Abstract
Autosomal dominant polycystic kidney disease (PKD) is characterized by cyst formation and growth, which are partially driven by abnormal proliferation of tubular cells. Proproliferative mechanistic target of rapamycin (mTOR) complexes 1 and 2 (mTORC1 and mTORC2) are activated in the kidneys of mice with PKD. Sirolimus indirectly inhibits mTORC1. Novel mTOR kinase inhibitors directly inhibit mTOR kinase, resulting in the inhibition of mTORC1 and mTORC2. The aim of the present study was to determine the effects of sirolimus versus the mTOR kinase inhibitor torin2 on cyst growth and kidney function in the Pkd1 p.R3277C (Pkd1RC/RC) mouse model, a hypomorphic Pkd1 model orthologous to the human condition, and to determine the effects of sirolimus versus torin2 on mTORC1 and mTORC2 signaling in PKD1−/− cells and in the kidneys of Pkd1RC/RC mice. In vitro, both inhibitors reduced mTORC1 and mTORC2 phosphorylated substrates and negatively impacted cellular metabolic activity, as measured by MTT assay. Pkd1RC/RC mice were treated with sirolimus or torin2 from 50 to 120 days of age. Torin2 was as effective as sirolimus in decreasing cyst growth and improving loss of kidney function. Both sirolimus and torin2 decreased phosphorylated S6 protein, phosphorylated eukaryotic translation initiation factor 4E-binding protein 1, phosphorylated Akt, and proliferation in Pkd1RC/RC kidneys. In conclusion, torin2 and sirolimus were equally effective in decreasing cyst burden and improving kidney function and mediated comparable effects on mTORC1 and mTORC2 signaling and proliferation in the Pkd1RC/RC kidney.
Keywords: apoptosis, autosomal dominant polycystic kidney disease, polycystic, proliferation, sirolimus, torin2
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most common life-threatening hereditary disorders (59). In ADPKD, a mutation in the PKD1 gene (in ~75–77% of cases) results in the slow development of kidney cysts, causing chronic kidney disease requiring dialysis or kidney transplantation, usually in the sixth decade of life (59). In a preclinical study (57) and subsequent human studies (55, 56), the drug tolvaptan was effective in slowing cyst growth and improving kidney function, resulting in United States Food and Drug Administration approval for the treatment of ADPKD (57). The development of tolvaptan as a treatment for ADPKD highlights the importance of preclinical studies for drug development for ADPKD.
Mechanistic target of rapamycin (mTOR) exists in two distinct structural and functional complexes: mTORC1 and mTORC2. mTORC1 downstream signaling is mainly via the proproliferative S6 ribosomal protein (S6) and eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) pathways. The mTORC2 protein complex associates with rapamycin-independent companion of mTOR (Rictor). mTORC2 signaling is mainly propagated via phosphorylation of Akt at Ser473.
In two large randomized human studies, the effect of the rapamycin analogs (rapalogs) sirolimus and everolimus (indirect mTORC1 inhibitors) on polycystic kidney disease (PKD) was disappointing (45, 58). Apart from dose limitations due to toxicity and inconsistent timing or duration of rapalog treatment, rapalogs may not impact major downstream proproliferative substrates of mTORC1, such as the translational repressor 4E-BP1. Sirolimus does not usually directly target 4E-BP1 (16, 47); instead, sirolimus is only a partial inhibitor of mTORC1 in most cell types (16, 47, 53, 54), and phosphorylation of 4E-BP1 is usually sirolimus resistant at Thr46 (30). Another possible reason for the disappointing effect of rapalogs in human studies is that rapalogs do not directly target mTORC2 or its downstream substrates (16, 47).
Second-generation mTOR inhibitors, mTOR kinase inhibitors (TORKi), inhibit both mTORC1, especially 4E-BP1 (34), and mTORC2. Studies have shown 4E-BP1 phosphorylation sensitivity to TORKi (8, 52). We have previously shown that an active site TORKi, PP242, decreases PKD and improves kidney function in the Han:SPRD (Cy/+) rat model of ADPKD (38). We have also previously shown that a mTOR antisense oligonucleotide that targets both mTORC1 and mTORC2 decreases PKD and improves kidney function in the Pkd2−/− mouse (37). However, in PKD, it is unknown whether there is aberrant phosphorylation of 4E-BP1 or whether phosphorylated 4E-BP1 (pE4-BP1) species (Thr70, Thr37/46, and Ser65) are sensitive to TORKi. Also, the effect of an active site TORKi has not been directly compared with the mTOR allosteric inhibitor sirolimus in PKD.
We hypothesized that a TORKi would inhibit proproliferative mTORC1 downstream substrates, such as 4E-BP1, and mTORC2, to a greater degree than the allosteric mTORC1 inhibitor sirolimus. Furthermore, we hypothesized that a TORKi would be as effective as, or more effective than, sirolimus in ameliorating PKD in a hypomorphic Pkd1RC/RC mouse model orthologous to the human disease. The aims of the study were to characterize mTORC1 and mTORC2 signaling in PKD1−/− cells in vitro and Pkd1RC/RC mice in vivo in response to sirolimus or treatment with a TORKi. This preclinical study of a TORKi in PKD may offer insights into performing future clinical studies in PKD.
METHODS
In vitro model.
Human primary cells from the normal renal cortical tubular epithelium (RCTE; PKD1+/+) and ADPKD cyst-lining epithelium [PKD1−/− (WT 9-12 cell line)] immortalized with ori-adeno-simian virus 40, as previously described (31), were used. PKD1−/− cells have increased proliferation (1) and a delayed proliferative response to antiproliferative agents (49), making them a translational in vitro PKD model to study the relationship between mTOR and proliferation. Cells were cultured as previously described (31). Doses of sirolimus (1, 10, 100, and 1,000 nM) and torin2 (1, 10, 100, and 1,000 nM) were increased over a 1-h period in 10-cm2 plates. Briefly, cells were plated 18 h in advance. Once plates had reached 80% confluence, cells were exposed to escalating doses of sirolimus or torin2. After 1 h, protein was isolated from cell lysates and immunoblotted for mTORC1 and mTORC2 substrates.
Metabolic activity was assayed using MTT according to the manufacturer’s instructions. Briefly, 5,000 cells were plated per well in a 96-well plate, allowed to adhere for 5 h, exposed to sirolimus (10 nM) or torin2 (100 nM) for 2 h, and then assayed for MTT reduction. Actively respiring cells convert water-soluble MTT to insoluble purple formazan. The formazan concentration was determined by optical density measured at 590 nm.
In vivo model.
Pkd1RC/RC mice have a hypomorphic Pkd1 gene mutation orthologous to that of PKD patient disease variant PKD1 p. R3277C (Pkd1RC/RC) (22). Pkd1RC/RC mice in the C57BL/6 background have cysts at 3 mo of age (21, 24). Cyst expansion and size correlate with increased tubular cell proliferation (22). Wild-type C57BL/6J mice (stock no. 000664) were purchased from Jackson Laboratories (Bar Harbor, ME). All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Animal Care and Use Committee of the University of Colorado at Denver. Mice were maintained on a standard diet under standard pathogen-free housing conditions, with food and water freely available.
