Keywords: biochemistry, cancer, heart, physiology, Sentrin, SUMO
Abstract
Sentrin/small ubiquitin-like modifier (SUMO) is protein modification pathway that regulates multiple biological processes, including cell division, DNA replication/repair, signal transduction, and cellular metabolism. In this review, we will focus on recent advances in the mechanisms of disease pathogenesis, such as cancer, diabetes, seizure, and heart failure, which have been linked to the SUMO pathway. SUMO is conjugated to lysine residues in target proteins through an isopeptide linkage catalyzed by SUMO-specific activating (E1), conjugating (E2), and ligating (E3) enzymes. In steady state, the quantity of SUMO-modified substrates is usually a small fraction of unmodified substrates due to the deconjugation activity of the family Sentrin/SUMO-specific proteases (SENPs). In contrast to the complexity of the ubiquitination/deubiquitination machinery, the biochemistry of SUMOylation and de-SUMOylation is relatively modest. Specificity of the SUMO pathway is achieved through redox regulation, acetylation, phosphorylation, or other posttranslational protein modification of the SUMOylation and de-SUMOylation enzymes. There are three major SUMOs. SUMO-1 usually modifies a substrate as a monomer; however, SUMO-2/3 can form poly-SUMO chains. The monomeric SUMO-1 or poly-SUMO chains can interact with other proteins through SUMO-interactive motif (SIM). Thus SUMO modification provides a platform to enhance protein-protein interaction. The consequence of SUMOylation includes changes in cellular localization, protein activity, or protein stability. Furthermore, SUMO may join force with ubiquitin to degrade proteins through SUMO-targeted ubiquitin ligases (STUbL). After 20 yr of research, SUMO has been shown to play critical roles in most, if not all, biological pathways. Thus the SUMO enzymes could be targets for drug development to treat human diseases.
Posttranslational protein modification emerges as a power mechanism in nature’s toolbox to regulate cellular physiology. Sentrin/SUMO (small ubiquitin-like modifier) is an excellent illustration of how basic understanding in biochemical mechanism reveals fascinating pathophysiological processes that cause human ailments, such as cancer, diabetes, heart failure, autoimmune diseases, and neurological disorders. Activation or inhibition of this pathway will herald in a new generation of therapeutics for human ailments.
I. INTRODUCTION
Ubiquitin is the prototype of posttranslational protein modification by small polypeptides that revolutionized the concept of energy-dependent protein degradation (34, 53). In 1996, a new ubiquitin-like protein called Sentrin, which binds to the death domain of FAS and regulates cell death signaling, was reported (137). The same protein was described by other laboratories as GMPI or PIC, which regulates protein nuclear transport or promyelocytic leukemia protein, respectively (16, 125). A subsequent paper coined the name SUMO (small ubiquitin-like modifier) for this ubiquitin-like protein that covalently modifies Ran-GTPase-activating protein, RanGAP1 (123). SUMO was later adopted by the field to describe this new ubiquitin-like protein.
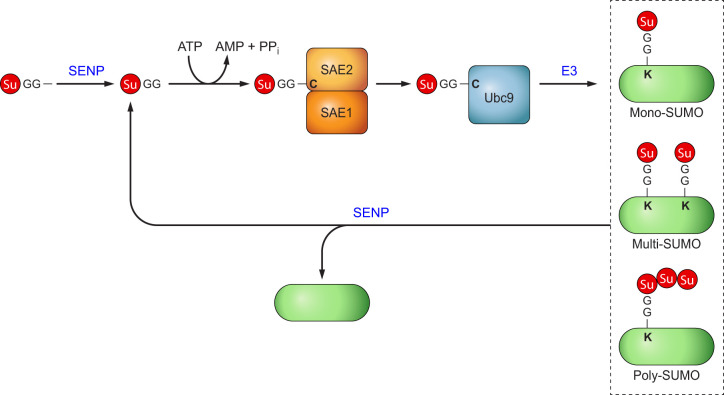
SUMOylation (SUMO modification) is catalyzed by SUMO-specific E1, E2, and E3 enzymes, whereas de-SUMOylation is regulated by the family of Sentrin-specific proteases (SENPs) (214). SUMO-1, SUMO-2, and SUMO-3 are the main SUMO proteins. The first step in the SUMO conjugation pathway involved cleavage of their COOH termini to reveal the di-glycine residues required for conjugation (FIGURE 1). The mature SUMOs are conjugated to a SUMO-activating enzyme (E1), which is composed of two subunits, SAE1/Aos1 and SAE2/Uba2 (TABLE 1). The activation step uses ATP hydrolysis to generate a high-energy SUMO-E1 thioester bond. This is followed by transfer of SUMO to UBC9, the only known SUMO E2. Subsequently, with the help of a SUMO E3 ligase, SUMO forms an isopeptide bond with specific lysine residues on the substrates (TABLE 2). Similar to phosphorylation and dephosphorylation, SUMO-modified proteins can be deconjugated by a family of SENPs. The SENPs prefer different SUMO paralogs, and several of the SENPs have editing function (TABLE 3).
FIGURE 1.
Biochemistry. Small ubiquitin-like modifier (SUMO) precursors are processed by Sentrin-specific protease 1 (SENP1), SENP2, and SENP5 to yield SUMO-GG, which is activated by SUMO E1 (SAE1/SAE2), transferred to SUMO E2 (UBC9), and through the help of a SUMO E3 ligase to conjugate to substrates. SUMO-1 is usually conjugated as a monomer, whereas SUMO-2/3 builds a poly-SUMO chain. A substrate can have multiple SUMO modification sites (Multi-SUMO). SUMO can be removed from SUMO-conjugated substrates through SENPs. SENP1 and SENP2 can deconjugate all SUMO-modified substrates, whereas SENP3, SENP5, SENP6, and SENP7 prefer SUMO-2/3 as substrate. SENP6 and SENP7 also have chain-editing activity, which removes SUMO-2/3 from poly-SUMO chains (poly-SUMO). Additional reviews are excellent sources for more mechanistic details (45, 192).
Table 1.
SUMO-activating and conjugation enzymes
| Enzyme | Name | Substrates | Regulation |
|---|---|---|---|
| E1 | SAE1/SAE2 | SUMO | Oxidation (19); small molecule activator (94); small molecule inhibitor (68, 111); Gam1 (ubiquitin-mediated degradation) (17) |
| E2 | Ubc9 | SUMO | Oxidation (19); acetylation (76); phosphorylation (178); SUMOylation (101); listerolysin O-induced degradation (152); HPV E6-induced degradation (69) |
HPV, human papillomavirus; SUMO, small ubiquitin-like modifier.
Table 2.
SUMO E3 ligases
| Name | Substrate | Function |
|---|---|---|
| PIAS1 | Foxp3 | Regulate Treg differentiation (116) |
| Sp3 | Silencing of Sp3 (160) | |
| PIAS3 | IRF-1 | Repress IRF-1 transcription activity (135) |
| PIASy | HIF1a | Increase HIF1α stability in hypoxia (90) |
| MMS21 | SMC6 | DNA repair (144) |
| RanBP2 | RanGAP1 | Nuclear transport (141) |
| HDAC4 | Modulate deacetylase activity (100) | |
| Pc2/CBX4 | CtBP1 | Polycomb body may be SUMOylation center (84) |
| Topors | p53 | Increase in p53 level (202) |
| MAPL | Drp1 | Regulate mitochondrial fission (20) |
| Rhes | Huntingtin | SUMOylated huntingtin is cytotoxic (179) |
| ZATT | Topoisomerase 2 | Resolution of the TOP2 cleavage complex (161) |
| KAP1 | IRF7 | Regulation of IRF7’s transactivation activity (30, 112) |
HIF1α, hypoxia-inducible factor 1α; SUMO, small ubiquitin-like modifier.
