAs and decreased levels of ω6-polyunsaturated fatty acids (PUFAs) when compared with females, and these differences become more pronounced following consumption of the HFD (50, 63). Interestingly, these sex-related differences appear to be brain specific, since the plasma FA profile did not reflect the sexual dimorphism observed in the brain (50, 63).
The mechanisms underpinning the brain-specific sexual dimorphic FA profiles are currently unknown and might involve a potential sexually dimorphic effect on either the trafficking of lipids across the blood-brain barrier (BBB), de novo FA biosynthesis, fatty acid oxidation, or lipid metabolism. These differences cannot be associated with 17β-E2 plasma concentrations. However, it is known that estrogens are produced locally in the brain, and thus such differences in FA content between male and female mice could be explained by the local brain production of estrogens. In addition, we are aware that a whole plethora of additional differences exist beyond 17β-E2 concentrations. Below we highlight why the sexual difference in FA profiles may be important, and we discuss a disease state that is sexually dimorphic and related to brain lipid content.
DO ESTROGENS INFLUENCE LIPID TRANSPORT ACROSS THE BLOOD-BRAIN BARRIER?
FA uptake across plasma membranes has been demonstrated to be sexually dimorphic (35, 55); however, the mechanisms involved in these differences are still unclear. In peripheral tissues such as in skeletal muscle, the levels of the fatty acid transporter CD36, as well as the fatty acid transport protein 1 (FATP1), and the fatty-acid binding protein (FABP) display higher levels in females than in males (35), yet less is known about whether these same sexual dimorphisms occur in the brain, and thus future studies investigating the presence of these differences in the brain are warranted.
One potential way by which estrogens may regulate FA uptake across plasma membranes into the brain may be through augmenting the permeability to the BBB. It has been reported that BBB transport is influenced by sex hormones (8, 57); however, the mechanisms of how sex hormones influence BBB biology are still unknown. In rats, ovariectomy increased the permeability of BBB (64), suggesting that estrogens may affect the transport of molecular species, including lipids from the periphery into the CNS. In the same study, old male mice (28–32 wk old) presented with a higher permeability of BBB compared with younger male mice (10–12 wk old). Although investigators have not investigated the permeability of BBB in older female mice, it would be interesting to know whether in older women the increased permeability of BBB may be due to aging, the fact they produce less estrogens, or both.
It was recently reported that 17β-E2 protects the BBB from inflammation-induced disruption (36). In a study by Maggioli et al. (36), the authors compared the response of male and female mice to a systemic inflammatory model (peripheral LPS challenge) and found that males, ovariectomized (OVX) females, and reproductively old females showed an inflammation-induced deficit in BBB integrity that was absent in young females and in OVX females that received 17β-E2 replacement. It was further suggested that this effect is mediated by the anti-inflammatory protein annexin A1 (ANXA1), which contributes to limited barrier permeability, promoting tight junction formation and enhancing interendothelial cell tightness (6, 36). Interestingly, ANXA1 protein was highly expressed in young female mice compared with older females and male mice; additionally, 17β-E2 replacement restored ANXA1 expression in OVX females. In vitro, the use of an anti-ANXA1 antibody in immortalized endothelial cells (hCMEC/D3) blocked the effect of 17β-E2 to reverse the impaired paracellular permeability induced by inflammation. However, it is not clear whether media without phenol was used, which could have been crucial since phenol has estrogenic activity and could be a confounding factor for these results. It is known that ANXA1-deficient mice are obese (1) and that HFD exposure results in reductions in ANXA1 protein levels in the endothelial cells forming the BBB, thereby enhancing leakage of the BBB and favoring the uptake of saturated FAs into the brain (63).
DO ESTROGENS MEDIATE DE NOVO FA BIOSYNTHESIS IN THE CNS?