Experimental in vivo protocol.
Male and female C57BL/6 Pkd1RC/RC mice were treated with torin2 (10 mg/kg ip, daily on weekdays), sirolimus (0.5 mg/kg ip, daily on weekdays), or vehicle (28% DMSO in polyethylene glycol 300) from 50 to 120 days of age. There were equal numbers of male and female mice per group. PKD does not differ significantly between male and female C57BL/6 Pkd1RC/RC mice (24). Sirolimus was obtained from Sigma-Aldrich (St. Louis, MO). Torin2 was a gift from the developer of the compound, Dr. Nathanael Gray (Dana Farber Cancer Institute, Harvard Medical School, Boston, MA).
Dosage of sirolimus and torin2.
In our previous study (61), Pkd2−/− mice treated with sirolimus (0.5 mg·kg−1·day−1) had a trough blood level of 20–22 ng/ml and a 51% decrease in the cyst index. Higher doses of sirolimus (5 mg·kg−1·day−1) resulted in a similar decrease in the cyst index in Pkd1−/− mice (46). The therapeutic blood level of sirolimus in humans is 5–15 ng/ml. In view of the sirolimus trough levels of 20–22 ng/ml with the 0.5 mg·kg−1·day−1 treatment, it is unlikely that a higher dose of sirolimus would mediate a more therapeutic effect on PKD.
In addition, a dose experiment was performed for sirolimus and torin2 in male C57BL/6 mice at 70 days of age (see Supplemental Fig. S2; all Supplemental Data for this article can be found at https://doi.org/10.6084/m9.figshare.7949153.v1). Mice were treated with vehicle (28% DMSO in polyethylene glycol 300), sirolimus (0.1, 0.5, or 1 mg/kg), or torin2 (0.1, 1, or 10 mg/kg) on the day before they were euthanized. After 24 h, mice received a second dose of the indicated treatment and were euthanized within 1 h. Kidney tissue was analyzed for mTORC1 and mTORC2 substrates. A 0.5 mg·kg−1·day−1 dose of sirolimus was used for long-term treatment based on the virtually complete inhibition of the mTORC1 substrate phosphorylated S6 (pS6) by sirolimus at 0.5 mg·kg−1·day−1 (Supplemental Fig. S2A). A 10 mg·kg−1·day−1 dose of torin2 was chosen for long-term treatment based on the virtually complete inhibition of mTORC1 and mTORC2 substrates: pS6, p4E-BP1 (Thr37/46), and phosphorylated Akt (pAkt) (Supplemental Fig. S2B).
Measurement of kidney function.
Blood urea nitrogen was measured with a urea assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions (DIUR-100). Serum creatinine was measured by HPLC-tandem mass spectrometry. 2H3 and creatinine were detected in multiple reaction-monitoring mode, examining transitions of m/z from 114 to 44.2 and from 117 to 47.2, respectively.
Immunoblot analysis.
Protein was isolated from cells and tissues using RIPA buffer and cOmplete protease and phoSTOP phosphatase inhibitor cocktails (Sigma). Homogenates were centrifuged, and the supernatant was obtained for protein quantification by DC protein assay (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Samples were mixed with Laemmli sample buffer, boiled for 5 min, and run on 4–20% precast polyacrylamide gels. Proteins were then transferred to 0.45-µm PVDF membranes, blocked with 2.5% evaporated milk, and probed with the antibodies provided in Supplemental Table S1. The specificity of each of the antibodies has been validated by the vendor (Cell Signaling Technology, Danvers, MA) and cited in previous publications (4, 6, 12, 18, 37, 38, 61). Blots were developed by chemiluminescence and analyzed for densitometry using ImageJ.
Routine histology.
Tissues were fixed overnight in 10% formalin at 4°C, transferred to fresh 70% ethanol, and left overnight at 4°C; this process was carried out a total of three times. Next, the tissues were processed and embedded in paraffin wax using Leica systems. Tissues were sectioned at 4 µm and baked at 60°C for 2 h. Kidneys were stained with hematoxylin and eosin, and the cystic index and number of cysts were quantified on 2 sections/mouse (left and right kidneys) using the NIS-Elements macro, as previously described (24). The cyst index and number of cysts were expressed as percentages of the cross-sectional area and numbers per cross-sectional area, respectively. Areas with tissue tears and bubbles that were identified with higher magnification (×40) were excluded from analysis.
Immunohistochemistry protocol.
After tissue sections were deparaffinized and rehydrated, antigen unmasking was performed in sodium citrate buffer (pH 6.0) for 25 min at 100°C. Sections were rinsed for 10 min in cold tap water, immersed in 3% hydrogen peroxide for 10 min, and then rinsed in deionized water for 5 min to block endogenous peroxidase activity. Blocking was performed using Vectastain Elite ABC kit blocking serum for 30 min at room temperature. Primary antibodies were diluted in Tris-buffered saline with Tween 20, as described in Supplemental Table S1, and incubated overnight at 4°C in a humidified chamber. Immunoreactions were detected using the Vectastain standard protocol with 3,3′-diaminobenzidine tetrahydrochloride hydrate (DAB) counterstained with hematoxylin. Slides were subsequently dipped one to three times in 0.3% acid alcohol, dehydrated, and mounted. DAB-positive staining was analyzed using macros provided by Aperio ImageScope.
TUNEL protocol.
TUNEL was performed on tissue sections using a DeadEnd colorimetric apoptosis detection system kit (Promega) according to the manufacturer’s instructions.
Quantitation of immunohistochemistry staining.
The number of positive-staining cells was counted using the Aperio ImageScope (Leica Biosystems) by an observer blinded to the treatment modality. Noncystic tubules were defined as <50-µm-diameter tubules; 15–20 fields of view (×40 magnification) devoid of cysts in the cortex per sample were randomly selected for noncystic quantitation. To avoid sensitivity and selection artifacts between noncystic and dilated, possibly precystic, tubules as well as potential changes in the tubular epithelium lining massive cysts, positive staining was counted in ~75- to 200-µm-diameter cysts. Fifty to seventy-five cortical cysts per tissue section were randomly selected for histological analysis.
Statistical analysis.
Values are means ± SE. Data sets were analyzed by a nonparametric unpaired Mann-Whitney test. Multiple group comparisons were performed using ANOVA with a Newman-Keuls post test. Single group comparisons were made using a Student’s t-test. P values of <0.05 were considered statistically significant.
RESULTS
Dose response of sirolimus versus torin2 on mTORC1 and mTORC2 in vitro.