Table 3.
SUMO proteases
| Name | Isoform Preference | COOH-Terminal Hydrolase | Iso-peptidase | Chain Editing | Regulation |
|---|---|---|---|---|---|
| SENP1 | All SUMO | Yes | Yes | No | Hypoxia (210); androgen (8); IL-6 (31) |
| SENP2 | All SUMO | Yes | Yes | No | Ubiquitin-mediated degradation in the cytosol (79); phosphorylation (72) |
| SENP3 | SUMO2/3 | No | Yes | No | Ubiquitin-mediated degradation in the nucleolus (78); oxidative stress stabilizes SENP3 (78) |
| SENP5 | SUMO2/3 | Yes | Yes | No | Mitochondrial fission (227) |
| SENP6 | SUMO2/3 | No | Yes | Yes | Homologous recombination (40) |
| SENP7 | SUMO2/3 | No | Yes | Yes | SENP7L, SENP7S (9) |
| DeSI1 | All SUMO | No | Yes | Weak | Limited substrate, BZEL (167) |
| DeSI2 | ? | No | ? | ? | ? |
| USPL1 | SUMO2/3 | Weak | Yes | Yes | Regulation of coilin localization independent of enzyme activity (162) |
IL-6, interleukin-6; SENP, Sentrin-specific protease; SUMO, small ubiquitin-like modifier.
Although the enzymatic mechanism of SUMOylation is similar to ubiquitination, the SUMO pathway enzymes are entirely distinct from enzymes involved in ubiquitination. The SUMO enzymes are fewer in number than their counterpart in the ubiquitin pathway; however, the number of SUMO substrates is surprisingly large. How does SUMOylation achieve specificity through a limited number of conjugating and deconjugating enzymes? This is still not convincingly answered. Another riddle in SUMO research is that a small amount of SUMOylation of a given substrate can lead to profound biological effects. The first 10 years of SUMO research regarded SUMOylation and ubiquitination as two distinct, not overlapping, pathways. However, recent literature illustrated a close interaction between SUMO and ubiquitin in many biological pathways, in particular protein degradation. Many of the early literature on SUMO were generated in cell-free or cell-based assays. These studies may not truly reflect the real biology or physiology. Animal models have been generated to search for biological relevance of SUMOylation or de-SUMOylation in different organ systems. Findings based on genetic murine models or human genetic studies have now firmly established the clinical relevance of the SUMOylation pathway in human diseases. In this review, the focus is on the link of the mammalian SUMO pathway to physiology and human diseases.
II. SUMO ISOFORMS
Four SUMO isoforms are present as precursors in mammalian cells. The SUMO-1 precursor contains 101 amino acids with a flexible NH2 terminus, followed by the ubiquitin fold (amino acids 22–97), and a short COOH-terminal tail (215). Four amino acids from the COOH terminus are cleaved by SUMO isopeptidase (FIGURE 1) to reveal the conserved di-Gly-Gly residues required for formation of isopeptide bond with lysine residue of target protein (89). SUMO-2 precursor is 103 amino acids long, and SUMO-3 precursor contains 95 amino acids; these two isoforms are 97% identical to each other, but only 46% identical to SUMO-1 (87). Because antibody specific for SUMO-2 or SUMO-3 is not available, these two isoforms are commonly referred as SUMO-2/3 (157). Both SUMO-2 and SUMO-3 also require COOH-terminal processing to yield the mature isoform required for protein conjugation. In addition, SUMO-2 and SUMO-3 share a conserved lysine 11 residue that can form poly-SUMO chains (185). The SUMO-4 precursor contains proline at amino acid 90, which limits its processing to yield a mature form suitable for conjugation (139). Furthermore, SUMO-4 is rapidly degraded under normal culture condition (204). However, in serum-starved cells, SUMO-4 is not degraded and appears to be conjugated to proteins that are involved in stress response (204). SUMO-4 (M55V) was shown to be highly associated with the development of type 1 diabetes (58). Although this association is controversial (143), recent meta-analysis confirmed the association with type 1 and type 2 diabetes in both Asian and European populations (173, 183).
SUMO-1 haplo-insufficiency was found in a patient with cleft lip and palate (3). When SUMO-1 transcripts were interrupted by β-galactosidase insertion (BayGenomics gene trap cell line RRQ016), 4 out of 46 pups and embryos of the heterozygous genotype developed cleft lip and palate (3). Furthermore, death during embryonic development and immediate postnatal death were observed in the heterozygous and homozygous genotypes. However, in the SUMO-1 knockout mice derived from BayGenomics XA024 ES cells targeted by β-galactosidase, homozygous SUMO-1 null mice developed normally and did not show early mortality or cleft palate (43). Thus the initial report of cleft palate is most likely due to genetic changes unrelated to SUMO-1 because SUMO-1 deficiency could be compensated by SUMO-2/3 (43). SUMO-1 null mice generated from other laboratories also demonstrated normal development and absence of cleft palate (193, 222). SUMO-2 null mice showed severe developmental delay and died at embryonic day 10.5 due to reduced cell proliferation and increased cell death, whereas SUMO-3 null mice were viable (195). The differential contribution of SUMO-2 versus SUMO-3 in development is likely due to the much higher expression of SUMO-2 during development (195). Thus, in the SUMO-3 null background, SUMO-2 haplo-deficiency also had developmental delay (195). Taken together, SUMO-2 appears to be the most important SUMO isoform in the mammalian cells.
III. SUMO-ACTIVATING ENZYME
The SUMO activating enzyme (E1) is composed of two subunits, SAE1/Aos1 and SAE2/Uba2 (55, 83). SAE1/Aos1 is 56% similar to the NH2 terminus of the ubiquitin E1, and SAE2/Uba2 resembles the remainder of the ubiquitin E1. The active-site cysteine 173 is located at the SAE2/Uba2 subunit. Activation of SUMO involves formation of SUMO-adenylate in an ATP-dependent reaction, followed by a 130º rotation and remodeling of the E1 active site, leading to formation of a high-energy SUMO-E1 thioester bond (138). SAE1/Aos1 can be recruited by an adenoviral protein GAM1 to a ubiquitin ligase for degradation (17, 18). Hydrogen peroxide can reversibly inactivate SUMO E1 through formation of disulfide bond between active site cysteine (19). Inactivation of SUMO E1 will lead to a global decrease in SUMOylation. A small molecule activator of SUMO E1 has been discovered that binds to a pocket in SAE1/Aos2 (94). This activator appears to enhance SUMOylation of SERCA2a and a limited number of substrates. The reason for this selectivity is not known. ML-792, a small molecule inhibitor of SUMO E1, has been reported that targeted the ATP-binding site of SAE2; S95N M97T mutant of SAE2 rescued the mitotic defect induced by ML-792 (68). COH000, attached covalently to Cys30 of SAE2 and induced ubiquitin-mediated degradation of SAE2, is a new class of SUMO E1 inhibitor (111). COH000 could induce apoptosis of cancer cells in a patient-derived xenograft model with colorectal carcinoma (111).