FA can be derived from the diet or generated through de novo synthesis. One of the enzymes required for the de novo FA synthesis is fatty acid synthase (FAS), which catalyzes the synthesis of long-chain fatty acids, including palmitate from malonyl-CoA and acetyl-CoA (34). FAS together with other FA metabolic enzymes, including acetyl-CoA carboxylase (ACC), which produces malonyl-CoA from acetyl-CoA, and malonyl-CoA decarboxylase (MCD), which degrades malonyl-CoA back to acetyl-CoA, are expressed in neurons and glial cells in the brain (11, 22, 25, 71). In the hypothalamus, an essential brain site in regulating global energy homeostasis and metabolism, these enzymes are abundantly expressed in rodents as well as in humans, implicating the significance of hypothalamic FA metabolic pathways in the regulation of energy balance and body weight homeostasis (14, 25, 67). A role for hypothalamic malonyl-CoA in the central control of feeding and body weight was proposed based on studies demonstrating inhibition of FAS by the compound C75 (a potent FAS inhibitor) administered either peripherally or centrally markedly reduced food intake and body weight (31). In this study, the inhibition of FAS led to inhibition of feeding by 90% over the first 24 h, which was normalized later, probably because of a blockade effect of neuropeptide Y (NPY). The reduction in body weight was associated with loss of water as well as fat and lean body mass. Because estrogens also have been shown to regulate food intake and body weight, one hypothesis tested was whether estrogens modulate FAS and malonyl-CoA. To begin to elucidate the intersection between estrogens and FAS, the effect of hypothalamic malonyl-CoA on feeding was explored by intracerebroventricular injections of the selective estrogen receptor modulator (SERM) tamoxifen. Data from these experiments demonstrated that tamoxifen treatment suppressed appetite and downregulated FAS expression specifically in the ventromedial nucleus of the hypothalamus (VMN) (33), although the identity of the specific form of ER involved is currently unknown. Still, little is known about sites and mechanism of action by which SERMs exert their effects on body weight. In addition, more recent research has demonstrated that peripheral treatment with 17β-E2 in OVX mice, but not other ovarian hormones such as progesterone, appears to inhibit FAS expression through an ESR1-mediated pathway in the VMN of the hypothalamus, resulting in lower food intake and weight loss (44). This effect appears to be dependent on the presence of estrogens and varies across the estrous cycle, with the magnitude of the effect being the greatest when estrogens are at their highest levels (during proestrous). Consistently, FAS expression in the VMN of proestrus females was significantly decreased when compared with male rats, confirming the correlation between 17β-E2 level and FAS expression (44). Within this study, these authors explored the role of tamoxifen and compared it with 17β-E2 with respect to their ability to modulate FAS expression, and these authors found a similar response that contradicted what might have normally been predicted. The role of SERMs and tamoxifen is currently unclear, as it appears there are conflicting data as to whether they are agonists or antagonists, which may differ based on the location of the receptor being targeted. Specifically, the authors directly injected tamoxifen in this series of experiments into the brain while 17β-E2 was peripherally injected, and therefore, their results may suggest differing signaling pathways impacting FAS based on the site of the injection. In addition, in the study by Martínez de Morentin et al. (44), which was conducted in pregnant rats, the authors found that increased estrogen levels were associated with low expression of FAS in the hypothalamus, which would be predicted to result in decreased food intake, and they instead found increased appetite and food intake, indicating that in this study the pregnant females were resistant to the decreased FAS expression and subsequently accumulation of malonyl-CoA in the hypothalamus. The mechanisms underlying this resistance are unclear, but Martínez de Morentin et al. (44) speculated that there may be an accumulation of specific lipid species other than malonyl-CoA in the hypothalamus of these rats.