PKD1−/− (WT 9-12) cells were treated with logarithmic doses of sirolimus and torin2, resulting in a marked decrease in phosphorylation of mTORC1 and mTORC2 substrates (Supplemental Fig. S1). Sirolimus at 10, 100, and 1,000 nM decreased pS6 and p4E-BP1 (Thr37/46) and had no effect on pAkt (Ser473) in PKD1−/− cells (Supplemental Fig. S1A). Torin2 at 1, 10, 100, and 1,000 nM significantly decreased pS6. Phosphorylation of 4E-BP1 (Thr37/46) was nearly eliminated at 10, 100, and 1,000 nM torin2; however, because of decreased total 4E-BP1 abundance, only 10 nM torin2 trended in the decreased p4E-BP1-to-total 4E-BP1 ratio (P = 0.05; Supplemental Fig. S1). The ratio of pAkt (Ser473) to total Akt in PKD1−/− cells was decreased with 100 nM torin2 (Supplemental Fig. S1). Based on the dose escalation, a dose of sirolimus mediating mTORC1 inhibition (10 nM) and a dose of torin2 mediating inhibition of both mTORC1 and mTORC2 (100 nM) were chosen for further study in PKD1+/+ and PKD1−/− cells.
Decreased phosphorylation of mTORC1 and mTORC2 substrates as well as decreased metabolic activity in vitro with sirolimus and torin2 treatment.
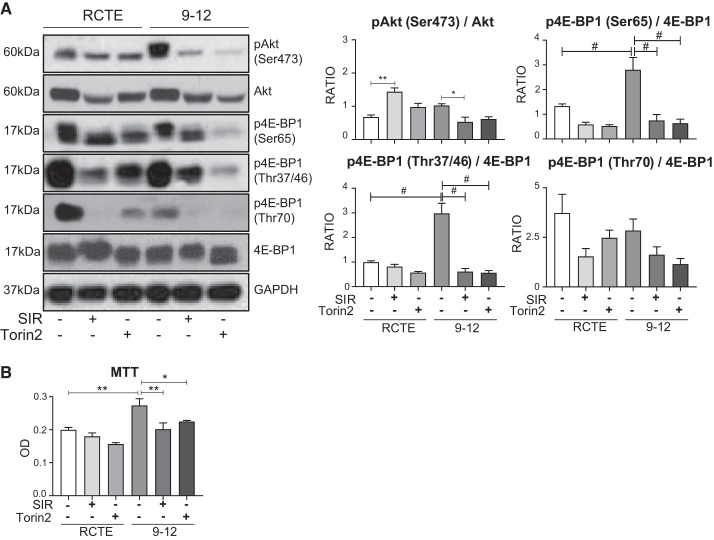
Binding of 4E-BP1 to the translation initiator eIF4E is reversible (18). Hypophosphorylated 4E-BP1 binds avidly to eIF4E; 4E-BP1 hyperphosphorylation retards this interaction. mTOR signaling is the major factor in release of 4E-BP1 from eIF4E (18) and, thus, promotes translation and proliferation. Therefore, we characterized three phosphorylated isoforms of 4E-BP1 most associated with the initial phospho-priming (18) and subsequent release of eIF4E (17), namely, Thr37/46, Thr70, and Ser65, in PKD1+/+ (RCTE) and PKD1−/− (WT 9-12) cells exposed to sirolimus and torin2. Relative to total 4E-BP1 abundance, the Thr37/46 and Ser65 phosphorylated isoforms were elevated uniquely in PKD1−/− compared with PKD1+/+ cells. Sirolimus resulted in a significant decrease in p4E-BP1 (Ser65 and Thr37/46) in PKD1−/− cells (Fig. 1A). Similarly, torin2 resulted in a significant decrease in p4E-BP1 (Ser65 and Thr37/46) in PKD1−/− cells (Fig. 1A). Sirolimus decreased the mTORC2 substrate Akt in PKD1+/+ cells but increased the ratio of pAkt to total Akt (Fig. 1A). In this regard, sirolimus-dependent inhibition of mTORC1 has been shown to increase phosphorylation of Akt (Ser473) (27, 36). Sirolimus significantly decreased phosphorylation of Akt (Ser473) in PKD1−/− cells (Fig. 1A). Finally, we assessed any possible suppressive metabolic effect of sirolimus and torin2 in PKD1−/− (WT 9-12) and PKD1+/+ (RCTE) cells by MTT assay (10, 48). PKD1−/− cells exhibited significantly higher metabolic activity than PKD1+/+ cells (Fig. 1B). Both sirolimus and torin2 decreased metabolic activity, as detected by MTT assay of PKD1−/− cells (Fig. 1B). Sirolimus inhibited phosphorylation of 4E-BP1 (Thr37/46 and Ser65) and phosphorylation of Akt (Ser473), a marker of mTORC2 activation, in PKD1−/− cells (Fig. 1A). From these data, we hypothesized that sirolimus and torin2 may exhibit anticystic effects in vivo, mediated through a combination of suppression of both mTORC1 and mTORC2 signaling and a decrease in metabolic activity in cystic renal epithelial cells.
Fig. 1.
Effect of sirolimus (SIR) versus torin2 on mechanistic target of rapamycin complex (mTORC)1 [phosphorylated eukaryotic translation initiation factor 4E-binding protein 1 (p4E-BP1)] and mTORC2 [phosphorylated Akt (pAkt) (Ser473)] in PKD1−/− cells. A: PKD1+/+ [renal cortical tubular epithelium (RCTE)] and PKD1−/− (WT 9-12) cells were treated with sirolimus (10 nM) or torin2 (100 nM), and the effect on substrates of mTORC1 signaling [p4E-BP1 (Ser65, Thr37/46, and Thr70)] and mTORC2 [pAkt (Ser473)] was determined by immunoblot analysis. Values of the ratio of total to phosphorylated protein abundance are means ± SE; n = 3 per group in triplicate. B: MTT assay of PKD1+/+ and PKD1−/− cells treated with sirolimus or torin2. OD, optical density (590 nm). Values are means ± SE; n = 4 per group, in triplicate. *P < 0.05, **P < 0.01, and #P < 0.0001 (by ANOVA with a Newman-Keuls post test).
Both sirolimus and torin2 decreased cyst burden and protected kidney function in Pkd1RC/RC mice.
