Abstract
Humanin is a small regulatory peptide encoded within the 16S ribosomal RNA gene (MT-RNR2) of the mitochondrial genome that has cellular cyto- and metabolo-protective properties similar to that of aerobic exercise training. Here we investigated whether acute high-intensity interval exercise or short-term high-intensity interval training (HIIT) impacted skeletal muscle and plasma humanin levels. Vastus lateralis muscle biopsies and plasma samples were collected from young healthy untrained men (n = 10, 24.5 ± 3.7 yr) before, immediately following, and 4 h following the completion of 10 × 60 s cycle ergometer bouts at V̇o2peak power output (untrained). Resting and postexercise sampling was also performed after six HIIT sessions (trained) completed over 2 wk. Humanin protein abundance in muscle and plasma were increased following an acute high-intensity exercise bout. HIIT trended (P = 0.063) to lower absolute humanin plasma levels, without effecting the response in muscle or plasma to acute exercise. A similar response in the plasma was observed for the small humanin-like peptide 6 (SHLP6), but not SHLP2, indicating selective regulation of peptides encoded by MT-RNR2 gene. There was a weak positive correlation between muscle and plasma humanin levels, and contraction of isolated mouse EDL muscle increased humanin levels ~4-fold. The increase in muscle humanin levels with acute exercise was not associated with MT-RNR2 mRNA or humanin mRNA levels (which decreased following acute exercise). Overall, these results suggest that humanin is an exercise-sensitive mitochondrial peptide and acute exercise-induced humanin responses in muscle are nontranscriptionally regulated and may partially contribute to the observed increase in plasma concentrations.
NEW & NOTEWORTHY Small regulatory peptides encoded within the mitochondrial genome (mitochondrial derived peptides) have been shown to have cellular cyto- and metabolo-protective roles that parallel those of exercise. Here we provide evidence that humanin and SHLP6 are exercise-sensitive mitochondrial derived peptides. Studies to determine whether mitochondrial derived peptides play a role in regulating exercise-induced adaptations are warranted.
Keywords: exercise, humanin, mitochondrial derived peptides, mitokine, small humanin-like peptides
INTRODUCTION
Exercise activates cellular signaling processes that, if repeatedly activated, lead to adaptations that enhance the functional capacity of various organs in the body (15, 16). Skeletal muscle is particularly plastic with its adaptive responses being proportional to the nature of the exercise stress. Adaptative stress responses can include, but are not limited to, the induction of muscle fiber hypertrophy, enhanced mitochondrial function, and alterations in substrate utilization and storage (15, 20, 31). Mitochondria are best recognized for supplying ATP to contracting muscle and responding to exercise training by increasing their volume/density (mitochondrial biogenesis) (8). However, the role of mitochondria extends beyond that of energy production to include control over some inflammatory responses, proteostasis, adaptive stress responses, and apoptosis (5, 35). Since mitochondria are sensitive to cellular energy demands and have diverse roles in controlling homeostatic processes, they are well placed to facilitate adaptive signaling in response to exercise stress.
Mitochondria have their own DNA (mtDNA), which contains only 13 protein-coding genes, all of which encode essential subunits of the oxidative phosphorylation (OXPHOS) complexes, CI, CIII, CIV, and CV (1). As the remaining 72 subunits of these complexes and all other mitochondrial proteins are encoded by nuclear DNA (13), it is essential that the mitochondria communicate with the nucleus. A number of mitocellular communication networks have been described, including physical interactions, metabolites, and signaling molecules (13). Recently, small peptides encoded by short open reading frames (sORFs) within the mitochondrial genome have been identified and suggested to mediate a novel pathway by which the mitochondrial genome can communicate directly with the cell nucleus and other cells (7, 12, 14, 28). The first described mitochondrial derived peptide (MDP) was humanin (14, 27), a 24-amino acid polypeptide encoded within the 16S ribosomal RNA gene (MT-RNR2). Since humanin's initial identification through a cDNA library generated from a human Alzheimer's disease brain (14), several more peptides have been reported to be encoded within the 16S ribosomal RNA gene [small-humanin-like peptides 1–6 (SHLP1–6)] (7) and one within the 12S ribosomal RNA (mitochondrial open reading frame of the 12S rRNA-c; MOTS-c) (28).
Primarily, rodent and cell studies have shown the mitochondrial derived peptides are largely cyto- and metabolo-protective (23) with humanin and its analogs initially being shown to confer protection against amyloid-β-related cytotoxicity (14) as well as having more generalized antiapoptotic effects (12, 21). Humanin also has beneficial impacts on cellular metabolism that parallel those of exercise, with exogenous treatment of rodents prompting insulin sensitivity, glucose stimulated-insulin release, fat mass loss, and improved cognitive function (10, 25, 36, 43). Furthermore, humanin and its analogs can promote mitochondrial respiration and mitogenesis in various cell models (22, 24, 39).
Consistent with these observations there is some evidence that exercise training may alter mitochondrial derived peptide levels, with 12 wk of resistance training elevating muscle humanin expression, while the more aerobic Nordic walking did not induce the same response (9). However, whether acute exercise stress modulates humanin expression and thus potentially implicates humanin as an exercise-sensitive peptide that may modulate metabolic adaptation to exercise training is not known. Therefore, we investigated the hypothesis that acute high-intensity exercise and short-term high-intensity interval training (HIIT) would increase humanin levels in plasma and skeletal muscle. We report that acute high-intensity exercise induces an increase in relative muscle and plasma humanin levels and that short-term HIIT trended to lower absolute levels of plasma humanin without affecting its response to acute exercise. Furthermore, contraction of mouse EDL muscle in isolation increases muscle levels of humanin.
MATERIALS AND METHODS
Participants.
Ten healthy young men [age: 24.5 ± 3.7 yr, body mass index (BMI): 24.1 ± 2.1 kg/m2, body mass, 79.7 ± 2.8 kg; V̇o2peak: 41 ± 2 ml·kg−1·min−1] were recruited and gave written informed consent to participate. All participants reported as nonsmokers, free of chronic illnesses including cardiovascular or metabolic disease and completed <4 h of physical activity/week. Participants were not taking any medication or dietary supplements within the past 6 mo. This study was approved by the Health and Disability Ethics Committee, New Zealand (16STH116).