AMP-activated protein kinase (AMPK) controls the metabolic lipid flux partly through impacting the de novo FA biosynthetic pathway (72). AMPK is expressed in various organs such as the brain, muscle, liver, and adipose tissues, where it exerts a control over global energy homeostasis (5, 59, 77). AMPK is a cellular energy sensor regulating a variety of metabolic pathways, including lipid metabolism. At the cellular level, an increase in the AMP/ATP ratio, which indicates a low energy status, enhances the activity of AMPK to activate catabolic pathways such as fatty acid oxidation to produce ATP while simultaneously suppressing anabolic pathways such as lipogenesis to reduce ATP consumption (32). Relevant to the discussion in this review is that 17β-E2 is one of the hormones that directly affects hypothalamic AMPK (43). Indeed, administration of 17β-E2 into the VMN of the hypothalamus, but not in the arcuate nucleus, inhibits AMPK through ESR1. Thus, in the brain, estrogens might modulate AMPK activity to impact de novo FA biosynthesis, ultimately resulting in changes in FA profiles. There is evidence showing that, at least in bronchial epithelial cells, AMPK action regulates the expression and the activity of certain desaturases in the PUFA synthetic pathways (70). In addition, there are data suggesting that in neuroblastoma cells exposure to 17β-E2 stimulates the formation of PUFAs [eicosapentaenoic acid (20:5-ω3) and docosapentaenoic acid (22:5-ω3)] from α-linolenic acid (18:3-ω3), suggesting that, in neuronal cells in culture, 17β-E2 is able to modulate the upstream production of membrane ω3 long-chain PUFA (2). In the periphery, it was reported that the activity of elongase 6, an essential enzyme catalyzing FA elongation (49), is higher in the liver of female compared with male rats, which may contribute to the sexually dimorphic concentrations of saturated FAs in the liver (42); however, no studies have evaluated whether a sex difference exists in the activity of enzymes involved in FA biosynthesis in the brain.
DO ESTROGENS INFLUENCE FATTY ACID OXIDATION IN THE BRAIN?
Although limited, FA oxidation counts for 20% of the total brain energy requirement (9). Until recently, astrocytes have been considered as the only cell type in which FA β-oxidation plays a quantitative role in energy metabolism (9), and relevant to this review, ESR1 has been localized in astrocytes, providing a direct link to estrogens and FA oxidation through astrocytes. Additionally, recent transcriptome data show that microglia also express different forms of acyl-CoA synthetase (79) and that both astrocytes and microglia are able to use FAs as a fuel source in substrate oxidation pathways influencing production of ketone bodies (18). Once released, ketone bodies affect hypothalamic neuronal activity and modulate neuronal output, resulting in regulation of energy homeostasis (28, 37). Importantly, the diet impacts processing of lipids in the brain, and as we have previously mentioned, there is a sexual dimorphism in brain FA levels following high-fat diet (HFD) exposure (50, 63). Astrocytes increase FA β-oxidation and ketogenesis, thereby influencing lipid distribution within the hypothalamus and hence, lipid sensing (21); however, this has been demonstrated only in males. The mechanisms that underline this process have not been fully elucidated in females. Noteworthy is the fact that we have observed sex differences in ketone body levels following exposure to HFD, supporting a possible role for estrogens (or additional sex-related factors) in modulating this process (51).
Additionally, estrogens influence FA oxidation by regulating expression of key enzymes involved in FA β-oxidation (24), as demonstrated by the finding that ovariectomy reduces the levels of acyl-CoA dehydrogenase, an essential enzyme in mitochondrial β-oxidation, in mouse tissues, including adipose tissue and skeletal muscle. Consistently, in a mouse model where aromatase has been knocked out (ArKO), resulting in endogenous estrogen levels that are undetectable, the mRNA expression and activity of enzymes involved in FA metabolism are significantly reduced, at least in the liver, leading to accumulation of lipid droplets in hepatocytes and lower FA β-oxidation. The mRNA expression of the enzymes involved with FA metabolism was restored following 17β-E2 treatment (69). In addition, basal constitutive FA β-oxidation activity was higher in the liver of ArKO female mice compared with males (69); however, these differences were not fully investigated. Future studies need to be performed to determine whether this is occurring also in the brain.
DO ESTROGENS INFLUENCE LIPID METABOLISM IN THE BRAIN?