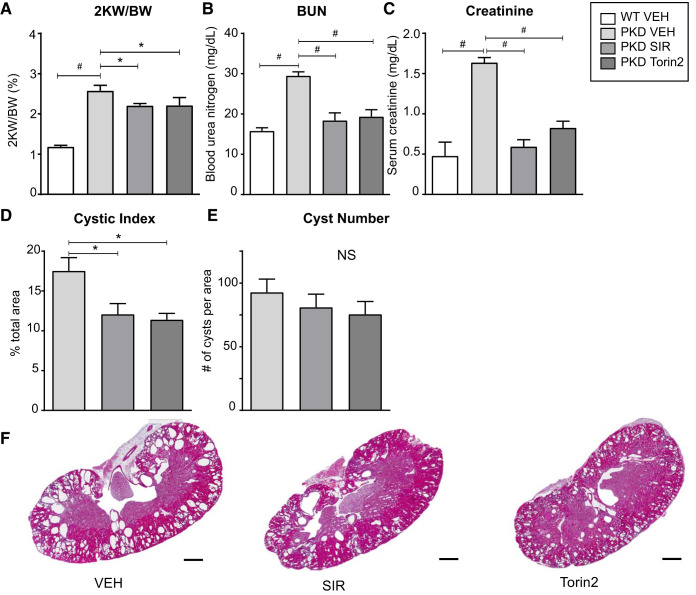
At 50 days of age, mice were treated with vehicle, sirolimus (0.5 mg·kg−1·day−1), or torin2 (10 mg·kg−1·day−1) for 70 weekdays. No side effects of treatment were noted on gross examination in either treatment group. Both sirolimus and torin2 resulted in a significant decrease in the kidney weight-to-body weight ratio (Fig. 2A), a significant improvement in kidney function, as measured by serum blood urea nitrogen and creatinine (Fig. 2, B and C), and a significant decrease in the cyst index (Fig. 2D), with no significant difference in efficacy between the two treatment approaches. Although the number of cysts was decreased, there was no significant reduction in the total number of cysts in PKD kidneys (Fig. 2E). Representative hematoxylin and eosin-stained sections showed less cystic area in both sirolimus- and torin2-treated mice (Fig. 2F). Thus, both sirolimus and torin2 resulted in an equivalent decrease in cyst burden and improvement of kidney function in Pkd1RC/RC mice, with no side effects on gross examination.
Fig. 2.
Effect of sirolimus (SIR) versus torin2 on cyst burden and kidney function in Pkd1RC/RC mice. A: 2-kidney weight-to-body weight ratio (2KW/BW) in wild-type (WT) versus Pkd1RC/RC (PKD) mice treated with vehicle (VEH), torin2, or sirolimus. B: blood urea nitrogen (BUN). C: serum creatinine. D: cystic index (percentage of cross-sectional area). E: cyst number (per cross-sectional area). Values are means ± SE; n = 4–5 per group. *P < 0.05 and #P < 0.0001 (by ANOVA with a Newman-Keuls post test). NS, not significant. F: representative hematoxylin and eosin-stained sections. Scale bar = 1 mm.
Both sirolimus and torin2 decreased proliferation and increased apoptosis in Pkd1RC/RC kidneys.
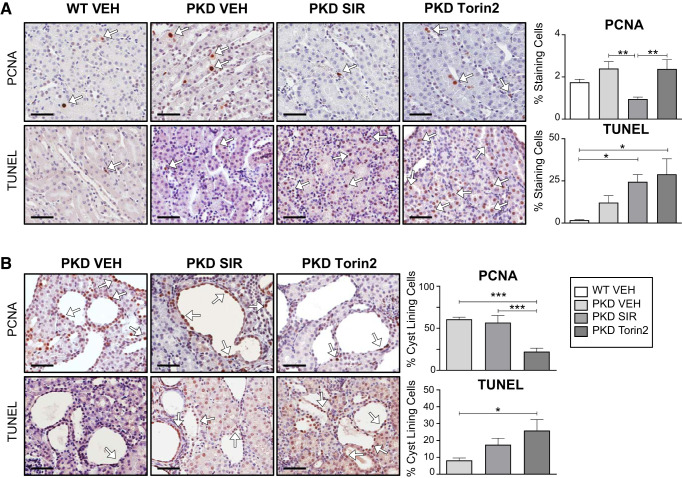
Proliferating cell nuclear antigen (PCNA) staining, a marker of proliferation, and TUNEL staining, a marker of apoptosis, were measured in noncystic cortical renal tubules and in the cyst-lining epithelium of PKD kidneys. PCNA staining in noncystic tubules was modestly increased in PKD compared with wild-type kidneys (Fig. 3A). Sirolimus, but not torin2, resulted in a significant decrease in PCNA-positive nuclei in noncystic areas of PKD kidneys (Fig. 3A). The increase in TUNEL staining in noncystic tubules in PKD kidneys compared with wild-type control kidneys did not reach statistical significance (Fig. 3A). In the cystic epithelium of PKD kidneys, torin2 resulted in a significant decrease in PCNA-positive nuclei compared with vehicle and sirolimus (Fig. 3B). In cells lining the cysts, torin2 significantly increased apoptosis (TUNEL staining) compared with vehicle (Fig. 3B).
Fig. 3.
Effect of sirolimus (SIR) versus torin2 on apoptosis (TUNEL) and proliferation [proliferating cell nuclear antigen (PCNA)]. A: PCNA and TUNEL staining in noncystic tubules from wild-type (WT) and Pkd1RC/RC [polycystic kidney disease (PKD)] kidneys, expressed as the percentage of cells staining positive per 240-µm2 area (%staining cells). B: PCNA and TUNEL staining in cells lining the cysts in PKD kidneys, expressed as the percentage of cells lining the cyst staining positive per cyst (%cyst-lining cells). White arrows indicate 3,3′-diaminobenzidine tetrahydrochloride hydrate-positive cells. VEH, vehicle. Scale bars = 50 µm. Values are means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.0001 (by ANOVA with a Newman-Keuls post test).
Both sirolimus and torin2 decreased mTORC1 and mTORC2 phosphorylated substrates in Pkd1RC/RC kidneys.