Exercise study protocol.
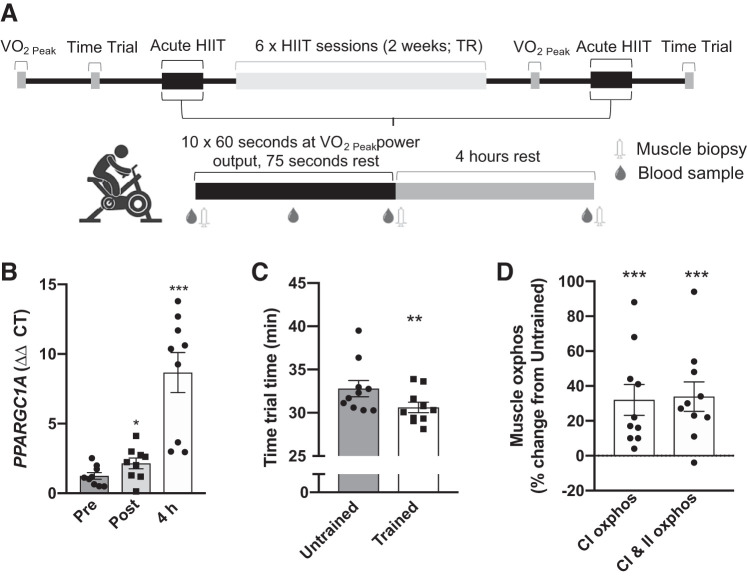
Acute exercise and exercise training experimental design are outlined in Fig. 1A. Prior to the first acute exercise session participants completed a ramped (starting at 60 W and increasing by 15 W per minute) exercise protocol to voluntary exhaustion using a electromagnetically braked cycle ergometer (Velotron, RacerMate, WA) with open-circuit spirometry (Parvo Medics True One 2400, Sandy, UT) to determine peak oxygen uptake (V̇o2peak) and associated peak power output, and on a separate day performed a 16 km time trial at least 2 days before and following the first and last acute exercise session, respectively. During the time trial participants were instructed to complete a 16 km cycle ergometer ride as fast as possible, and they received information on distance traveled but not time. In the acute exercise sessions before (untrained) and following (trained) the short-term high-intensity interval training (HIIT) period fasted (overnight) participants completed 10× 60 s cycling intervals at V̇o2peak power output interspersed with 75 s low-intensity (30 W) intervals on a cycle ergometer. HIIT consisted of six training sessions, using the same protocol as in the acute exercise bouts (starting with 8 intervals and increasing to 12 by the last session) on nonconsecutive weekdays over 2 wk (Fig. 1A).
Fig. 1.
Human exercise experimental design. Overview of the acute exercise bout and high-intensity interval training (HIIT) study design (A). The effect of acute exercise protocol on muscle PPARGC1A (PGC1α) mRNA expression (B), and HIIT period (trained vs. untrained) on 16 km time trial performance (C), and mitochondrial respiration (D). **P < 0.01 vs. Pre-TR; ***P < 0.001 vs. untrained or Pre. Pre, preacute exercise; Post, postacute exercise; 4 h, 4 h postacute exercise; untrained, before HIIT period; Trained, after HIIT training period; CI oxphos, complex 1 oxidative phosphorylation; CII oxphos, complex 2 oxidative phosphorylation.
Blood and muscle sampling.
Blood samples were collected during the acute exercise sessions from a cannula in an antecubital vein into a K2 EDTA vacutainer tube before, after five exercise intervals (Mid), immediately after, and 4 h following exercise (Fig. 1A). Whole blood samples were centrifuged immediately (2,000 g for 10 min at 4°C), and plasma was recovered and placed on ice until storage at −80°C. Percutaneous muscle biopsies were obtained from the vastus lateralis muscle (thigh) using a suction-modified Bergstrom biopsy needle under local anesthesia (3a). Muscle biopsy samples were either placed in ice-cold histidine-tryptophan-ketoglutarate (HTK, in mM: 180 histidine, 30 mannitol, 18 histidine-HCl, 15 NaCl, 9 KCl, 4 MgCl, 2 tryptophan, 1 KH-2-oxoglutarate, 0.015 CaCl2) transplant buffer (Custodiol, Alsbech Hähnlein, Germany) for mitochondrial respiration analysis or snap-frozen in liquid nitrogen and subsequently stored at −80°C until further analysis. Mitochondrial respiration was measured in permeabilized skeletal muscle fibers from basal (pre-exercise) samples before (untrained) and following (trained) 2 wk of HIIT using a Oroboros O2k oxygraph (Oroboros Instruments, Innsbruck, Austria), as described previously in detail for this study with full respiration data presented (17).
Plasma mitochondrial derived peptide analysis.
Plasma humanin, SHLP 2 and 6 were extracted in 90% acetonitrile and 10% 1 N HCl and measured using an in-house sandwich ELISA developed at the University of California, Los Angeles (UCLA) (6, 7, 42). A standard curve was produced using synthetic humanin, SHLP 2 and 6 (GenScript, HK), respectively, to allow measurement of endogenous concentrations within a range of 0.1–50 ng/mL. Intra- and interassay coefficient variations were reported as <10%. Following ELISA, sample absorbances were measured on a plate spectrophotometer (Molecular Devices, CA) at 490 nm. For humanin the capture antibody used in the ELISA was a rabbit anti-humanin analog (HNG) anti-serum that was produced by Harlan Laboratories (Indianapolis, IN). IgG subclasses were purified with a protein A column chromatography (Pierce, Rockford, IL). Total IgG was further purified with a humanin peptide conjugated ligand affinity column and labeled with biotin. This biotinylated ligand affinity purified IgG is used as a detection antibody (6). There is no similarity among SHLP fragments and the full-length humanin, with details on these peptides being reported previously (7). Antibodies we produced in the same way for SHLP2 and SHLP6 (7). We have not detected SHLP peptide with the humanin ELISA, thus there is little cross-activity between these ELISAs.
Murine housing and isolated muscle experiments.