Lipid metabolism in the brain may also influence activation of estrogen signaling cascades. A recent study demonstrated that mice with neuron-specific deletion of lipoprotein lipase (LPL; NEXLPL−/− mice), the enzyme hydrolyzing circulating triglyceride-rich lipoproteins to release free fatty acids (FFA) for uptake and oxidation in different tissues, have upregulated expression of ESR1 in the hypothalamus, and this appears to be selectively true only in females (76). A previous study has shown that neuron-specific deletion of LPL in both female and male mice resulted in increased food intake, reduced energy expenditure, and obesity in both sexes (75). Intriguingly, when pair-fed mice were compared with the wild-type control mice, obviating the body weight phenotype, the male LPL-deficient mice were still obese, indicating that in males LPL deficiency reduced energy expenditure (76). In contrast, female LPL-deficient mice did not develop obesity, suggesting females are sensitive to dietary modifications and limiting total food intake, which prevented obesity independent of neuronal LPL expression. As previously mentioned, LPL deletion promotes upregulation of ESR1 specifically in females, suggesting a female-specific LPL-mediated control of hypothalamic ESR1 expression that regulates body weight homeostasis. The mechanisms underlying the potential LPL-mediated regulation of ESR1 expression are unknown, but it is possible that PUFAs might be involved, acting downstream of LPL and upstream of ESR1 expression. Indeed, it has been demonstrated that neuronal LPL deficiency is associated with a reduction in the level of PUFAs, indicating that LPL action impacts PUFA metabolism (75). It would be interesting to further evaluate whether ESR1 expression and activity in the hypothalamus influences lipid metabolism in the brain, consistent with our findings of a sexual dimorphism in lipid content.
DO ESTROGENS INFLUENCE THE INCORPORATION OF FA INTO LIPID RAFTS?
An example of how the estrogenic and lipid environment interact is evidenced by the findings that extranuclear ERs can be localized in lipid raft domains, which are dynamic regions of the plasma membrane that serve as a platform for intracellular signaling by promoting protein-protein and protein-lipid interactions (Fig. 1) (45). ESR1 has been identified within lipid rafts in the CNS in human frontal cortex, the hippocampus, and neuronal cell culture (7, 40, 62). Lipid rafts are comprised of different classes of lipids, and alterations of these lipids within the lipid rafts such as sterols, gangliosides, and polyunsaturated fatty acids (PUFAs) have been identified in neuropathologies such as Alzheimer’s disease (AD) (10, 78). Relative to this review, sex hormones appear to influence the incidence of AD on the prevalence of the disease being significantly higher in women compared with men; two-thirds of individuals diagnosed with AD are women (48). One potential reason for this sex difference is that women live longer than men. Also, following menopause, women undergo a rapid loss of estrogens (48), and estrogens are thought to protect against neurodegeneration and provide neuroprotection (39) During the aging process, men also experience a significant decrease in estrogens due to the fact that there is a reduction in testosterone, which can be converted to 17β-E2 by aromatase. However, the decrease in estrogens is significantly more gradual in men (a 2–3% reduction/year in testosterone concentration after the age of 30) (12), and thus men do not experience the abrupt loss of estrogens experienced by women after menopause. Additionally, the content of the lipids in the lipid raft changes with disease progression, and in AD, lipid rafts show significant reductions in ω3-FAs and ω3-long-chain PUFAs such as docosahexaenoic acid (DHA). Modifications of lipid content within lipid rafts affect the physical properties of the lipid raft (permeability and fluidity among others) (68), thus changing the behavior of the proteins that localize in these regions. The accessibility of protein binding sites might affect the changing lipid milieu as well as the degree of protein-protein and protein-lipid interaction (16, 26). Importantly, data have demonstrated that ESR1 in neuronal lipid rafts interacts with the proteins caveolin-1 and the voltage-dependent anion channel (VDAC) and modulates VDAC activation, thereby preventing amyloid-β-induced toxicity (38), which is often associated with AD. The interaction with ESR1 is lost in the lipid rafts of cortical neurons from AD brains, promoting VDAC activity and contributing to neuronal impairment (38). What is still unknown and requires future study is how estrogens and/or ESR1 may influence the lipid environment within the lipid raft and thereby impact protein-protein and protein-lipid interactions and, therefore, signaling pathways that might enhance AD susceptibility.