IV. SUMO-CONJUGATING ENZYME
There is only a single SUMO conjugating enzyme, UBC9 (54), in contrast to 35 mammalian ubiquitin E2s (190). UBC9 binds to SAE2/Uba2’s ubiquitin fold domain to accept transfer of SUMO from E1 to E2 (120). UBC9 forms thiol ester conjugates with SUMO, but not with ubiquitin or NEDD8 (54). SUMO-1 and SUMO-2/3 bind to the NH2 terminus of UBC9 with equal affinity (187). Complete loss of UBC9 is embryonal lethal; the embryo died following uterine implantation presumably after exhaustion of maternal UBC9 (134). However, haplo-deficiency of UBC9 does not affect survival. UBC9 can be reversibly inactivated by hydrogen peroxide through formation of disulfide bond between active site cysteine (19). UBC9 can be degraded by the human papillomavirus E6 through recruitment of a ubiquitin E3 ligase, E6AP (69), or by listeriolysin O from Listeria monocytogenes in a proteasome-independent manner (152). UBC9 can also be regulated by acetylation, phosphorylation, and SUMOylation (76, 101, 178). Acetylation of UBC9 at K65 reduces UBC9 binding to substrates with negatively charged amino acid-dependent SUMOylation motif (NDSM) (76). K65 acetylation is also regulated through SIRT1 by hypoxia; UBC9 acetylation controls the SUMOylation status of CBP and ELK1 to limit activation of hypoxia-responsive genes, such as vascular endothelial growth factor (VEGF) (76). CDK1/cyclin B phosphorylates UBC9 at serine 71 to increase the thioester bond formation between phosphorylated UBC9 and SUMO-1 (178). UBC9 can also be modified by SUMO at lysine 14. SUMOylated UBC9 has reduced activity for RanGAP1, but enhanced activity for Sp100 (101).
V. SUMO-CONSENSUS MOTIF
The surface of UBC9 involved in SUMO-1 binding is positively charged, which binds to a negatively charged surface in SUMO-1 (51). Moreover, UBC9 also contains a surface that interacts with target proteins (51). Indeed, UBC9 has been shown to bind to a large number of proteins using yeast two-hybrid interaction assay (215). Many of the UBC9-interacting proteins contain the SUMO consensus motif composed of ψK(Q/T)E, and this motif is required for direct UBC9 binding (88, 158). ψ is used to denote any large hydrophobic amino acid. An inverted consensus motif (E/D)XKψ has been reported for SUMO-2 conjugates (124). UBC9 binding can be enhanced by negatively charged amino acid patch downstream of the SUMO consensus motif, ψKXEXXEEEE (NDSM) (213), or by a proline-directed phosphorylation site adjacent to the SUMO consensus motif, ψKX(pS)P (PDSM) (74, 130), or by a hydrophobic cluster SUMO motif, ψψψKXE (HCSM) (124). Many predicted algorithms for the SUMOylation sites are available on the internet. The sensitivity of these algorithms ranges from 0.54 to 0.70, and the specificity ranges from 0.53 to 0.89 (28). A large-scale proteomic study identified 954 SUMO-3 modified lysine resides, and 86% of the sites have not been reported (105). A recent study using mass spectrometry to detect endogenous SUMOylation sites revealed 1,209 unique sites (122). Sixty percent of the proteins have multiple SUMOylation sites, and 5% have more than six SUMO conjugation sites. Interesting, 60% of these SUMO sites do not follow the ψK(Q/T)E or (E/D)XKψ motif (122). The non-consensus sites could be generated through a nearby SUMO-interacting motif or other unknown determinants (15). Furthermore, 30% of the lysine residues are also modified by ubiquitin. The same lysine residue can be modified by ubiquitin or acetylated to create a competition for posttranslational protein modification (113, 189).
VI. SUMO-INTERACTING MOTIF
In addition to covalent conjugation of substrates, SUMO can also interact with other proteins through a SUMO-interaction motif (SIM) (127). SIM was initially defined as hhXSXS/Taaa, where h is a hydrophobic amino acid and a is an acidic amino acid. However, with the use of nuclear magnetic resonance spectroscopy analysis, SXS followed by several acidic residue was not involved in binding to SUMO1. Instead, V/I-X-V/I-X-V/I was found to interact strongly with SUMO1 and was designated as a SUMO-binding motif (SBM) (174). SBM binds to a deep groove in SUMO-1 that is lined with hydrophobic and aromatic amino acids (175). SBM-derived different peptides can bind in reverse orientation to the same groove in SUMO-1 (175). However, noncovalent interaction of SUMO-1 with UBC9 or dipeptidyl peptidase 9 (DPP9) occurs at a patch surrounding E67 of SUMO-1 (25, 102, 142). This interaction occurs on the opposite surface of the active site cysteine residue of UBC9 and serves to activate DPP9. Some of the SUMO and SIM interaction is paralog-specific as SIM of DPP9 binds to SUMO-1, but less to SUMO-2. Another example is the DAXX SIM, I-I-V-L-S-D-S-D, which binds to a concave surface of SUMO-1 (114), and phosphorylation of the two serine residues promotes binding to SUMO-1, but not SUMO-2/3 (27). The main function of SIM, however, is to recruit effectors downstream of a SUMOylated protein. For example, SUMO-1 modification of a key residue of LKB1 is required to activate the downstream kinase AMP kinase (153). Furthermore, multiple SIMs can be used to target poly-SUMO chains chain modified PML for ubiquitin-mediated degradation by SUMO-targeted ubiquitin ligase RNF4 (104, 181, 184). SUMO-SIM interaction can be regulated by phosphorylation (177) and acetylation (188).
VII. SUMO LIGASES
Although UBC9 can catalyze conjugation of SUMO to many target proteins in vitro, SUMO-specific ligases are often required for in vivo SUMOylation. The major SUMO E3 ligases are the protein inhibitors of activated signal transducer and activator of transcription (STAT) (PIAS) (66, 82, 86, 129). It contains the SP-RING and SP-CTD domains that bind to UBC9 or SUMO, respectively (221). The NH2-terminal PINIT domain is required for binding of a PIAS-dependent substrate, such as proliferating cell nuclear antigen (PCNA). The PIAS family includes PIAS1, PIAS2 (PIASx), PIAS3, and PIAS4 (PIASy) that serve as SUMO E3 ligases for a large number of substrates (66, 86, 129, 144).
Although individual PIAS gene is not essential for life (155, 159, 206), each PIAS could have a unique substrate and function. For example, PIAS1 plays a critical role in the suppression of nuclear factor (NF)-κB target genes (182). The SUMO E3 ligase activity of PIAS1 is required in pro-inflammatory stimuli-induced phosphorylation of PIAS1 to repress inflammatory gene activation (117). This could be due to SUMOylation of IKKα, which is the kinase for PIAS1 phosphorylation (115). Furthermore, PIAS1 is the E3 ligase for peroxisome proliferator-activated receptor (PPAR)γ; SUMOylated PPARγ binds to nuclear receptor corepressor-histone deacetylase 3 complex on the promoters of inflammatory genes to repress transcription (140). PIAS4 can also collaborate with PIAS1 to control STAT1 activation and pro-inflammatory gene suppression (182). PIAS1 and PIAS4 are recruited at the site of DNA double-strand break and play an important role in DNA repair (47). PIAS4 is involved in the SUMOylation of ORC2, which is important in maintaining genomic stability (196).