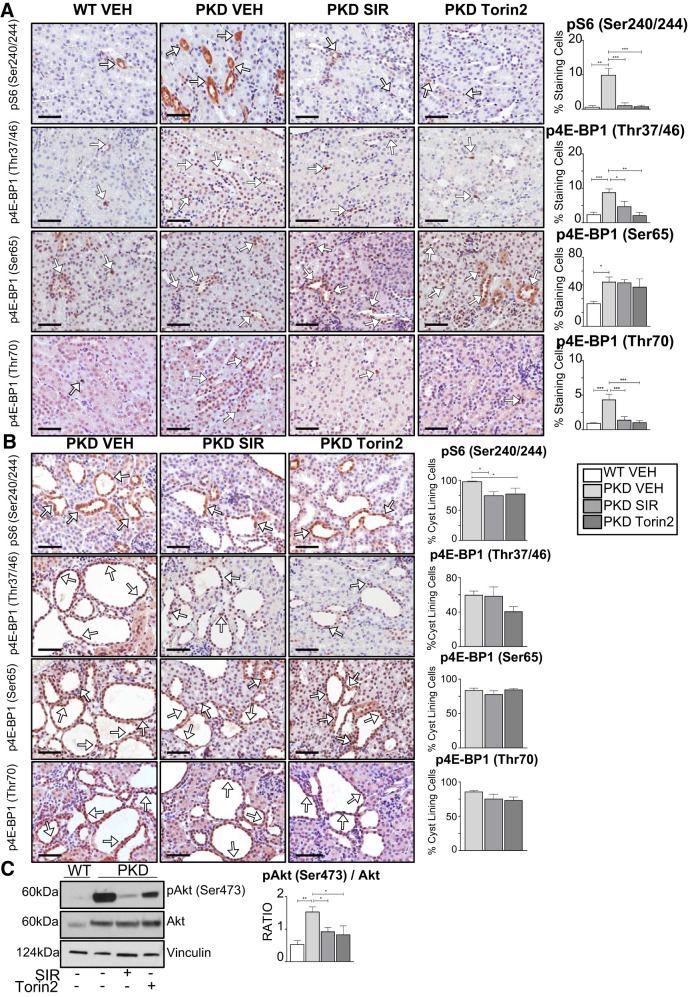
Increased mTOR substrates 4E-BP1 and S6 have been associated with worsening prognosis in oncology. For example, high 4E-BP1 and S6 protein levels predict reduced benefit from treatment, poor prognosis, and endocrine resistance in breast cancer (23). For this reason, we performed immunohistochemical analysis of noncystic tubules in PKD kidneys and cells lining the cysts for p4E-BP1 isoforms and pS6. There was an increase in pS6 and p4E-BP1 (Thr37/46 and Thr70) staining in noncystic areas of PKD compared with wild-type kidneys and in cells lining kidney cysts (Fig. 4, A and B). The increase in pS6 and p4E-BP1 (Thr37/46 and Thr70) staining in noncystic areas of PKD kidneys was decreased by both sirolimus and torin2 (Fig. 4A). There was an increase in p4E-BP1 (Ser65) staining in noncystic areas of PKD kidneys compared with wild-type control kidneys (P = 0.05). Staining of p4E-BP1 (Thr37/46, Ser65, and Thr70) in cells lining kidney cysts was not significantly affected by sirolimus or torin2 (Fig. 4B). Finally, immunoblot analysis of whole kidney homogenates revealed that pAkt (Ser473) was significantly increased in PKD compared with wild-type kidneys and significantly decreased in PKD kidneys treated with both sirolimus and torin2 (Fig. 4C). These data show, for the first time, that staining of p4E-BP1 (Thr37/46, Ser65, and Thr70) was increased in noncystic tubules in PKD kidneys and present in cells lining the cysts and was sensitive to mTORC1 and mTORC2 inhibition in vivo.
Fig. 4.
Effect of sirolimus (SIR) versus torin2 on mechanistic target of rapamycin complex (mTORC)1 [phosphorylated S6 (pS6) and phosphorylated eukaryotic translation initiation factor 4E-binding protein 1 (p4E-BP1)] and mTORC2 [phosphorylated Akt (pAkt) (Ser473)] in Pkd1RC/RC kidneys. The effect of sirolimus or torin2 treatment on pS6 and p4E-BP1 (Thr37/46, Ser65, and Thr70) was determined in kidneys from mice with polycystic kidney disease (Pkd1RC/RC) and wild-type (WT) mice by immunohistochemical analysis. A: pS6 (Ser240/244) and p4E-BP1 (Thr37/46, Ser65, and Thr70) staining in noncystic tubules, expressed as the percentage of cells staining positive per 240-µm2 area (%staining cells). White arrows indicate 3,3′-diaminobenzidine tetrahydrochloride hydrate-positive cells. Scale bars = 50 µm. B: pS6 (Ser240/244) and p4E-BP1 (Thr37/46, Ser65, and Thr70) staining in cyst-lining cells, expressed as the percentage of positive-staining cells per cyst (%cyst-lining cells). White arrows indicate 3,3′-diaminobenzidine tetrahydrochloride hydrate-positive cells. Scale bars = 50 µm. C: pAkt (Ser473) Akt sensitivity to torin2 and sirolimus in Pkd1RC/RC kidneys. The ratio of pAkt to total Akt was quantitated by densitometry. Values are means ± SE; n = 4–5 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 (by ANOVA with a Newman-Keuls post test).
DISCUSSION
mTORC1 is associated with cell proliferation and protein synthesis via phosphoregulation of the downstream substrates phosphorylated 70-kDa S6 kinase (and, in turn, S6) and 4E-BP1. 4E-BP1 has three main phosphorylated isoforms (18) resulting in increased translation and proliferation. Sirolimus is only a partial inhibitor of mTORC1 in most cell types (16, 47, 53, 54), and phosphorylation of 4E-BP1, especially in cancer studies, has been shown to be sirolimus resistant but sensitive to TORKi (8, 52). mTORC2 directly phosphorylates Akt at Ser473, and a reduction in Rictor expression inhibits phosphorylation of Akt (Ser473). Phosphoregulation of Akt (Ser473) plays a crucial role in proliferation, cell survival, and growth. Both mTORC1 and mTORC2 contain a mTOR kinase that is inhibited by TORKi. Torin2 is a second-generation ATP-competitive active site inhibitor that is both potent and highly selective for mTOR kinase, exhibiting a superior pharmacokinetic profile compared with previous TORKi (63). In support of the use of combined mTORC1 and mTORC2 inhibition in PKD, our previously published data demonstrated that a mTOR antisense oligonucleotide blocks both mTORC1 and mTORC2 decreases in cyst burden and improves kidney function in Pkd2−/− mice (37). Whether p4E-BP1 and pAkt (Ser473) are resistant to sirolimus in kidney cells and in the kidney is not known. Also, the effects of a TORKi versus sirolimus on p4E-BP1 isoforms and pAkt (Ser473) in kidney cells and in the kidney are not known. The major aims of the present study were to determine the effect of sirolimus versus a TORKi on cyst burden and kidney function and to characterize mTORC1 and mTORC2 signaling in in vitro and in vivo Pkd1 models.