CONCLUSIONS
Lipid metabolism constitutes a key component in energy homeostasis, and here we discuss the potential impact of estrogens on FA metabolic pathways in the brain. A number of studies indicate that estrogens modulate the activity of enzymes involved in de novo FA synthesis and FA oxidation, thereby influencing the FA levels in brain, but to draw conclusions, additional work in this area needs to be accomplished. The involvement of ESR1 in FA oxidation is largely unknown and needs to be elucidated. Furthermore, at the subcellular level, lipid rafts act as a potential mediator in ESR1 signaling actions, which might be involved not only in metabolism/lipid homeostasis but also in neuronal preservation. Altogether these studies indicate that, although it appears estrogens affect lipid metabolism in the brain, there is still a gap in the knowledge as to the mechanisms by which estrogens regulate these processes.
GRANTS
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico GRants 1160820 (to E. Morselli) and 1171075 (to A. Criollo), FONDAP-ACCDis Grant 15130011 (to A. Criollo), the CONICYT Grant PCI 170147 (to Y. Ávalos), and International Centre for Genetic Engineering and Biotechnology Grant CRP/CHL16-06 (to E. Morselli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.M. and A.C. prepared figures; E.M. and D.J.C. drafted manuscript; E.M., R.d.S.S., S.G., Y.A., A.C., B.F.P., and D.J.C. edited and revised manuscript; E.M., R.d.S.S., S.G., Y.A., A.C., B.F.P., and D.J.C. approved final version of manuscript.
REFERENCES
- 1.Akasheh RT, Pini M, Pang J, Fantuzzi G. Increased adiposity in annexin A1-deficient mice. PLoS One 8: e82608, 2013. doi: 10.1371/journal.pone.0082608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessandri JM, Extier A, Langelier B, Perruchot MH, Heberden C, Guesnet P, Lavialle M. Estradiol favors the formation of eicosapentaenoic acid (20:5n-3) and n-3 docosapentaenoic acid (22:5n-3) from alpha-linolenic acid (18:3n-3) in SH-SY5Y neuroblastoma cells. Lipids 43: 19–28, 2008. doi: 10.1007/s11745-007-3117-6. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Chambliss KL, Mineo C, Shaul PW. Recent insights into non-nuclear actions of estrogen receptor alpha. Steroids 81: 64–69, 2014. doi: 10.1016/j.steroids.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab 14: 289–299, 2011. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond) 124: 491–507, 2013. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 6.Cristante E, McArthur S, Mauro C, Maggioli E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J, Christian HC, Weksler BB, Malaspina A, Solito E. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci USA 110: 832–841, 2013. doi: 10.1073/pnas.1209362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deecher DC, Swiggard P, Frail DE, O’Connor LT. Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12). Endocrine 22: 211–223, 2003. doi: 10.1385/ENDO:22:3:211. [DOI] [PubMed] [Google Scholar]
- 8.Diler AS, Uzüm G, Akgün Dar K, Aksu U, Atukeren P, Ziylan YZ. Sex differences in modulating blood brain barrier permeability by NO in pentylenetetrazol-induced epileptic seizures. Life Sci 80: 1274–1281, 2007. doi: 10.1016/j.lfs.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 23: 5928–5935, 2003. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabelo N, Martín V, Marín R, Santpere G, Aso E, Ferrer I, Díaz M. Evidence for premature lipid raft aging in APP/PS1 double-transgenic mice, a model of familial Alzheimer disease. J Neuropathol Exp Neurol 71: 868–881, 2012. doi: 10.1097/NEN.0b013e31826be03c. [DOI] [PubMed] [Google Scholar]