RanBP2 is another SUMO E3 ligase that binds to the cytoplasmic filament of the nuclear pore complex (141). It is quantitatively associated with SUMOylated RanGAP1 and UBC9 in a stable complex (205). UBC9 in this complex serves as glue for stable complex formation; a second UBC9 is required to catalyze the transfer of SUMO to RanBP2 substrates. The internal repeat 2 (IR2) region of RanBP2, which binds to SUMO-1 and UBC9, is considered as the catalytic center. Free RanBP2 does not have the SUMO E3 ligase activity. This complex is analogous to the multi-subunit ubiquitin ligase, such as the cullin-RING ubiquitin E3 ligase. RanBP2 preferentially binds to SUMO-1, but not SUMO-2/3; thus RanBP2 could be a paralog-specific SUMO E3 ligase (186). The RanBP2 E3 ligase complex also dissembles the crm1-dependent nuclear export complexes (154) and is poised to play an important role in the regulation of nuclear transport.
The PIAS family and RanBP2 complex can catalyze SUMOylation of a large number of substrates. However, there also exist a number of ligases that only enhance SUMOylation of limited number of substrates. For example, PC2 increases SUMOylation of the transcription co-repressor CtBP1 by recruiting both UBC9 and CtBP to the polycomb body (84) and RSUME enhances SUMOylation of hypoxia-inducible factor 1α (HIF1α) and IKβ by increasing the binding of SUMO to UBC9 (26). Some of the SUMO E3 ligases are organelle-specific, such as MAPL (mitochondrial-associated protein ligase), which enhances SUMOylation of dynamin-related protein 1 (Drp1) in the mitochondria (20), or tissue-specific, such as Rhes, which catalyzes SUMOylation of mutant Huntingtin in the corpus striatum (179). Topors, a ubiquitin ligase, is a dual-specific E3 ligase as it also enhances SUMOylation of p53 and topoisomerase 1 (60, 149, 202). ZATT binds to the topoisomerase 2 cleavage complex to increase its SUMOylation and to enhance the binding of a tyrosyl-DNA phosphodiesterase to SUMO-Top2 to resolve the cleavage complex (161). It is clear that the number of SUMO ligases is far below that of the ubiquitin ligases. Thus it remains to be determined how a small number of SUMO ligases are capable of distinguishing the large number of SUMO substrates.
VIII. SUMO-TARGETED UBIQUITIN LIGASE
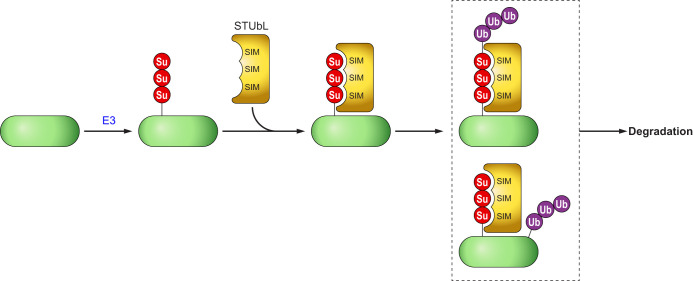
In the first decade of SUMO research, SUMOylation and ubiquitination were considered two parallel pathways carrying different biological functions (215). In particular, SUMOylation was considered important in the regulation of protein localization and function, but not stability. This view was changed in 2007 with the discovery of SUMO-targeted ubiquitin ligase (STUbL), which binds to the SIM domains of SUMOylated proteins to catalyze ubiquitin-conjugation on the SUMO chains or on the target proteins (FIGURE 2) (104, 113, 147, 181, 184). RNF4 was initially identified as a RING-finger protein that binds to PML3 in a SUMO-1-dependent manner (59). RNF4 was later shown to ubiquitinate SUMO-conjugated substrates dependent on SUMO-SIM interaction (181). RNF4 binds to SUMO-2 chains to ubiquitinate PML-RAR on lysine 48 leading to arsenic-induced degradation of PML-RAR (104, 184). In acute promyelocytic leukemia, PML is fused to the RAR gene to form a hybrid protein PML-RAR, which affects maturation of myeloid cells (216). Arsenic trioxide, an ancient Chinese medication, is effective in degrading PML-RAR in a proteasome-dependent manner (29, 166). Mechanistically, arsenic binds to the histidine residues in the RING finger domain of PML-RAR to induce PML-RAR oligomerization, which facilitates binding of UBC9 to SUMOylate PML-RAR (224). RNF4 catalyzes formation of ubiquitin chains on PML-RAR itself or the poly-SUMO chain on PML-RAR (184). The finding of RNF4 as the STUbL that ubiquitinates SUMO2-modified PML-RAR provides an elegant demonstration of the SUMO and ubiquitin cross talk. Arkadia (RNF111) is another example of a STUbL that binds to poly-SUMO chains. There is a strong affinity of Arkadia for SUMO-1-capped SUMO2/3 chains (176). However, the significance of the mixed SUMO chains is currently unknown.
FIGURE 2.
Small ubiquitin-like modifier (SUMO)-targeted ubiquitin ligases (STUbL). A poly-SUMOylated substrate provides the platform for binding to a STUbL that binds to the SUMO chains through SUMO-interactive motif (SIM). The STUbL can conjugate ubiquitin either on the poly-SUMO chain itself or on a separate lysine residue of the substrate, leading to degradation through the ubiquitin/proteasome pathway. Another excellent review provides more detail in STUbL mechanism (113).
IX. SENTRIN/SUMO-SPECIFIC PROTEASES
The main SUMO deconjugating enzymes in the mammalian cells are the Sentrin/SUMO-specific proteases (SENPs) (215). Two additional protease families have been identified that can de-conjugate SUMO from limited number of substrates (162, 167). There are six SENPs (215). They shared a conserved catalytic domain in the COOH terminus that contains the active-site pocket of His-Asp-Cys (131). They can be grouped into three separate families: SENP1 and SENP2, SENP3 and SENP5, as well as SENP6 and SENP7. SENP1, SENP2, SENP3, and SENP5 resemble Ulp1 in yeast, whereas SENP6 and SENP7 function more like Ulp2 (133) (FIGURE 3).
FIGURE 3.

Small ubiquitin-like modifier (SUMO)-protease family. The green cylinder represents the catalytic domain of Sentrin/SUMO proteases. Several reviews are excellent sources for more mechanistic details (73, 133).
SENP1 is the first mammalian enzyme reported (56); it is 21% identical and 50% similar to the yeast Ulp1 (109). SENP1 was found to be localized mostly to the nucleus (56). It catalyzed the de-SUMOylation of SUMO-modified PML, but not RanGAP1 in vivo. However, SENP1 can deconjugate SUMOylated RanGAP1 in vitro. This could be due to the observation that SUMOylated RanGAP1 is an integral part of the RanBP2 E3 ligase complex and may be protected from SENP1 in vivo (205). SENP1 contains both nuclear localization and export signals in the NH2 or COOH terminus, respectively (6, 99). Thus SENP1 could also regulate cytoplasmic substrates (44). SENP1 can process all SUMO precursors to mature form through its COOH-terminal hydrolase activity (32). In the SENP1-null embryo, SUMO-1 precursors are poorly processed suggesting that SENP1 is the main COOH-terminal hydrolase for SUMO-1 (32). SUMO conjugates bind to a tryptophan channel in SENP1 that leads to isomerization of the scissile peptide bond and kinking of the target to a right angle that facilitate SENP1 cleavage (164). SENP1 preferentially cleaves SUMO-1 over SUMO-2 due to the ability of SENP1 to orient the scissile bond of SUMO-1 conjugates more efficiently (165). SENP1 is highly expressed in the prostatic intraepithelial neoplasia and prostate carcinoma tissues (31), and its expression is regulated by androgen (8). SENP1 is also induced by interleukin (IL)-6, which is elevated in prostate cancer patients (31).