In-house bred male C57Bl/6j mice were maintained in a temperature-controlled (20°C) animal facility with 12 h light-dark cycle and had ad libitum access to water and a standard rodent chow diet (Teklad TB 2018; Harlan, Madison, WI). All experiments were approved by the University of Auckland Animal Ethics Committee, New Zealand. At 12–20 wk of age, littermates were anesthetized with pentobarbitone diluted in saline to a final concentration of 3 mg/mL. Sufficient surgical anesthesia was determined by lack of response to toe pinch, at which time the extensor digitorum longus (EDL) muscle was excised and then mounted in an incubation chamber (Radnoti, Monrovia, CA) containing Krebs-Henseleit buffer (118.5 mM NaCl, 24.7 mM NaHCO3, 4.74 mM KCl, 1.18 mM MgSO4, 1.18 mM KH2PO4, 2.5 mM CaCl2, 8 mM mannitol, and 2 mM pyruvate, 0.1% BSA). As described previously (32), muscles were brought to optimal tension, incubated for 10 min before being contracted [pulse durations: 350 ms at a frequency of 150 Hz, every 5 s for 10 min with an electrical stimulator (Radnoti, Monrovia, CA)] or left under basal tension for 10 min. Immediately following rest/contraction period muscles were snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
Immunoblotting.
Muscle protein samples were extracted with modified RIPA buffer (50 mM Tris, pH 8.0, 75 mM NaCl, 0.3% NP-40, 1% sodium deoxycholate, 0.1% SDS) with EDTA-free protease and phosphatase inhibitors (Roche). Protein concentration was determined by BCA assay, and sample concentrations were adjusted with Laemmli buffer and then heated at 95°C for 5 min. Proteins were separated in 15% Tris-glycine gels and transferred to 0.2 μm PVDF membranes (Bio-Rad) using a semidry transfer system (Transblot Turbo, Bio-Rad) at a constant 9 V for 15 min. Membranes were blocked in 2% fish gelatin (Sigma G7041) and then incubated with primary antibody overnight at 4°C [Humanin (Sigma Aldrich H2414, 1:500, validated by peptide blocking; Supplementary Fig. S2) (all Supplementary Material is available at https://github.com/TMerryNZ/Humain_JAP_Supps.git), ERK1/2 phosphor-Thr202/Tyr204 (Cell Signaling Technologies 9101; 1:1,000), total ERK (Cell Signaling Technologies 9107; 1:5,000), β-actin (Sigma Aldrich A-2228; 1:5,000), and α-tubulin (Thermo Fisher Inc. Scientific A11126), 1:10,000]. The following day membranes incubated with HRP-conjugated secondary antibodies (1:10,000) for 1 h at room temperature and then developed and imaged using Clarity Western ECL (Bio-Rad) chemiluminescence and Chemidoc XRS+ system (Bio-Rad). Band intensities were quantified using ImageJ software and each band was normalized to a gel control sample in each respective gel and then normalized to β-actin as a loading control protein.
Real-time polymerase chain reaction.
Total RNA from muscle was extracted using an AllPrep DNA/RNA/miRNA Universal Kit (QIAGEN, Hilden, Germany) as per the manufacturer’s instructions. Total RNA was fractioned into small (<200 nucleotides) and large RNAs (>200 nucleotides) using a Zymo RNA Clean & Concentrator Kit (cat. R1016, Zymo Research, Irvine, CA) as per the manufacturer’s instructions. Total RNA used for SYBR green quantitative real-time PCR (qRT-PCR) was reverse-transcribed using a High-Capacity RNA-to-cDNA kit (Life Technologies, Carlsbad, CA). Fractioned RNA (50 ng) used for TaqMan small RNA assays were reverse-transcribed using a TaqMan microRNA RT kit 4366596 (Life Technologies, Carlsbad, CA) and multiplexed with a combined pool of the small RNA assay targets (Humanin, RNU44 and RNU48) (26, 33). Quantification of genes of interest from both total and fractioned samples were measured by a QuantStudio 6 PCR System using SYBR green select master mix (Applied Biosystems, Foster City, CA) and TaqMan Universal PCR Master Mix with UNG (PE Applied Biosystems), respectively. Each sample was loaded in triplicate and normalized to the geometric mean of two reference genes for both total and small RNA gene expression assays (B2M and 36B4 for SYBR green and RNU44 and RNU48 genes for small RNA assays, respectively). Genes of interest were expressed using the delta-delta Ct (ΔΔCt) method. Primer sequences for genes SYBR green assays and assay ID’s for TaqMan assays in Supplementary Tables S1 and S2.
Cell culture.
143B human osteosarcoma and 143B Rho 0 cells obtained from Dr. Mike Murphy (University of Otago, Dunedin, New Zealand) and cultured in MEM media supplemented with 10% fetal calf serum (FCS), 3.5 mg/ml D-glucose, 20 mM HEPES, 1 mM sodium pyruvate, and 50 µg/ml uridine. Cells were incubated at 37°C with 5% CO2. 143B Rho 0 cells, which are devoid of mtDNA, were generated by culturing 143B cells in low doses of ethidium bromide and characterized and described previously (2, 19).
Statistical analyses.
Statistical analyses were performed using Prism 8 (GraphPad Software), with statistical significance determined as P ≤ 0.05. Data are presented as individual data points and means ± SE. Statistical significance was determined with linear regression, one-way (time) or two-way (time × training status) ANOVA with Holm-Sidak’s post hoc analysis, or Student’s t test as indicated.
RESULTS
Humanin levels increase in muscle and plasma with acute exercise.
To determine the effect of high-intensity interval exercise on acute responses to exercise, skeletal muscle samples were collected before and following acute exercise prior (untrained) to and after (trained) short-term high-intensity interval training (HIIT) (Fig. 1A). Acute high-intensity exercise substantially increased mRNA expression of the exercise responsive gene PPARGC1A (PGC1α), and short-term HIIT improved exercise performance and muscle mitochondrial function (Fig. 1, B–D and (17)), indicating that the exercise protocol induced a potent training stimulus.