- 11.Fantino M. Role of lipids in the control of food intake. Curr Opin Clin Nutr Metab Care 14: 138–144, 2011. doi: 10.1097/MCO.0b013e3283437b78. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 589–598, 2002. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 13.Frank A, Brown LM, Clegg DJ. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol 35: 550–557, 2014. doi: 10.1016/j.yfrne.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao S, Lane MD. Effect of the anorectic fatty acid synthase inhibitor C75 on neuronal activity in the hypothalamus and brainstem. Proc Natl Acad Sci USA 100: 5628–5633, 2003. doi: 10.1073/pnas.1031698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 142: 4751–4757, 2001. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 16.Gross RW, Jenkins CM, Yang J, Mancuso DJ, Han X. Functional lipidomics: the roles of specialized lipids and lipid-protein interactions in modulating neuronal function. Prostaglandins Other Lipid Mediat 77: 52–64, 2005. doi: 10.1016/j.prostaglandins.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med 346: 340–352, 2002. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 18.Guzmán M, Blázquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab 12: 169–173, 2001. doi: 10.1016/S1043-2760(00)00370-2. [DOI] [PubMed] [Google Scholar]
- 19.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann K, Lamberz C, Piotrowitz K, Offermann N, But D, Scheller A, Al-Amoudi A, Kuerschner L. Tanycytes and a differential fatty acid metabolism in the hypothalamus. Glia 65: 231–249, 2017. doi: 10.1002/glia.23088. [DOI] [PubMed] [Google Scholar]
- 22.Hu Z, Dai Y, Prentki M, Chohnan S, Lane MD. A role for hypothalamic malonyl-CoA in the control of food intake. J Biol Chem 280: 39681–39683, 2005. doi: 10.1074/jbc.C500398200. [DOI] [PubMed] [Google Scholar]
- 23.Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab 29: 557–568, 2015. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Kamei Y, Suzuki M, Miyazaki H, Tsuboyama-Kasaoka N, Wu J, Ishimi Y, Ezaki O. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr Sci Vitaminol (Tokyo) 51: 110–117, 2005. doi: 10.3177/jnsv.51.110. [DOI] [PubMed] [Google Scholar]
- 25.Kim EK, Miller I, Landree LE, Borisy-Rudin FF, Brown P, Tihan T, Townsend CA, Witters LA, Moran TH, Kuhajda FP, Ronnett GV. Expression of FAS within hypothalamic neurons: a model for decreased food intake after C75 treatment. Am J Physiol Endocrinol Metab 283: E867–E879, 2002. doi: 10.1152/ajpendo.00178.2002. [DOI] [PubMed] [Google Scholar]
- 26.Lee AG. Lipid-protein interactions. Biochem Soc Trans 39: 761–766, 2011. doi: 10.1042/BST0390761. [DOI] [PubMed] [Google Scholar]
- 27.Lemieux C, Phaneuf D, Labrie F, Giguère V, Richard D, Deshaies Y. Estrogen receptor alpha-mediated adiposity-lowering and hypocholesterolemic actions of the selective estrogen receptor modulator acolbifene. Int J Obes 29: 1236–1244, 2005. doi: 10.1038/sj.ijo.0803014. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152: 2552–2557, 2011. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19: 1951–1959, 2005. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord 26: 1103–1109, 2002. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 31.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288: 2379–2381, 2000. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 32.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116: 1776–1783, 2006. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S, Blount M, Vázquez MJ, Finer N, Powles TJ, O’Rahilly S, Saha AK, Diéguez C, Vidal-Puig AJ. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 55: 1327–1336, 2006. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- 34.López M, Lelliott CJ, Vidal-Puig A. Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. BioEssays 29: 248–261, 2007. doi: 10.1002/bies.20539. [DOI] [PubMed] [Google Scholar]
- 35.Lundsgaard AM, Kiens B. Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front Endocrinol (Lausanne) 5: 195, 2014. doi: 10.3389/fendo.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggioli E, McArthur S, Mauro C, Kieswich J, Kusters DH, Reutelingsperger CP, Yaqoob M, Solito E. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun 51: 212–222, 2016. doi: 10.1016/j.bbi.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Magnan C, Levin BE, Luquet S. Brain lipid sensing and the neural control of energy balance. Mol Cell Endocrinol 418: 3–8, 2015. doi: 10.1016/j.mce.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Marin R. Signalosomes in the brain: relevance in the development of certain neuropathologies such as Alzheimer’s disease. Front Physiol 2: 23, 2011. doi: 10.3389/fphys.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marin R, Casañas V, Pérez JA, Fabelo N, Fernandez CE, Diaz M. Oestrogens as modulators of neuronal signalosomes and brain lipid homeostasis related to protection against neurodegeneration. J Neuroendocrinol 25: 1104–1115, 2013. doi: 10.1111/jne.12068. [DOI] [PubMed] [Google Scholar]