SENP2 is 57% identical to SENP1 in the catalytic domain. The NH2-terminal 70 amino acids promote binding of SENP2 to nucleoporin 153 (Np153), which is localized at the nucleoplasmic surface of the nuclear pore complex (62, 223). Binding to Np153 restricts SENP2’s access to other nuclear target; thus removing the NH2-terminal 70 amino acids of SENP2 would markedly increase the ability SENP2 to deconjugate SUMOylated substrates (62). In addition to the nuclear localization signal (NLS) in the NH2 terminus, SENP2 also possesses a nuclear export signal (NES) localized before the catalytic domain (79). SENP2 was shown to shuttle between the nucleus and cytoplasm, and SENP2 in the cytosol can be ubiquitinated and degraded by the proteasome (79). The localization of SENP2 is also regulated by disturbed flow in endothelial cells. Disturbed flow activates the serine/threonine kinase p90RSK, which phosphorylates SENP2 at threonine 368 that promotes export of SENP2 from the nucleus to cytosol (72).
SENP3 and SENP5 belong to the second SENP family and are localized predominantly in the nucleolus (31, 220). Both SENP3 and SENP5 bind to B23/nucleophosmin, a nucleolar protein involved in ribosome biogenesis (220). SENP3 is degraded by ubiquitin/proteasome constitutively; however, mild oxidative stress stabilizes SENP3 to allow SENP3 to redistribute from the nucleolus to nucleoplasm (78). Low oxidative stress also enhances the localization of SENP3 to the nuclear body to deconjugate SUMO2/3 from PML, which accelerates cell proliferation (61). SENP5 was found to deconjugate SUMO-1 modified DRP1, a key regulator of mitochondrial fission (64, 227).
SENP6 and SENP7 belong to the third family of the SENP; these two proteases are characterized by insertion of 50–200 amino acids within the catalytic domain (215). Full-length SENP7L and a shorter SENP7S, lacking exon 6-encoded amino acids, have distinct biological function (9). The SENP7L transcript increases with the onset of metastatic breast cancer in human patient samples (9). Tumor metastasis further increases SENP7L transcript in the primary tumor samples (9). SENP6 possesses SUMO chain-editing activity, but no COOH-terminal hydrolase activity (132).
De-SUMOylating isopeptidase-1 (DeSI-1) is a new SUMO-deconjugating enzyme distinct from the SENPs (167). DeSI-1 deconjugates SUMO-modified BZEL, but not PML or ΔNP63. DeSI-1 does not have COOH-terminal hydrolase activity, but possesses weak chain-editing activity. DeSI-1 belongs to the PPPDE (permuted papain fold peptidases) family. A closely related DeSI-2 was also identified; however, DeSI-2 was not characterized in detail. Both DeSI-1 and DeSI-2 are shorter in length than the SENPs (168 amino acids for DeSI-1 and 573 for SENP3). DeSI-1 crystal structure reveals a homodimer with the conserved cysteine and histidine residues in a groove between the two subunits (180). Another SUMO protease, USPL1, has been shown to have potent isopeptidase and chain-editing activity, but weak COOH-terminal hydrolase activity (162). USPL1 colocalizes with coilin in the Cajal body; deletion of USPL1 affects coilin distribution in a SUMO-protease-independent manner. The biological significance of USPL1 is unknown. Again, the number of SUMO deconjugating enzymes is limited compared with the ubiquitin deconjugating enzymes. Regulation of SENPs by other posttranslational modification or through redox control is critical in providing specificity of de-SUMOylation.
X. SUMO AND OXYGEN SENSING
SENP1-null embryo died between day 13 and 15 of gestation due to severe fetal anemia, resulting from an increase of apoptosis of erythroid precursors (32). Erythropoietin (EPO) production is reduced in the SENP1 null fetal liver, and restoration of EPO prevents apoptosis in SENP1 null fetal liver cells. SENP1 regulates EPO gene transcription through HIF1α, and HIF1α protein level is markedly reduced in the absence of SENP1. It is well known that in the presence of oxygen, HIF1α is proline-hydroxylated which allows for binding of VHL-containing ubiquitin E3 ligase to degrade HIF1α through the ubiquitin/proteasome pathway (FIGURE 4, left panel) (80). Importantly, the SUMO pathway provides another layer of control over oxygen sensing. Under hypoxic condition, HIF1α is SUMOylated and can be degraded by the ubiquitin/proteasome pathway (32). SENP1 is required for removal SUMO from SUMO-conjugated HIF1α to prevent its degradation; thus SENP1 null embryo had marked reduction in HIF1α level and severe fetal anemia (FIGURE 4, right panel). Since HIF1α regulates erythropoiesis, angiogenesis, and gluconeogenesis, SENP1 null MEF cells are also defective in hypoxia-induced expression of VEGF and glucose transporter 1 (Glut-1) (32) (FIGURE 4). HIF1α also induces SENP1 expression by binding to two hypoxia-responsive elements in the SENP1 promoter (210). Thus SENP1 and HIF1α form a feed-forward loop under the hypoxic condition. In another SENP1 knockout mouse, defective erythropoiesis was attributed to hyper-SUMOylation of GATA1, which resulted in reduced EPO receptor transcription (218). A SENP1 polymorphism has been linked to susceptibility of chronic mountain sickness in the Andean highlanders (75).
FIGURE 4.
Sentrin-specific protease 1 (SENP1) controls hypoxia-inducible factor 1α (HIF1α) stability under the hypoxic condition. Under hypoxic condition, HIF1α is modified by SUMO-1 and can still be degraded by the ubiquitin-proteasome pathway. SENP1 is required to remove SUMO from SUMO-HIF1α to stabilize it. Epo, erythropoietin; Glut-1, glucose transporter 1; PHD, prolyl hydroxylase domain; VEGF, vascular endothelial growth factor; VHL, Von Hippel-Lindau. [From Cheng et al. (32), with permission from Elsevier.]
XI. SUMO AND CANCER
Both SUMOylation and de-SUMOylation enzymes have been implicated in cancer pathogenesis (11, 92, 217). This most likely represents the delicate homeostasis of the SUMOylation status of proteins that are involved in DNA repair, cell division, and cellular signaling in normal cells and its dysregulation in cancer cells. Dysregulation of SUMO enzymes is associated with multiple types of cancer; furthermore, specific mutant protein, such as microphthalmia-associated transcription factor (E318K), is associated with melanoma and renal cell carcinoma. They are discussed in detail below.
Activating mutations of the Ras oncogene are frequently observed in pancreatic, lung, colorectal, and other cancers. With the use of genome-wide shRNA screen, SAE1 and Ubc9 (SUMO E1 and E2 conjugating enzymes) were identified as KRAS synthetic lethal partners (217). This was dependent on SUMO-1, but not SUMO-2/3, modification of a group of KRAS-associated SUMOylated proteins (KASPs). A prime example of the KASPs is KAP1, a transcription repressor with three major SUMOylation sites. With the use of a similar screen with Myc-driven tumors, SAE2, a component of SUMO E1, was shown as the most significant synthetic lethal partner (92). This is probably due to the requirement for SUMOylation of proteins involved in mitotic spindle formation. In human breast cancer tumors with high Myc expression, low SAE1 and SAE2 expression correlated with longer metastasis-free survival. Thus targeting SUMO E1 or E2 enzymes may be useful in treating Ras- or Myc-driven tumors.