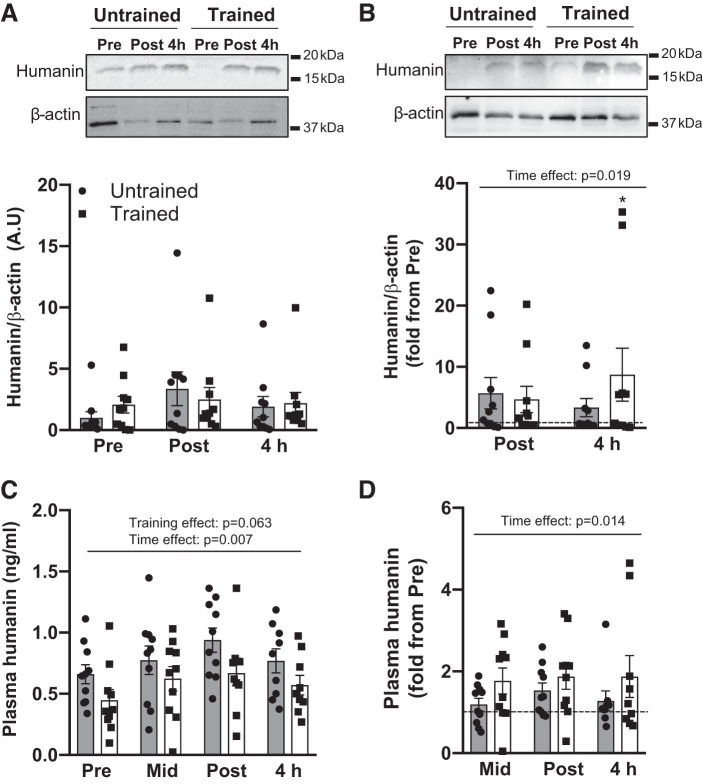
Neither acute exercise nor exercise training altered the absolute expression of humanin in skeletal muscle (Fig. 2A; time effect = 0.193, training effect 0.870); however, relative (individual fold change from pre exercise) muscle humanin increased with acute exercise (time effect P = 0.019), most prominently 4 h following exercise for trained (post hoc P < 0.013 vs. pre-exercise, Fig. 2B). Humanin absolute and relative plasma concentration increased with acute exercise (time effect P < 0.05), and absolute plasma levels tended to be lower overall following short-term HIIT (trained, P = 0.063) despite showing a similar acute exercise response (Fig. 2, C and D).
Fig. 2.
The effect of acute high-intensity exercise and short-term high-intensity interval training (HIIT) on skeletal muscle and plasma humanin levels. Vastus lateralis humanin absolute (A) and relative (B) muscle levels, and absolute (C) and relative (D) plasma levels at rest (Pre), after five exercise intervals (Mid), immediately (Post) and 4 h (4 h) following acute high-intensity exercise before (untrained) and after (trained) short-term HIIT. Horizontal dotted line represents pre-exercise. Representative Western blots (A and B) are from two different participants. Significant main 2-way ANOVA effects are given in figures and *P < 0.05 for post hoc vs. Pre of same training status.
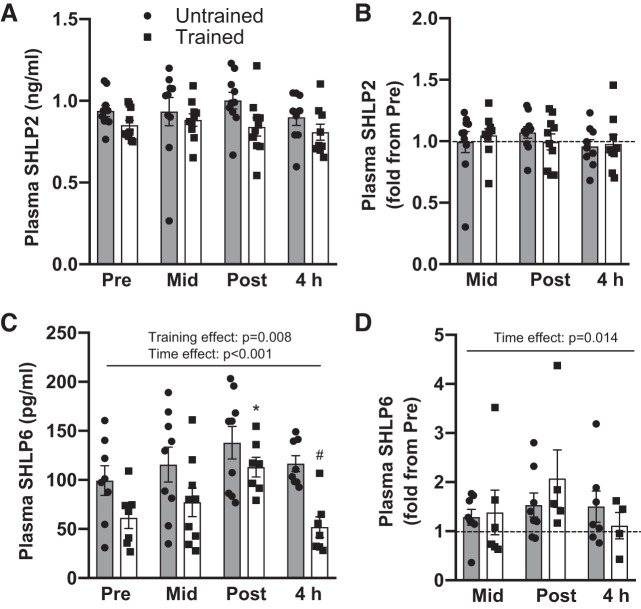
To determine if the effect of exercise on mitochondrial derived peptides was unique to humanin, we measured the plasma concentrations of small humanin-like peptides (SHLP) 2 and 6, which are also encoded within the 16S ribosomal RNA (MT-RNR2) (7). In contrast to humanin, SHLP2 plasma concentrations were not affected by acute exercise or exercise training (Fig. 3, A and B). However, SHLP6 showed a significant time effect with acute exercise (P < 0.001) appearing to increase with acute exercise then return to baseline during recovery (Fig. 3, C and D). Similar to humanin, short-term HIIT lead to an overall lower plasma concentration of SHLP6 (Fig. 3C, training effect P = 0.008) but did not change the response to exercise (Fig. 3D). Plasma SHLP6 levels were below the detection limit of the assay at various time points for three participants when they were untrained (before HIIT) and for six participants when they were trained (following HIIT). These data points were not included in analysis (Fig. 3C), and data for all time-points have been removed for fold change data (Fig. 3D) if the pre-exercise sample was below the detection limit.
Fig. 3.
The effect of acute high-intensity exercise and short-term term high-intensity interval training (HIIT) on skeletal muscle and plasma SHLP levels. Absolute and relative plasma SHLP2 (A, B) and SHLP6 (C, D) concentrations at rest (Pre), after five exercise intervals (Mid), immediately (Post) and 4 h (4 h) following acute high-intensity exercise before (untrained) and after (trained) HIIT. Horizontal dotted line represents Pre level. Significant main 2-way ANOVA effects are given in figures and #P < 0.05 for post hoc vs. untrained at same time-point. SHLP, small humanin-like peptides.
Skeletal muscle humanin correlates with plasma humanin during acute exercise.