First, mTORC1 and mTORC2 signaling and the effect of mTOR inhibition were determined in immortalized human cells from the normal RCTE and ADPKD cyst-lining epithelium (31). Metabolism was increased in PKD1−/− compared with PKD1+/+ cells, as determined by the MTT assay. Recent studies have shown that defective glucose metabolism might be a hallmark of ADPKD, offering a new opportunity for therapy. Metabolomic profiling using nuclear magnetic resonance of the extracellular medium of wild-type and Pkd1−/− cells revealed that Pkd1−/− cells consume high levels of glucose and preferentially use it in aerobic glycolysis for energy production (40). These data demonstrating that Pkd1−/− cells consume high levels of glucose and preferentially use it in aerobic glycolysis are in line with an upregulation of mTORC1 in ADPKD, as this complex regulates transcriptional programs involved in energy metabolism (33, 43). mTORC1 inhibition with sirolimus benefits glucose metabolism in five mouse models of type 2 diabetes (39), reducing weight gain, improving insulin sensitivity, and reducing hyperinsulinemia. The literature is lacking as to the glucose metabolic effects of a TORKi in diabetes or ADPKD. Thus, in wild-type and PKD1−/− cells, we compared the effects of sirolimus and a TORKi on NAD(P)H-dependent cellular oxidoreductase activity (a direct measurement of MTT assay) and metabolic activity (an indirect measurement of MTT assay) (48). The increase in metabolism in PKD1−/− cells was associated with an increase in p4E-BP1 (Ser65 and Thr37/46). Both sirolimus and torin2 caused a reduction in metabolic rates in PKD1−/− cells that was associated with decreased phosphorylation of 4E-BP1 at Ser65 and Thr37/46 but not at Thr70. Sirolimus decreased phosphorylation of Akt, a marker of mTORC2 in PKD1−/− cells. Thus, in human PKD1−/− cells, it is likely that metabolism is mediated by additional mTORC1-independent proproliferative pathways, as sirolimus is able to inhibit phosphorylation of 4E-BP1 and phosphorylation of Akt (Ser473) (mTORC2). Also, in vitro models may have inherent artifacts of immortalization and, therefore, may not reflect an effect on the mTORC1/2 system in an in vivo model of PKD.
Human studies of sirolimus in PKD were plagued by side effects, especially mucositis (45, 58). In animal studies, the side effect profile of a TORKi was better than that of rapalogs, perhaps due to a lesser immunosuppressive effect (no bone marrow suppression and no effect on T and B cell proliferation) (2, 7, 9, 25). Human safety and tolerability studies of TORKi have shown that the side effect profile is different from that of rapalogs and may be milder (3, 35). Thus, based on the data that a TORKi can inhibit both mTORC1 and mTORC2, including 4E-BP1, and are well tolerated in preclinical and clinical studies, a study of the TORKi torin2 versus sirolimus was performed in Pkd1RC/RC mice. Both torin2 and sirolimus mediated similar effects in terms of reducing kidney cyst index and maintaining kidney function, despite potentially different specificities/mechanisms of action of the two drugs. Many signaling pathways, in addition to mTOR, are involved in ADPKD (20, 41). However, both drugs effectively target pS6 in noncystic and cystic tubules, and both drugs target p4E-BP1 (Thr37/46 and Thr70) in noncystic tubules. Thus, although torin2 and sirolimus have different specificities and mechanisms of action for the effective inhibition of mTOR signaling, they do not specifically target the other pathways dysregulated in PKD (28). Regardless, the possibility that TORKi treatment may have fewer side effects than sirolimus in patients with ADPKD is encouraging.
Sirolimus decreased PCNA staining in noncystic tubules in PKD kidneys, whereas torin2 decreased PCNA staining in cystic tubules. As the mechanism for torin2 and sirolimus cellular uptake is ill defined, it is plausible that cellular uptake for sirolimus versus torin2 may differ between cystic and noncystic tubules. Human and experimental data provide strong evidence that abnormal proliferation and apoptosis in tubular epithelial cells play a crucial role in cyst development and/or growth in PKD (59). Thus, both sirolimus and torin2 likely decreased cyst burden in Pkd1RC/RC mice, at least in part, by retarding cystic and noncystic cortical tubular cell proliferation.
S6 ribosomal protein is the main mTORC1 proproliferative substrate that is inhibited by sirolimus. In the present study, pS6 was increased in PKD compared with wild-type kidneys, markedly inhibited by both sirolimus and torin2 in noncystic areas of PKD kidneys, and inhibited to a lesser extent in cyst-lining epithelial cells. These data suggest that both sirolimus and torin2 may decrease proliferation in PKD kidneys via inhibition of pS6.
The role of apoptosis in cyst growth is controversial (14). Pharmacological (50) or genetic (51) inhibition of apoptosis results in less cyst growth. However, induction of apoptosis in two Pkd1−/− models also resulted in stunted cyst growth (15). Protection against PKD with high-dose sirolimus (1.67 or 5 mg·kg−1·day−1) was associated with increased apoptosis (46). Our previously published data showed that sirolimus at a much lower dose, 0.5 mg·kg−1·day−1, had no effect on cleaved caspase-3 or apoptosis in Pkd2−/− mice (61) and female Cy/+ rats (6). In the present study, torin2 resulted in increased apoptosis in cells lining the cysts. These data suggest that therapeutic effects of torin2 on PKD may be related in part to increased apoptosis in addition to decreased proliferation. While both inhibitors had effects on proliferation and apoptosis and decreased cystic index, neither significantly decreased the total number of cysts, suggesting that these inhibitors may act by preventing cyst expansion driven by proliferation and apoptosis more so than de novo cystogenesis.
4E-BP1 inhibits cap-dependent translation by binding to the translation initiation factor eIF4E. Hyperphosphorylation of 4E-BP1 disrupts this interaction and results in the activation of cap-dependent translation. Phosphorylation of 4E-BP1 at Thr37 and Thr46 does not prevent the binding of 4E-BP1 to eIF4E, but it does prime 4E-BP1 for subsequent phosphorylation at Ser65 and Thr70. PKD rat models (Cy/+) (60) and renal tissues from both patients with autosomal recessive PKD (5, 32) or ADPKD exhibit an increase in 4E-BP1 proteins. It has been shown that p4E-BP1 is increased in the kidney of a nonorthologous PKD mouse model, transmembrane protein 67 knockout mice (13), and in Pkd1−/− mouse embryonic fibroblasts (11). We aimed to characterize the sensitivity of p4E-BP1 isoforms to mTORC1 and mTORC2 inhibition in a hypomorphic Pkd1RC/RC in vivo model. Immunohistochemical analysis revealed increased p4E-BP1 (Thr37/46 and Thr70) in Pkd1RC/RC compared with wild-type kidneys. Both sirolimus and torin2 decreased p4E-BP1 (Thr37/46 and Thr70) staining of noncystic tubular cells of PKD kidneys. These data suggest that sirolimus is as effective as torin2 at reducing 4E-BP1 phosphorylation events in Pkd1RC/RC kidneys.
mTORC2 directly phosphorylates Akt on Ser473, and a reduction in Rictor expression inhibits phosphorylation of Akt (Ser473). Phosphoregulation of Akt (Ser473) plays a crucial role in proliferation, cell survival, and growth. Knockout of mTORC2 does not affect mTORC1, suggesting that mTORC2 can function independently of mTORC1, and, as such, can activate a pool of Akt that is not upstream of mTORC1. We previously demonstrated that pAkt (Ser473) increased in the kidney in the Han:SPRD (Cy/+) rat model and Pkd2−/− mouse model of ADPKD (6, 37, 38). We have previously demonstrated that silencing of Rictor in Madin-Darby canine kidney cells results in a decrease in pAkt (Ser473) and a decrease in cyst size that is reversed by the introduction of constitutively active Akt (37). In the present study, in vitro, the increase in the ratio of pAkt (Ser473) to total Akt in PKD1−/− compared with PKD1+/+ cells did not reach statistical significance. However, our immunoblot analysis showed an increase in pAkt (Ser473) in Pkd1RC/RC compared with wild-type kidneys. Both sirolimus and torin2 decreased phosphorylation of Akt (Ser473) in Pkd1RC/RC kidneys. Sirolimus does not usually directly target mTORC2 or downstream substrates (16, 47); however, prolonged sirolimus treatment has been shown to inhibit mTORC2 assembly (42). Similarly, long-term administration of sirolimus can inhibit mTORC2 in some, but not all, in vitro and in vivo systems (44). In the present study, in Pkd1RC/RC kidneys, long-term (70 days) treatment with sirolimus was able to inhibit pAkt (Ser473), a marker of mTORC2 activation.