SENP1 is highly expressed in prostatic intraepithelial neoplasia and prostate carcinoma, but not in normal prostate epithelia (8). Chronic androgen exposure induces SENP1 expression through an androgen-responsive element in the promoter of SENP1. Knocking down SENP1 markedly reduces prostate cancer cell proliferation (8). SENP1 also enhances androgen-receptor-mediated transcription by deconjugating SUMO-1 from SUMO-1 modified histone deacetylase 1, forming a feed-forward loop (33).
In a SENP1 transgenic model, in which the murine SENP1 is driven by a probasin promoter, HIF1α is stabilized (7). An increase in expression in the prostate gland leads to an increase in VEGF expression and an increase in angiogenesis (FIGURE 5). Moreover, there is also an increase in cyclin D1 expression and an increase cell proliferation (7). By 12 mo, high-grade intraepithelial neoplasia is present; however, prostate pathology does not progress to carcinoma (7). Upon closer examination, the prostate lumen of the 12-mo-old SENP1 transgenic mice accumulated apoptotic epithelial cells (10). This is most likely due to SENP1-induced stabilization of phosphatase and tensin homolog (PTEN); in normal homeostasis, PTEN is degraded through a SUMO-targeted ubiquitin ligase, WWP2 (10). Thus prostate carcinoma can be induced in the SENP1 transgenic mice by mating them with PTEN+/− (10). In humans, higher SENP1 expression correlates with higher Gleason score and an increase in recurrence after prostatectomy (110). Momordin, a SENP1 inhibitor, suppresses prostate cancer PC3 tumor growth in xenograft model (207). In addition to prostate cancer, SENP1 overexpression has also been implicated in the development of bladder cancer (21), breast cancer (23, 128, 200), neuroblastoma (208), osteosarcoma (118), and lung cancer (197).
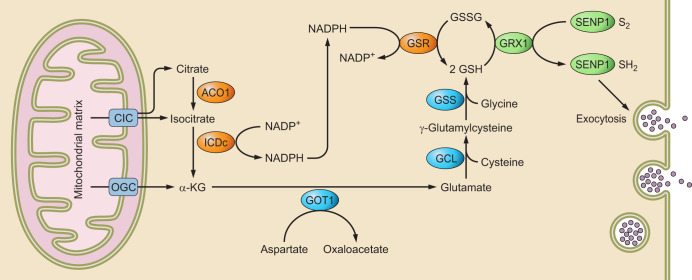
FIGURE 5.
Redox regulation of Sentrin-specific protease 1 (SENP1) activity controls insulin secretion. Cytosolic isocitrate dehydrogenase (ICDc)-dependent generation of NADPH is followed by glutathione (GSH) reduction and glutaredoxin 1 (GRX1) activation, leading to reduction of the SENP1 active site cysteine that de-SUMOylate proteins that are involved in exocytosis of insulin from insulin-containing granules. α-KG, α-ketoglutarate; ACO1, aconitase; CIC, citrate carrier; GCL, glutamate-cysteine ligase; GOT1, glutamic-oxaloacetic transaminase 1; OGC, oxoglutarate/malate carrier. [From Ferdaoussi et al. (44), with permission from American Society of Clinical Investigation.]
SENP3 is phosphorylated by p19ARF, a tumor suppressor that inhibits the Mdm2 ubiquitin E3 ligase for p53 (121). SENP3 phosphorylation is followed by ubiquitination and proteosomal degradation (103). Both SENP3 and ARF are predominantly localized in the nucleolus and bind to nucleoplasmin, which is modified by SUMO-2 at lysine 263 (201). Loss of the ARF locus on chromosome 9p21 is the most frequent cytogenetic changes in human cancer (97). Thus SENP3 expression is increased in prostate, ovarian, lung, rectal, and colon cancers most likely due to loss of ARF regulation (61). One of the SENP3 substrates is p300, which facilitates HIF1α-mediated expression of VEGF (78). Overexpression of SENP3 in Hela cells generated more aggressive tumors in a xenograft mouse model (78). In addition to regulation by ARF-mediated ubiquitination and degradation, SENP3 is also regulated by oxidative stress (78). SENP3 is constitutively degraded by the proteasome in the nucleolus; mild oxidative stress inhibits the ubiquitin-proteosomal mediated degradation of SENP3 (78). H2O2 promotes the redistribution of SENP3 from the nucleolus to the nucleoplasm to regulate nuclear substrates, such as SUMO2/3-modified p300, which in conjunction with HIF1α induces the expression of VEGF to increase angiogenesis (78).
SENP7 is expressed in breast epithelia as a full-length transcript (SENP7L) or a shorter transcript (SENP7S), due to alternative splicing of exon 6 (9). In breast carcinoma, SENPL is highly expressed with an even higher expression in metastatic breast cancer cells. SENP7L contains an interaction domain for the epigenetic remodeler heterochromatin protein 1α (HP1α). This interaction domain is encoded by exon 6, which is deleted in SENP7S. SENP7L binds to HP1α to deconjugate SUMO-modified HP1α. SUMOylated HP1α is enriched at mesenchymal gene promoters to silence these genes, promoting cellular senescence. SENP7L renders HP1α hypo-SUMOylated, which removes transcriptional repression of the mesenchymal genes and concurrently decreases transcription of epithelial-promoting genes via an HP1α-independent mechanism (9). Thus SENP7L level correlates with epithelial mesenchymal transition and invasiveness of breast cancer cells. Stable knockdown of SENP7L reduces the dissemination of highly metastatic breast cancer cells to the lungs from primary implantation sites. Thus differential splicing of the SENP7 regulates either breast cancer suppression or progression. SENP7 also promotes chromatin relaxation to create a permissive environment for DNA repair by removing SUMO2/3 from KAP1, which binds to the chromatin remodeler CHD3 (48). SENP7 also stabilizes c-Myc by removing SUMO-2 from c-Myc, which is ubiquitinated by a STUbL, RNF4, for proteasomal degradation (57).
Origin recognition complex 2 (ORC2) is essential for DNA replication initiation in eukaryotic cells (12). In addition, ORC2 is localized to the centromere during the G2/M phase (35). ORC2 is modified by SUMO-2, but not SUMO-1, at the G2/M phase of the cell cycle catalyzed by PIAS4 and reversed by SENP2 (196). SUMO2 modification of ORC2 is important for the recruitment of KDM5A to convert H3K4me3 to H3K4me2, a “permissive” histone marker for α-satellite transcription at the centromere (77). Persistent expression of ORC2 that cannot be conjugated by SUMO-2 led to reduced α-satellite transcription and impaired pericentric heterochromatin silencing, resulting in re-replication of heterochromatin DNA. DNA re-replication activated the DNA damage response, bypassing mitosis to form polyploid cells. Thus SUMOylation of ORC2 is essential for centromere histone methylation to prevent DNA re-replication and genomic stability. SUMOylation and DNA repair have been extensively reviewed and will not be discussed further (13, 40, 41, 81).