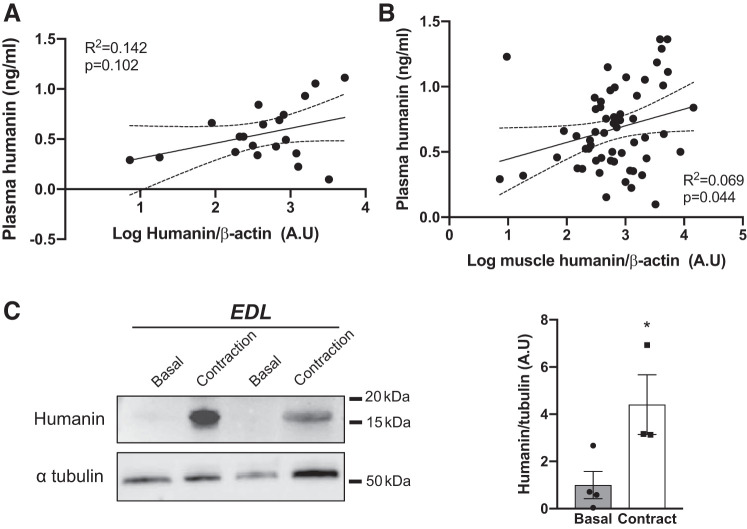
Having observed that humanin responds to exercise in muscle and in plasma we next correlated humanin muscle and plasma, finding there was no relationship between plasma and muscle humanin at rest (Fig. 4A) and a weak association between muscle and plasma humanin (R2 = 0.07, P = 0.04) levels when exercise samples were also considered (Fig. 4B). We then tested for the accumulation of humanin within exercising muscle by electrically stimulating contraction of isolated mouse extensor digitorum longus (EDL) muscle ex vivo. EDL humanin levels were fourfold higher immediately following 10 min of contraction compared with basal EDL levels (Fig. 4C).
Fig. 4.
Correlation of plasma and muscle Humanin levels and increased humanin expression within isolated mouse extensor digitorum longus (EDL) muscle during contraction. Correlation between plasma and muscle humanin levels at rest (A) and in response to exercise (B) from trained and untrained acute high-intensity exercise. Muscle humanin levels in mouse EDL (C) muscles at rest (basal) and following ex vivo contractions (contract). Curved dotted lines represent 95% confidence intervals. *P < 0.05 vs. basal for Student’s t test.
Skeletal muscle transcriptional regulation of humanin during acute exercise.
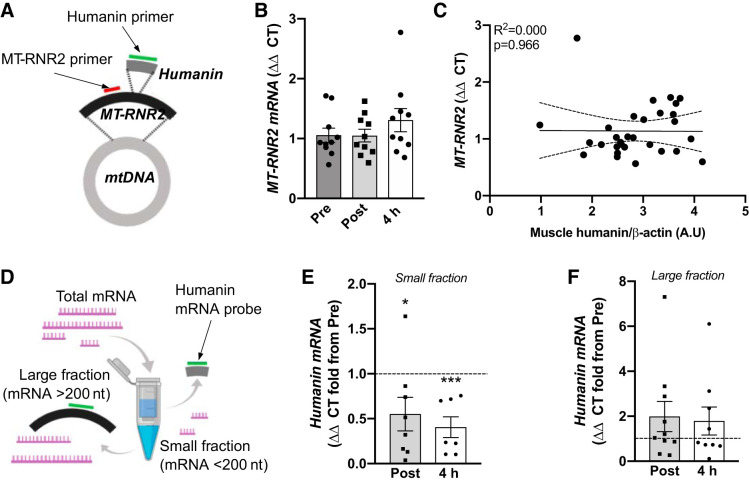
Since humanin appeared to be produced locally in muscle tissue during acute exercise/contraction, we next investigated its transcriptional regulation by using primers designed against MT-RNR2 mRNA (16S rRNA) and specifically against the humanin sequence within the 16S rRNA (Fig. 5A). MT-RNR2 mRNA expression was not affected by acute exercise (untrained) and there was no correlation between MT-RNR2 expression and muscle humanin levels (Fig. 5, B and C). To determine if humanin transcripts exist independent of MT-RNR2, we enriched fractions for small (<200 nucleotides) and large (>200 nucleotides) mRNA’s and used a custom TaqMan small RNA assay with a probe designed specifically against humanin sequence to measure Humanin mRNA (Fig. 5D). Control experiments show that this probe specifically detects a mitochondrial sequence because no amplification was evident in cells devoid of the mitochondrial genome (143B Rho 0 cells, Supplementary Fig. S1A). The expression of Humanin mRNA was greater in the large fraction, which only contains sequences >200 nt, such as the full-length MT-RNR2 mRNA, than the small fraction (Supplementary Fig. S1B). With acute exercise the Humanin mRNA was decreased in the small fraction, but similar to MT-RNR2 Humanin mRNA did not change in the large fraction (Fig. 5, E and F).
Fig. 5.
mRNA levels of MT-RNR2 and humanin following acute high-intensity exercise untrained. Primers were used against sequences within the MT-RNR2 gene that were independent of (MT-RNR2 primer) or matched to the humanin mRNA sequence (humanin primer) (A). MT-RNR2 mRNA muscle levels (B) and association with humanin muscle levels (C) in response to untrained acute high-interval exercise. Muscle mRNA from acute high-intensity exercise pretraining was fractioned into small (<200 nucleotides) and large (>200 nucleotides) mRNA fragments (D), and these fragments were assayed with the humanin primer probe (E, F). Curved dotted lines represent 95% confidence intervals (C). Horizontal dotted line represents Pre level (E, F). n = 7–8 for small mRNA faction assay due to limited amount of sample available. *P < 0.05 ***P < 0.01 vs. for one-way ANOVA.