- 40.Marin R, Ramírez CM, González M, Alonso R, Díaz M. Alternative estrogen receptors homologous to classical receptor alpha in murine neural tissues. Neurosci Lett 395: 7–11, 2006. doi: 10.1016/j.neulet.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 41.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7: 497–508, 2006. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks KA, Kitson AP, Stark KD. Hepatic and plasma sex differences in saturated and monounsaturated fatty acids are associated with differences in expression of elongase 6, but not stearoyl-CoA desaturase in Sprague-Dawley rats. Genes Nutr 8: 317–327, 2013. doi: 10.1007/s12263-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez de Morentin PB, González-García I, Martins L, Lage R, Fernández-Mallo D, Martínez-Sánchez N, Ruíz-Pino F, Liu J, Morgan DA, Pinilla L, Gallego R, Saha AK, Kalsbeek A, Fliers E, Bisschop PH, Diéguez C, Nogueiras R, Rahmouni K, Tena-Sempere M, López M. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 20: 41–53, 2014. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez de Morentin PB, Lage R, González-García I, Ruíz-Pino F, Martins L, Fernández-Mallo D, Gallego R, Fernø J, Señarís R, Saha AK, Tovar S, Diéguez C, Nogueiras R, Tena-Sempere M, López M. Pregnancy induces resistance to the anorectic effect of hypothalamic malonyl-CoA and the thermogenic effect of hypothalamic AMPK inhibition in female rats. Endocrinology 156: 947–960, 2015. doi: 10.1210/en.2014-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maselli A, Pierdominici M, Vitale C, Ortona E. Membrane lipid rafts and estrogenic signalling: a functional role in the modulation of cell homeostasis. Apoptosis 20: 671–678, 2015. doi: 10.1007/s10495-015-1093-5. [DOI] [PubMed] [Google Scholar]
- 46.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 203: 259–269, 2011. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol 6: 37–48, 2014. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem 276: 45358–45366, 2001. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- 50.Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A, Clegg DJ. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes 40: 206–209, 2016. doi: 10.1038/ijo.2015.114. [DOI] [PubMed] [Google Scholar]
- 51.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschöp MH, Clegg DJ. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Reports 9: 633–645, 2014. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mueller SO, Korach KS. Estrogen receptors and endocrine diseases: lessons from estrogen receptor knockout mice. Curr Opin Pharmacol 1: 613–619, 2001. doi: 10.1016/S1471-4892(01)00105-9. [DOI] [PubMed] [Google Scholar]
- 53.Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta). Horm Metab Res 34: 758–763, 2002. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 54.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 81: 1535–1565, 2001. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 55.Ockner RK, Burnett DA, Lysenko N, Manning JA. Sex differences in long chain fatty acid utilization and fatty acid binding protein concentration in rat liver. J Clin Invest 64: 172–181, 1979. doi: 10.1172/JCI109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly-Y M, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278: 640–645, 2000. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 57.Pakulski C, Drobnik L, Millo B. Age and sex as factors modifying the function of the blood-cerebrospinal fluid barrier. Med Sci Monit 6: 314–318, 2000. [PubMed] [Google Scholar]