Microphthalmia-associated transcription factor (MITF) is a transcription factor that regulates genes essential for melanin biosynthesis in melanocytes and HIF1α. MITF was shown to bind to UBC9 and modified by SUMO-1 at lysine 182 or 216 by PIAS3 (126, 209). A germline missense substitution in MITF(E318K) was shown to occur at a significantly higher frequency in patients affected with melanoma, renal cell carcinoma, or both (14). Codon 318 is located in a SUMO consensus site; thus MITF(E318K) cannot be SUMOylated, but binds to the HIF1α promoter more avidly to enhance HIF1α transcription. Gene expression profiling in a renal cell carcinoma cell line showed a MITF(E318K) signature promoting cell growth, proliferation, and inflammation (14). Furthermore, the MITF(E318K) increases melanocytic and renal cell clonogenicity, migration, and invasion.
SUMO-1-modified heat shock protein 90β isoform α (SUMO-1-HSP90) is a dominantly inherited risk factor for plasma cell dyscrasia, including monoclonal gammopathies of undetermined significance, multiple myelomas, and Waldenstrom’s macroglobulinemias (146). Paraproteins derived from patients with plasma cell dyscrasia bind to SUMO-1-HSP90, which accumulates in patients with deficient SENP2 activity. However, SENP2 message and protein levels were not altered in patients carrying SUMO-1-HSP90 (146). Thus additional factors that prevent SENP2 from de-conjugating SUMO-1 from SUMO-1-HSP90 must be identified. This association highlights the complexity of implicating the SUMOylation pathway in cancer pathogenesis. Nonetheless, the SENPs, especially SENP1, are excellent drug targets for cancer therapy. SUMO-pathway-related alterations that are associated with each type of cancer are summarized in TABLE 5.
Table 5.
Alterations in SUMO enzyme or substrates in cancer
| Cancer | Alterations in SUMO Enzyme or Substrate |
|---|---|
| Breast cancer | Low SAE1 and SAE2 expression correlated with longer metastasis-free survival, SENP7L (9) |
| Colorectal cancer | ↑SAE1, ↑Ubc9, ↑SENP3 (61, 92, 217) |
| Lung cancer | ↑SAE1, ↑Ubc9, ↑SENP3 (61, 92, 217) |
| Melanoma | MITF (E318K) (14) |
| Ovarian cancer | ↑SENP3 (61) |
| Pancreatic cancer | ↑SAE1, ↑Ubc9 (92, 217) |
| Plasma cell dyscrasia | ↑SUMO-1-HSP90 (146) |
| Prostate carcinoma | ↑SENP1, ↑SENP3 (31, 61) |
| Renal cell carcinoma | MITF (E318K) (14) |
SENP, Sentrin-specific protease; SUMO, small ubiquitin-like modifier; SUMO-1-HSP90, SUMO-1-modified heat shock protein 90β isoform α.
XII. SUMO AND HEART FAILURE
Endogenous SUMO-1 expression was shown to be reduced in experimental animal and human heart failure (93). This leads to reduced SUMO-1-modified SERCA2a, the key ATPase for calcium reuptake into sarcoplasmic reticulum during excitation-contraction coupling in the cardiomyocytes (93). SUMO-1 modification of SERCA2a at lysine 480 and 585 increased SERCA2a stability and increased its ATPase activity. Adenoviral-mediated delivery of SUMO-1 restored SERCA2a expression and activity in animal model of heart failure. Most importantly, SUMO-1 gene delivery improved cardiac ejection fraction in large animal models. Knocking down SERCA2a caused severe impairment of cardiac function both in vitro and in vivo, which is not restored by SUMO-1 overexpression (93). Thus the beneficial effect of SUMO-1 gene therapy is mediated by SERCA2a. N106, a small molecule activation of SERCA2a SUMOylation, was discovered in an activity screen that appeared to function as a SUMO E1 activator (94). N106 binds to a pocket in SAE1 that consists of glutamine 312 and valine 315. In a transverse aortic constriction model of heart failure, N106 increased SERCA2a level and reversed the decline in contractility. N106 appears to be specific for SERCA2a as it has no effect on SERCA2a knockout mice (94). The importance of SUMO-1 in cardiomyocyte function is also demonstrated by miR-146a, which negatively regulated SUMO-1 expression. miR-146a was found to be increased in animal and human heart failure, and overexpression of miR-146a reduced SUMO-1, SERCA2a, and cardiac contractility in vitro and in vivo (136). Interestingly, fibroblasts from failed heart secrete exosome that delivers miR-146 to cardiac myocytes to reduce SUMO-1 expression (136).
SUMO2/3-modified proteins were also shown to be increased in the heart samples from patients with end-stage heart failure (96). In the SUMO-2 transgenic mice (SUMO-2 GG under the control of cardiac myosin heavy chain promoter), the hearts show marked hypertrophy and increase in apoptosis. Calpain 2 and Calpastatin (an inhibitor of Calpain) are both modified by SUMO-2 (96). SUMO-2-modified Calpain 2 has increased enzymatic activity, whereas SUMO-2-modified Calpastatin is more prone to caspase cleavage. The net effect of SUMO-2 overexpression is to enhance Calpain activity to increase apoptosis. The overall balance of the SUMOylation and de-SUMOylation is critical for the homeostasis of cardiomyocytes. Thus overexpression of SUMO-2 causes development of cardiomyopathy, whereas overexpression of SENP5 also causes cardiomyopathy (95). In the heart of SENP5 transgenic mice, Drp1 is maintained in a hypo-SUMOylated state to decrease mitochondrial fission. Thus the mitochondria of SENP5 transgenic hearts at an early developmental stage were significantly larger compared with those in the control hearts. Overexpression of Bcl2 in SENP5 transgenic hearts improved cardiac function, perhaps through its regulation of mitochondrial function.
In a diabetes/myocardial infarction (MI) animal model, ERK5 was shown to be highly modified by SUMO-2/3 (168). SUMOylation of ERK5 can also be observed in cardiomyocytes exposed to H2O2 or high glucose, two well-known mediators of diabetes. SUMOylated ERK5 has reduced transcription activity, which may be responsible for the development of worsened heart failure in the diabetic patients following MI. ERK5 SUMOylation is blunted by binding to MEK5a, and transgenic mice with MEK5a overexpression have reduced ERK5 SUMOylation after MI. The MEK5a overexpressing mice also have preserved left ventricular (LV) ejection fraction after MI. The importance of ERK5 in cardiac physiology is also evidenced by the poor LV function in mice with cardiomyocyte-specific deletion of ERK5 following metabolic stress with high-fat diet (119). ERK5 regulates the expression of pGC1a to control mitochondrial metabolism. Thus SUMOylation of ERK5 is a key determinant of the development of stress-induced diabetic cardiomyopathy.
XIII. SUMO AND ATHEROSCLEROSIS
Atherosclerotic lesions appear predominantly in regions of blood vessels with disturbed blood flow, which activates the serine/threonine kinase p90RSK to phosphorylate SENP2 at threonine 368 (T368) (72). T368 phosphorylation of SENP2 promotes nuclear export of SENP2 and inactivates SENP2’s enzymatic activity. Export of phosphorylated SENP2 from the nucleus causes p53 and ERK5 hyper-SUMOylation, leading to downregulation of endothelial nitric oxide synthase expression and upregulation of proinflammatory adhesion molecule expression and apoptosis. Thus p90RSK-mediated phosphorylation of SENP2 is a key regulator in disturbed-flow-induced signaling, responsible for endothelial dysfunction and atherosclerosis (72). Disturbed flow also increases protein kinase C (PKC)-ζ activation, leading to SUMOylation of p53. SUMOylation of p53 is catalyzed by PIASy and reversed by SENP2. SUMOylated p53 is exported from nucleus to cytosol to enhance endothelial cell apoptosis (71). SENP2+/− mice demonstrated increased leukocyte rolling and accelerated formation of atherosclerotic plaques. Thus SENP2 phosphorylation under disturbed flow leads to SUMOylation of p53 and ERK5 to promote atherosclerotic plaque formation (71).