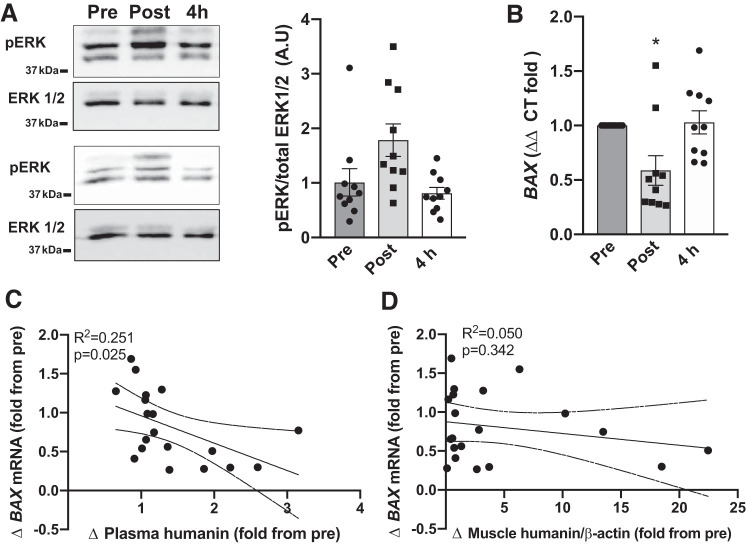
Muscle BAX mRNA expression correlates with plasma humanin concentration.
Some of the reported primary downstream targets of humanin are BAX and ERK (27). Acute HIIT did not significantly affect muscle ERK phosphorylation; however, BAX mRNA decreased immediately following exercise and returned to baseline levels during recovery (Fig. 6, A and B). Since BAX mRNA had a similar response pattern in response to acute exercise as humanin, we correlated BAX with plasma and muscle humanin levels. There was a significant but weak (P = 0.025, R2 = 0.251) correlation between plasma humanin and BAX mRNA, but not BAX mRNA and muscle humanin levels (Fig. 6, C and D). Neither plasma nor muscle humanin levels were associated with phosphorylated-ERK (P > 0.60, R2 <0.02; data not shown).
Fig. 6.
Association between muscle and plasma humanin levels and ERK phosphorylation and BAX mRNA expression in untrained. ERK (extracellular signal-regulated kinases) phosphorylation (A) and BAX mRNA (B) expression at rest (Pre) Pre and immediately (Post) and 4 h (4 h) following untrained acute high-intensity exercise before high-intensity interval training (HIIT). Correlations between humanin plasma and muscle levels and BAX mRNA expression (C, D) in response to acute high-intensity exercise before HIIT. Curved dotted lines represent 95% confidence intervals. *P < 0.01 vs. Pre for one-way ANOVA.
DISCUSSION
The mitochondrial derived peptide humanin has been shown to respond to cellular stress (23) and can facilitate mitochondrial and metabolic adaptions when given exogenously to mice (22, 24, 39). Here, we provide evidence that muscle and plasma abundance of humanin increase in response to acute high-intensity exercise in young men, and this appears to be independent of transcription. Furthermore, short-term high-intensity interval training (HIIT) tends to lower absolute plasma humanin and SHLP6 levels but does not affect the acute increase in response to exercise. Finally, we provide evidence that humanin plasma concentrations during exercise correlate with muscle humanin levels, and an increase in humanin levels also occurred in isolated contracting mouse EDL muscle.
Our finding that humanin is an exercise-sensitive mitochondrial peptide is partially supported by the observation that 12 wk of resistance exercise training increases skeletal muscle humanin expression, which also correlated with parameters of metabolic health in older men (54 ± 7 yr) with prediabetes (9). While we did not observe an effect of short-term HIIT on intramuscular humanin levels, a trend for decrease in plasma concentration was observed with short-term HIIT. Since humanin plasma levels have been reported to decrease with advancement of metabolic disease (9, 29, 38), it is likely that participants in the Gidlund et al. study (9) had lower baseline humanin levels than the younger cohort in this study, and the increase observed related to resistance exercise-associated improvements in metabolic health. This is unlikely to have occurred in our already healthy cohort that only undertook short-term (2 wk) training. Alternatively, this may reflect the differences in exercise modalities, with short-term HIIT having a greater aerobic component than resistance training or the duration of training period (2 vs. 12 wk). Interestingly, within the same study, 12 wk of Nordic walking, which is primarily an aerobic endurance exercise, also did not alter muscle humanin levels (9). Further studies are therefore required to more comprehensively identify the regulation of humanin in response to differing durations, intensities, and modes of exercise, in both healthy and metabolically unhealthy individuals.
Compared with high-intensity interval training, acute exercise had a more pronounced effect on humanin muscle and plasma levels. This suggests that the increase in humanin levels during acute exercise is likely to be in response to acute exercise-related mitochondrial stress and might explain why the increase is transient, with humanin levels generally trending toward baseline 4 h postexercise. This is in agreement with the short (<30 min) half-life of humanin (6). Similarly, the mitochondrial peptide MOTS-c has been shown to rapidly (within 30 min) and transiently translocate from the mitochondria to the nucleus in response to metabolic stress (glucose restriction and oxidative stress) to directly interact with DNA and facilitate an antioxidant response (28). While the current study was not designed to elucidate the molecular targets of humanin, we did observe a weak association between plasma humanin and muscle BAX mRNA levels. Humanin can form a complex with BAX and inhibit its translocation to the mitochondrial outer membrane, preventing its proapoptotic function (12, 34). While we do not have cellular localization data, it is possible that by decreasing BAX’s proapoptotic role, this feeds back to the nucleus to reduce transcription. Therefore, it is tempting to speculate that exercise-induced increases in humanin may play a role in regulating exercise-related protection against mitochondrial-mediated apoptosis (18).
The weak correlation between muscle and plasma humanin levels during acute exercise may indicate that either humanin from the circulation (potentially being released from other tissues) is entering the muscle or that humanin is being released from the muscle into the circulation. While both processes are possible and implicate humanin as a “mitokine,” the increase in humanin levels following contraction of isolated EDL muscle strongly suggests that the muscle is capable of intrinsically increasing humanin expression within minutes of contraction. Surprisingly, 16S rRNA (MT-RNR2) gene transcription was not affected by exercise, and small Humanin mRNA levels decreased. This suggests that acute regulation of humanin expression during exercise/contraction is nontranscriptional, but the decrease in transcription may begin to explain why a reduction in plasma humanin was observed following HIIT. Since expression of muscle humanin increased quickly during exercise/contraction (within 10 min), this suggests exercise/contraction may be acting to suppress the degradation of humanin leading to its increase in muscle and in circulation. While humanin has been reported to colocalize with lysosomes (11), its endogenous expression appears to be primarily regulated via ubiquitin-mediated degradation with binding of TRIM11 (Tripartite Motif Containing 11) promoting proteolysis (37). TRIM11 is highly expressed in skeletal muscle (3, 41), and ubiquitin proteasome-dependent proteolysis appears to be downregulated by endurance exercise, while in contrast, muscle disuse upregulates the ubiquitin-proteasome pathway (40). However, whether TRIM11 activity is modulated by exercise/contraction is not known.