- 58.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Invest 121: 604–612, 2011. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465, 2012. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 60.Pedram A, Razandi M, Blumberg B, Levin ER. Membrane and nuclear estrogen receptor α collaborate to suppress adipogenesis but not triglyceride content. FASEB J 30: 230–240, 2016. doi: 10.1096/fj.15-274878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem 284: 3488–3495, 2009. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramírez CM, González M, Díaz M, Alonso R, Ferrer I, Santpere G, Puig B, Meyer G, Marin R. VDAC and ERalpha interaction in caveolae from human cortex is altered in Alzheimer’s disease. Mol Cell Neurosci 42: 172–183, 2009. doi: 10.1016/j.mcn.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez-Navas C, Morselli E, Clegg DJ. Sexually dimorphic brain fatty acid composition in low and high fat diet-fed mice. Mol Metab 5: 680–689, 2016. doi: 10.1016/j.molmet.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saija AP, Princi P, D’Amico N, De Pasquale R, Costa G. Aging and sex influence the permeability of the blood-brain barrier in the rat. Life Sci 47: 2261–2267, 1990. doi: 10.1016/0024-3205(90)90157-M. [DOI] [PubMed] [Google Scholar]
- 65.Saito K, Cao X, He Y, Xu Y. Progress in the molecular understanding of central regulation of body weight by estrogens. Obesity (Silver Spring) 23: 919–926, 2015. doi: 10.1002/oby.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331: 1056–1061, 1994. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 67.Sorensen A, Travers MT, Vernon RG, Price NT, Barber MC. Localization of messenger RNAs encoding enzymes associated with malonyl-CoA metabolism in mouse brain. Brain Res Gene Expr Patterns 1: 167–173, 2002. doi: 10.1016/S1567-133X(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 68.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 126: 1–27, 2003. doi: 10.1016/S0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 69.Toda K, Takeda K, Akira S, Saibara T, Okada T, Onishi S, Shizuta Y. Alternations in hepatic expression of fatty-acid metabolizing enzymes in ArKO mice and their reversal by the treatment with 17beta-estradiol or a peroxisome proliferator. J Steroid Biochem Mol Biol 79: 11–17, 2001. doi: 10.1016/S0960-0760(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 70.Umunakwe OC, Seegmiller AC. Abnormal n-6 fatty acid metabolism in cystic fibrosis is caused by activation of AMP-activated protein kinase. J Lipid Res 55: 1489–1497, 2014. doi: 10.1194/jlr.M050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volpe JJ, Marasa JC. Regulation of palmitic acid synthesis in cultured glial cells: effects of lipid on fatty acid synthetase, acetyl-CoA carboxylase, fatty acid and sterol synthesis. J Neurochem 25: 333–340, 1975. doi: 10.1111/j.1471-4159.1975.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 72.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 50, Suppl: S138–S143, 2009. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR J 45: 455–461, 2004. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- 74.Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr 131: 2351–2357, 2001. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Astarita G, Taussig MD, Bharadwaj KG, DiPatrizio NV, Nave KA, Piomelli D, Goldberg IJ, Eckel RH. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab 13: 105–113, 2011. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Wang Y, Taussig MD, Eckel RH. Sex differences in obesity development in pair-fed neuronal lipoprotein lipase deficient mice. Mol Metab 5: 1025–1032, 2016. doi: 10.1016/j.molmet.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye J. Mechanisms of insulin resistance in obesity. Front Med 7: 14–24, 2013. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young G, Conquer J. Omega-3 fatty acids and neuropsychiatric disorders. Reprod Nutr Dev 45: 1–28, 2005. doi: 10.1051/rnd:2005001. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–11947, 2014. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]