Here, we report a study of the TORKi torin2 and sirolimus in PKD. One of the limitations of the study is the much shorter half-life (~1 h) of torin2 than sirolimus (63 h) (26, 28). As the half-life is the time required for half of a compound to be removed by biological processes, it is possible that the amount of functional torin2 present to inhibit mTOR signaling and, thus, impact PKD burden chronically may have been suboptimal compared with sirolimus. In an attempt to compensate for its short half-life, torin2 was administered at the highest tolerable dose, 10 mg/kg, once daily on weekdays. It is possible that the dose of torin2 administered in a slower or more stable format, e.g., subcutaneous minipump, slow-release formulations, or compound manipulation to increase stability, will have a stronger therapeutic effect.
In summary, we report the first study of 4E-BP1 in a hypomorphic Pkd1RC/RC mouse model, orthologous to human PKD, as well as a study of sirolimus versus a TORKi in PKD. Contrary to our hypothesis that torin2 would have a more potent antiproliferative effect than sirolimus by additional inhibition of both 4E-BP1 and mTORC2, we found that both drugs mediated comparable therapeutic effects, such as decreased cyst growth and improved kidney function, and both drugs increased apoptosis, decreased mTORC1 and mTORC2 signaling, and suppressed proliferation in PKD kidneys.
GRANTS
This work was supported by a Department of Veterans Affairs Merit Award under Grant BX003803-01A1 (to C. L. Edelstein), Department of Defense Grant W81XWH-16-1-0172 (to C. L. Edelstein), and a PKD Foundation Fellowship Award (to S. J. Holditch).
DISCLOSURES
C. L. Edelstein is on the Data Safety Monitoring Board for Conatus Pharmaceuticals.
AUTHOR CONTRIBUTIONS
S.J.H., C.N.B., and C.L.E. conceived and designed research; S.J.H., C.N.B., D.J.A., A.M.L., K.N.N., and H.W.T. performed experiments; S.J.H., C.N.B., D.J.A., A.M.L., and K.N.N. analyzed data; S.J.H., C.N.B., K.H., and C.L.E. interpreted results of experiments; S.J.H., C.N.B., D.J.A., and A.M.L. prepared figures; S.J.H., C.N.B., and C.L.E. drafted manuscript; S.J.H., C.N.B., D.J.A., K.H., and C.L.E. edited and revised manuscript; S.J.H., C.N.B., K.H., and C.L.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Nathanael Gray for generously providing the torin2 compound.
REFERENCES
- 1.Aguiari G, Varani K, Bogo M, Mangolini A, Vincenzi F, Durante C, Gessi S, Sacchetto V, Catizone L, Harris P, Rizzuto R, Borea PA, Del Senno L. Deficiency of polycystic kidney disease-1 gene (PKD1) expression increases A3 adenosine receptors in human renal cells: implications for cAMP-dependent signalling and proliferation of PKD1-mutated cystic cells. Biochim Biophys Acta 1792: 531–540, 2009. doi: 10.1016/j.bbadis.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Hussain AR, Bavi P, Ahmed SO, Al Sobhi SS, Al-Dayel F, Uddin S, Al-Kuraya KS. High prevalence of mTOR complex activity can be targeted using Torin2 in papillary thyroid carcinoma. Carcinogenesis 35: 1564–1572, 2014. doi: 10.1093/carcin/bgu051. [DOI] [PubMed] [Google Scholar]
- 3.Asahina H, Nokihara H, Yamamoto N, Yamada Y, Tamura Y, Honda K, Seki Y, Tanabe Y, Shimada H, Shi X, Tamura T. Safety and tolerability of AZD8055 in Japanese patients with advanced solid tumors; a dose-finding phase I study. Invest New Drugs 31: 677–684, 2013. doi: 10.1007/s10637-012-9860-4. [DOI] [PubMed] [Google Scholar]
- 4.Ayuso MI, Hernández-Jiménez M, Martín ME, Salinas M, Alcázar A. New hierarchical phosphorylation pathway of the translational repressor eIF4E-binding protein 1 (4E-BP1) in ischemia-reperfusion stress. J Biol Chem 285: 34355–34363, 2010. doi: 10.1074/jbc.M110.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker JU, Opazo Saez A, Zerres K, Witzke O, Hoyer PF, Schmid KW, Kribben A, Bergmann C, Nürnberger J. The mTOR pathway is activated in human autosomal-recessive polycystic kidney disease. Kidney Blood Press Res 33: 129–138, 2010. doi: 10.1159/000314380. [DOI] [PubMed] [Google Scholar]
- 6.Belibi F, Ravichandran K, Zafar I, He Z, Edelstein CL. mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol 300: F236–F244, 2011. doi: 10.1152/ajprenal.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry T, Luther W, Bhatnagar N, Jamin Y, Poon E, Sanda T, Pei D, Sharma B, Vetharoy WR, Hallsworth A, Ahmad Z, Barker K, Moreau L, Webber H, Wang W, Liu Q, Perez-Atayde A, Rodig S, Cheung NK, Raynaud F, Hallberg B, Robinson SP, Gray NS, Pearson AD, Eccles SA, Chesler L, George RE. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 22: 117–130, 2012. doi: 10.1016/j.ccr.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Hughes G, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R, Jacobsen M, Smith GC, Guichard S, Pass M. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 70: 288–298, 2010. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 9.Codeluppi S, Fernandez-Zafra T, Sandor K, Kjell J, Liu Q, Abrams M, Olson L, Gray NS, Svensson CI, Uhlén P. Interleukin-6 secretion by astrocytes is dynamically regulated by PI3K-mTOR-calcium signaling. PLoS One 9: e92649, 2014. doi: 10.1371/journal.pone.0092649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dias N, Nicolau A, Carvalho GS, Mota M, Lima N. Miniaturization and application of the MTT assay to evaluate metabolic activity of protozoa in the presence of toxicants. J Basic Microbiol 39: 103–108, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Distefano G, Boca M, Rowe I, Wodarczyk C, Ma L, Piontek KB, Germino GG, Pandolfi PP, Boletta A. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol 29: 2359–2371, 2009. doi: 10.1128/MCB.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328: 1172–1176, 2010. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du E, Li H, Jin S, Hu X, Qiu M, Han R. Evidence that TMEM67 causes polycystic kidney disease through activation of JNK/ERK-dependent pathways. Cell Biol Int 37: 694–702, 2013. doi: 10.1002/cbin.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein CL. What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle 4: 1550–1554, 2005. doi: 10.4161/cc.4.11.2185. [DOI] [PubMed] [Google Scholar]