For the first time, we also assessed plasma levels of the small humanin-like peptides (SHLP) 2 and 6 following exercise. Interestingly, while SHLP6 showed a similar trend as humanin (increasing in the plasma with acute exercise but showing lower absolute levels following training) SHLP2 concentration was not altered by acute exercise or HIIT. This suggests independent and stimulus-specific regulation of peptides encoded within the same region (16S rRNA) of the mitochondrial genome. Consistent with this, SHLP2 and 6 are differentially expressed across mouse tissues, and like humanin, SHLP2 appears to have a greater cytoprotective role than SHLP6 (7), and both humanin and SHLP2 can lower several markers of age-associated metabolic conditions (30). One reason potentially why humanin and SHLP6 showed a similar response to exercise is that they are both encoded by the heavy strand of mitochondrial DNA, while SHLP2 is encoded by the light strand (7). We note that a number of humanin homolog open reading frames (ORF) reside within the nuclear genome and could also transcribe humanin-like peptides (4). However, the antibodies used in the ELISA for humanin and SHLPs, and the humanin mRNA probe do not to detect these peptides in cells missing mitochondrial DNA (143B Rho 0 cells) [(7) and Supplementary Fig. 1A), indicating that our results can be attributed to primarily mitochondrially transcribed peptides.
The results of our study demonstrate that the mitochondrial encoded peptide, humanin, is regulated by acute high-intensity exercise and short-term high-intensity interval training (HIIT) in young healthy males. Furthermore, we suggest that the increase in plasma humanin levels is partially the result of increased levels of humanin within skeletal muscle, which may be regulated independent of transcription.
GRANTS
This study was funded by a Marsden Fast-start grant (to T. L. Merry). T. L. Merry is supported by a Rutherford Discovery Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.C.-S., P.C., A.J.R.H., C.J.M., and T.L.M. conceived and designed research; J.S.T.W., R.F.D., C.P.H., J.W., C.J.M., and T.L.M. performed experiments; J.S.T.W., R.F.D., C.P.H., and T.L.M. analyzed data; J.S.T.W., C.P.H., and T.L.M. interpreted results of experiments; J.S.T.W. and T.L.M. prepared figures; J.S.T.W. and T.L.M. drafted manuscript; J.S.T.W., R.F.D., C.P.H., M.V.B., D.C.-S., P.C., A.J.R.H., C.J.M., and T.L.M. edited and revised manuscript; J.S.T.W., R.F.D., C.P.H., J.W., M.V.B., D.C.-S., P.C., A.J.R.H., C.J.M., and T.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study participants for donating time and tissue samples.
REFERENCES
- 1.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature 290: 457–465, 1981. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Appleby RD, Porteous WK, Hughes G, James AM, Shannon D, Wei YH, Murphy MP. Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur J Biochem 262: 108–116, 1999. doi: 10.1046/j.1432-1327.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- 3.Human Protein Atlas http://www.proteinatlas.org. 2020.
- 3a.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975. [PubMed] [Google Scholar]
- 4.Bodzioch M, Lapicka-Bodzioch K, Zapala B, Kamysz W, Kiec-Wilk B, Dembinska-Kiec A. Evidence for potential functionality of nuclearly-encoded humanin isoforms. Genomics 94: 247–256, 2009. doi: 10.1016/j.ygeno.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Chandel NS. Mitochondria as signaling organelles. BMC Biol 12: 34, 2014. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin YP, Keni J, Wan J, Mehta H, Anene F, Jia Y, Lue YH, Swerdloff R, Cobb LJ, Wang C, Cohen P. Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents. Endocrinology 154: 3739–3744, 2013. doi: 10.1210/en.2012-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, Mehta HH, Gao Q, Ashur C, Huffman DM, Wan J, Muzumdar R, Barzilai N, Cohen P. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 8: 796–809, 2016. doi: 10.18632/aging.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J 30: 13–22, 2016. doi: 10.1096/fj.15-276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gidlund EK, von Walden F, Venojärvi M, Risérus U, Heinonen OJ, Norrbom J, Sundberg CJ. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol Rep 4: e13063, 2016. doi: 10.14814/phy2.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Z, Su K, Cui L, Tas E, Zhang T, Dong HH, Yakar S, Muzumdar RH. Central effects of humanin on hepatic triglyceride secretion. Am J Physiol Endocrinol Metab 309: E283–E292, 2015. doi: 10.1152/ajpendo.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong Z, Tasset I, Diaz A, Anguiano J, Tas E, Cui L, Kuliawat R, Liu H, Kühn B, Cuervo AM, Muzumdar R. Humanin is an endogenous activator of chaperone-mediated autophagy. J Cell Biol 217: 635–647, 2018. doi: 10.1083/jcb.201606095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423: 456–461, 2003. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson CM, Falkenberg M, Larsson NG. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu Rev Biochem 85: 133–160, 2016. doi: 10.1146/annurev-biochem-060815-014402. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA 98: 6336–6341, 2001. [Erratum in Proc Natl Acad Sci USA 98: 12854, 2001] doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell 159: 738–749, 2014. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Hawley JA, Lundby C, Cotter JD, Burke LM. Maximizing Cellular Adaptation to Endurance Exercise in Skeletal Muscle. Cell Metab 27: 962–976, 2019. doi: 10.1016/j.cmet.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Hedges CP, Woodhead JST, Wang HW, Mitchell CJ, Cameron-Smith D, Hickey AJR, Merry TL. Peripheral blood mononuclear cells do not reflect skeletal muscle mitochondrial function or adaptation to high-intensity interval training in healthy young men. J Appl Physiol 126: 454–461, 2018. doi: 10.1152/japplphysiol.00777.2018. [DOI] [PubMed] [Google Scholar]