- 15.Fan LX, Zhou X, Sweeney WE Jr, Wallace DP, Avner ED, Grantham JJ, Li X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J Am Soc Nephrol 24: 2010–2022, 2013. doi: 10.1681/ASN.2013020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7: e1000038, 2009. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev 13: 1422–1437, 1999. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15: 2852–2864, 2001. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol 26: 39–47, 2015. doi: 10.1681/ASN.2013121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson E, Pérez-Tenorio G, Amin R, Bostner J, Skoog L, Fornander T, Sgroi DC, Nordenskjöld B, Hallbeck AL, Stål O. The mTOR effectors 4EBP1 and S6K2 are frequently coexpressed and associated with a poor prognosis and endocrine resistance in breast cancer: a retrospective study including patients from the randomised Stockholm tamoxifen trials. Breast Cancer Res 15: R96, 2013. doi: 10.1186/bcr3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleczko EK, Marsh KH, Tyler LC, Furgeson SB, Bullock BL, Altmann CJ, Miyazaki M, Gitomer BY, Harris PC, Weiser-Evans MCM, Chonchol MB, Clambey ET, Nemenoff RA, Hopp K. CD8+ T cells modulate autosomal dominant polycystic kidney disease progression. Kidney Int 94: 1127–1140, 2018. doi: 10.1016/j.kint.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Lee PS, Sun Y, Gu X, Zhang E, Guo Y, Wu CL, Auricchio N, Priolo C, Li J, Csibi A, Parkhitko A, Morrison T, Planaguma A, Kazani S, Israel E, Xu KF, Henske EP, Blenis J, Levy BD, Kwiatkowski D, Yu JJ. Estradiol and mTORC2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient LAM cells. J Exp Med 211: 15–28, 2014. doi: 10.1084/jem.20131080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, Hur W, Zhang J, Sim T, Sabatini DM, Gray NS. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 53: 7146–7155, 2010. doi: 10.1021/jm101144f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strateg 6: 47–55, 2009. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Xu C, Kirubakaran S, Zhang X, Hur W, Liu Y, Kwiatkowski NP, Wang J, Westover KD, Gao P, Ercan D, Niepel M, Thoreen CC, Kang SA, Patricelli MP, Wang Y, Tupper T, Altabef A, Kawamura H, Held KD, Chou DM, Elledge SJ, Janne PA, Wong KK, Sabatini DM, Gray NS. Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res 73: 2574–2586, 2013. doi: 10.1158/0008-5472.CAN-12-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livingstone M, Bidinosti M. Rapamycin-insensitive mTORC1 activity controls eIF4E:4E-BP1 binding. F1000 Res 1: 4, 2012. doi: 10.12688/f1000research.1-4.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loghman-Adham M, Nauli SM, Soto CE, Kariuki B, Zhou J. Immortalized epithelial cells from human autosomal dominant polycystic kidney cysts. Am J Physiol Renal Physiol 285: F397–F412, 2003. doi: 10.1152/ajprenal.00310.2002. [DOI] [PubMed] [Google Scholar]
- 32.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10: 594–601, 2004. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 33.Mao Z, Zhang W. Role of mTOR in glucose and lipid metabolism. Int J Mol Sci 19: 2043, 2018. doi: 10.3390/ijms19072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moorman NJ, Shenk T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J Virol 84: 5260–5269, 2010. doi: 10.1128/JVI.02733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naing A, Aghajanian C, Raymond E, Olmos D, Schwartz G, Oelmann E, Grinsted L, Burke W, Taylor R, Kaye S, Kurzrock R, Banerji U. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer 107: 1093–1099, 2012. doi: 10.1038/bjc.2012.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: 1500–1508, 2006. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravichandran K, Zafar I, He Z, Doctor RB, Moldovan R, Mullick AE, Edelstein CL. An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2. Hum Mol Genet 23: 4919–4931, 2014. doi: 10.1093/hmg/ddu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravichandran K, Zafar I, Ozkok A, Edelstein CL. An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol Dial Transplant 30: 45–53, 2015. doi: 10.1093/ndt/gfu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reifsnyder PC, Flurkey K, Te A, Harrison DE. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY) 8: 3120–3130, 2016. doi: 10.18632/aging.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013. doi: 10.1038/nm.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saigusa T, Bell PD. Molecular pathways and therapies in autosomal-dominant polycystic kidney disease. Physiology (Bethesda) 30: 195–207, 2015. doi: 10.1152/physiol.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [Erratum in Cell 169: 361–371, 2017. 10.1016/j.cell.2017.03.035. 28388417]. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 14: 265–273, 2015. doi: 10.1111/acel.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 46.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471, 2006. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle 8: 3831–3837, 2009. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 48.Stockert JC, Horobin RW, Colombo LL, Blázquez-Castro A. Tetrazolium salts and formazan products in cell biology: viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem 120: 159–167, 2018. doi: 10.1016/j.acthis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Ta MH, Liuwantara D, Rangan GK. Effects of pyrrolidine dithiocarbamate on proliferation and nuclear factor-κB activity in autosomal dominant polycystic kidney disease cells. BMC Nephrol 16: 212, 2015. doi: 10.1186/s12882-015-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease. Proc Natl Acad Sci USA 102: 6954–6959, 2005. doi: 10.1073/pnas.0408518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao Y, Zafar I, Kim J, Schrier RW, Edelstein CL. Caspase-3 gene deletion prolongs survival in polycystic kidney disease. J Am Soc Nephrol 19: 749–755, 2007. doi: 10.1681/ASN.2006121378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485: 109–113, 2012. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy 5: 725–726, 2009. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 55.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators . Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators . Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017. doi: 10.1056/NEJMoa1710030. [DOI] [PubMed] [Google Scholar]
- 57.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH II. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 58.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 59.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 60.Wu M, Arcaro A, Varga Z, Vogetseder A, Le Hir M, Wüthrich RP, Serra AL. Pulse mTOR inhibitor treatment effectively controls cyst growth but leads to severe parenchymal and glomerular hypertrophy in rat polycystic kidney disease. Am J Physiol Renal Physiol 297: F1597–F1605, 2009. doi: 10.1152/ajprenal.00430.2009. [DOI] [PubMed] [Google Scholar]
- 61.Zafar I, Ravichandran K, Belibi FA, Doctor RB, Edelstein CL. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int 78: 754–761, 2010. doi: 10.1038/ki.2010.250. [DOI] [PubMed] [Google Scholar]