- 18.Heo JW, Yoo SZ, No MH, Park DH, Kang JH, Kim TW, Kim CJ, Seo DY, Han J, Yoon JH, Jung SJ, Kwak HB. Exercise Training Attenuates Obesity-Induced Skeletal Muscle Remodeling and Mitochondria-Mediated Apoptosis in the Skeletal Muscle. Int J Environ Res Public Health 15: E2301, 2018. doi: 10.3390/ijerph15102301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herst PM, Berridge MV. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim Biophys Acta 1767: 170–177, 2007. doi: 10.1016/j.bbabio.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Hyldahl RD, Chen TC, Nosaka K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc Sport Sci Rev 45: 24–33, 2017. doi: 10.1249/JES.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 21.Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA 100: 13042–13047, 2003. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, Mehta HH, Wan J, Kuehnemann C, Chen J, Hu JF, Hoffman AR, Cohen P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY) 10: 1239–1256, 2018. doi: 10.18632/aging.101463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SJ, Xiao J, Wan J, Cohen P, Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J Physiol 595: 6613–6621, 2017. doi: 10.1113/JP274472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein LE, Cui L, Gong Z, Su K, Muzumdar R. A humanin analog decreases oxidative stress and preserves mitochondrial integrity in cardiac myoblasts. Biochem Biophys Res Commun 440: 197–203, 2013. doi: 10.1016/j.bbrc.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, Bastie C, Su K, Huffman D, Surana M, Barzilai N, Fleischer N, Muzumdar R. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. FASEB J 27: 4890–4898, 2013. doi: 10.1096/fj.13-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Carré J, Lamon S, Léger B. Validation of a multiplex reverse transcription and pre-amplification method using TaqMan(®) MicroRNA assays. Front Genet 5: 413, 2014. doi: 10.3389/fgene.2014.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab 24: 222–228, 2013. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21: 443–454, 2015. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Li S, Wei X, Huang J, Lai M, Wang N, Huang Q, Zhao L, Peng Y, Wang Y. Comparison of serum concentrations of humanin in women with and without gestational diabetes mellitus. Gynecol Endocrinol 34: 1064–1067, 2018. [Erratum in Gynecol Endocrinol 34: 1097, 2018] doi: 10.1080/09513590.2018.1482869. [DOI] [PubMed] [Google Scholar]
- 30.Mehta HH, Xiao J, Ramirez R, Miller B, Kim SJ, Cohen P, Yen K. Metabolomic profile of diet-induced obesity mice in response to humanin and small humanin-like peptide 2 treatment. Metabolomics 15: 88, 2019. doi: 10.1007/s11306-019-1549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 594: 5135–5147, 2016. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab 298: E577–E585, 2010. doi: 10.1152/ajpendo.00239.2009. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell CJ, D’Souza RF, Schierding W, Zeng N, Ramzan F, O’Sullivan JM, Poppitt SD, Cameron-Smith D. Identification of human skeletal muscle miRNA related to strength by high-throughput sequencing. Physiol Genomics 50: 416–424, 2018. doi: 10.1152/physiolgenomics.00112.2017. [DOI] [PubMed] [Google Scholar]
- 34.Morris DL, Kastner DW, Johnson S, Strub MP, He Y, Bleck CKE, Lee DY, Tjandra N. Humanin induces conformational changes in the apoptosis regulator BAX and sequesters it into fibers, preventing mitochondrial outer-membrane permeabilization. J Biol Chem 294: 19055–19065, 2019. doi: 10.1074/jbc.RA119.011297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mottis A, Herzig S, Auwerx J. Mitocellular communication: Shaping health and disease. Science 366: 827–832, 2019. doi: 10.1126/science.aax3768. [DOI] [PubMed] [Google Scholar]
- 36.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, Budagov T, Cui L, Einstein FH, Poduval A, Hwang D, Barzilai N, Cohen P. Humanin: a novel central regulator of peripheral insulin action. PLoS One 4: e6334, 2009. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niikura T, Hashimoto Y, Tajima H, Ishizaka M, Yamagishi Y, Kawasumi M, Nawa M, Terashita K, Aiso S, Nishimoto I. A tripartite motif protein TRIM11 binds and destabilizes Humanin, a neuroprotective peptide against Alzheimer’s disease-relevant insults. Eur J Neurosci 17: 1150–1158, 2003. doi: 10.1046/j.1460-9568.2003.02553.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramanjaneya M, Bettahi I, Jerobin J, Chandra P, Abi Khalil C, Skarulis M, Atkin SL, Abou-Samra AB. Mitochondrial-Derived Peptides Are Down Regulated in Diabetes Subjects. Front Endocrinol (Lausanne) 10: 331, 2019. doi: 10.3389/fendo.2019.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR. The Mitochondrial-Derived Peptide Humanin Protects RPE Cells From Oxidative Stress, Senescence, and Mitochondrial Dysfunction. Invest Ophthalmol Vis Sci 57: 1238–1253, 2016. doi: 10.1167/iovs.15-17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taillandier D, Combaret L, Pouch MN, Samuels SE, Béchet D, Attaix D. The role of ubiquitin-proteasome-dependent proteolysis in the remodelling of skeletal muscle. Proc Nutr Soc 63: 357–361, 2004. doi: 10.1079/PAR2004358. [DOI] [PubMed] [Google Scholar]
- 41.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 42.Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, Lerman LO, Lerman A. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol 304: H393–H397, 2013. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yen K, Wan J, Mehta HH, Miller B, Christensen A, Levine ME, Salomon MP, Brandhorst S, Xiao J, Kim SJ, Navarrete G, Campo D, Harry GJ, Longo V, Pike CJ, Mack WJ, Hodis HN, Crimmins EM, Cohen P. Humanin Prevents Age-Related Cognitive Decline in Mice and is Associated with Improved Cognitive Age in Humans. Sci Rep 8: 14212, 2018. doi: 10.1038/s41598-018-32616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]