Disturbed-flow-activated p90RSK also phosphorylates membrane-associated guanylate kinase with inverted domain structure-1 (MAGI1), which activates Rap1 to induce endothelial cell activation (1). Furthermore, p90RSK binds to MAGI1 to facilitate transport of this complex to the nucleus to phosphorylate SENP2. MAGI1 also binds to ATF6 to transport ATF6 to the nucleus and activate the ER stress response and endothelial cell apoptosis. Interestingly, MAGI1 is modified by SUMO-2/3 in the cytosol, and its SUMOylation status is regulated by SENP2. Thus disturbed flow activates p90RSK to regulate SENP2 localization through phosphorylation. SENP2 then regulates the SUMOylation status of MAGI1, ERK5, p53 to control endothelial cell activation and apoptosis.
XIV. SUMO AND DIABETES
SENP1 plays important roles in insulin secretion in type 2 diabetes (44) and adipocyte-derived inflammatory cytokine production in type 1 diabetes (163). In type 2 diabetes, insulin secretion from β cells of the pancreatic islets of Langerhans is defective (85). Insulin secretion from β islet cells required a metabolism-dependent closure of ATP-sensitive potassium (KATP) channels (4) and a KATP-independent amplifying step (145). SENP1, a redox-sensitive enzyme, is localized with the insulin granules in the β islet cells and is activated by mitochondrial-derived NADPH and glutathione (44). SENP1 regulates the SUMOylation status of synaptotagmin VII (37) and several proteins involved in granule trafficking and exocytosis (36, 49, 50). These studies showed that inactivation of SENP1 by oxidative stress may be a crucial link to impaired insulin secretion in type 2 diabetes (5) (FIGURE 5).
Deletion of SENP1 from adipocytes, but not smooth muscle cells or endothelial cells, causes elevated glucose in animal models of type 1 diabetes (163). There is a sharp decline in the number of β cells in the islet when SENP1 is deleted from the adipocytes. Both CD4+ and CD8+ T cells are recruited in the islets, leading to immune-mediated β cell destruction. The pancreatic adipose tissue in the SENP1 null mice secretes large amount of IL-1β, IL-6, tumor necrosis factor-α, and CCL5 (RANTES, CCR5 ligand), which increases recruitment of inflammatory cells to the islet. In the SENP1 null adipocytes, NF-κB signaling is elevated due to an increase in SUMOylation of NEMO, a component of the IKK complex (106). Interestingly, the inflammation-driven destruction of the β islet cells is exacerbated by high-fat diet and ameliorated by inhibition of NF-κB signaling.
SUMOylation may also play a role in maintaining the health of β cells to prevent development of diabetes (67). NRF2, a key regulator of cellular response to oxidative stress, is SUMOylated at K525 and K595, and SUMOylated NRF2 is transcriptionally more active and more stable (67). When UBC9 is conditionally deleted from β cells, NRF2 expression and activity in the nucleus are both reduced, resulting in a lower antioxidative response and accumulation of reactive oxygen species. 90% of the β cell mass was lost 75 days after inducible deletion of UBC9, suggesting that SUMOylation plays a critical role in the viability of β cells. Ten weeks after induction of UBC9 deficiency, all mice developed diabetes. However, it is not known whether additional SUMOylated proteins are involved in the preservation of β cell viability.
PPARγ2 is modified by SUMO-1 at K107 in the NH2 terminus, causing a strong repression of its transcriptional activity (46, 140, 211). SUMOylation increases binding of nuclear receptor corepressor (NCoR) and histone deacetylase 3 (HDAC3) complex to PPARγ, preventing degradation of the repressive complex (140). The role of SUMO modification in K107 of PPARγ2 in adipocyte differentiation and insulin sensitivity was investigated in the K107R knock-in mice (91). The K107R mice indeed had higher insulin sensitivity as evidenced by increase in glucose-infusing rate without an increase in adiposity and adipocyte differentiation in vitro. However, the K107R mutation is less efficacious than rosiglitazone treatment in increasing insulin sensitivity and glucose tolerance. PPARγ coactivator 1α (PGC-1α) is a master regulator of mitochondrial biogenesis and is known to be modified by SUMO-1 (156). SUMOylated PGC-1α has reduced transcription activity. SENP1 maintains PGC-1α in a hypo-SUMOylated state to enhance PGC-1α transcription activity and to increase the expression of mitochondrial electron transport chain genes (24).
Fasting induces SENP1 to translocate to the mitochondria (198). SENP1 translocation is dependent on heat shock protein 90 (HSP90) and can be blocked by 17-allylaminogeldanammycin, an HSP90 catalytic inhibitor. In the mitochondria, SENP1 will remove SUMO-1 from SUMO-1-modified Sirt3 to increase Sirt3 deacetylase activity to enhance fatty acid oxidation. Thus the SENP1/Sirt3 axis plays an important role in the regulation of fatty acid oxidation and energy production in the fasting state. Mice with Sirt3 that cannot be SUMOylated do not gain weight with high-fat diet presumably due to absence of the SENP1/Sirt3 axis in metabolic regulation.
SUMOylation also regulates metabolic response to energy stress through the LKB1/AMP kinase axis. When exposed to metabolic stress with glucose starvation or phenformin treatment (mitochondrial complex 1 inhibitor), LKB1 is modified by SUMO-1 at lysine 178 (153). SUMOylation of LKB1 promotes its interaction with AMP kinase through a SIM domain on AMP kinase. Substitution of K178 with arginine abolished SUMO-1 modification of LKB1 and affects downstream signaling of AMP kinase to regulate mitophagy and apoptosis. AMP kinase is composed of a catalytic subunit, AMP kinase α, and two regulatory subunits, AMP kinase β and AMP kinase γ (65). SUMOylation of AMP kinase α is catalyzed by PIAS4, and SUMOylation reduces AMP kinase activity (212). Thus SUMOylation can play a complex role in the regulation of a signaling cascade by affecting both the upstream and downstream kinases.
XV. SUMO AND IMMUNOLOGY
SUMO has been shown to play an important role in both innate and adaptive immunity (63). This review will focus on the role of SUMO enzymes in early T and B cell development, in the differentiation of T cell subsets, and in the activation of T cells. Although SENP1 deletion is embryonal lethal, examination of early lymphoid precursors in the fetal thymus and liver reveals severe impairment in early T and B cell development (189). When SENP1−/− hematopoietic stem cells in the fetal liver were cocultured with OP9-DL1 stromal cells and Flt3L and IL-7 to induce T cell differentiation in vitro, T cell development was arrested at the CD4−/CD8−/CD25−/CD44high (DN1) stage. When cocultured with OP9 stromal cells and cytokines to induce B cell development, SENP1−/− stem cells were arrested at the Pre pro-B stage. Importantly, myeloid development was not affected in the SENP1−/− hematopoietic stem cells. SENP1 deficiency causes accumulation of SUMO-2/3-modified STAT5, which marked downregulation of STAT5 downstream messages, such as bcl-2 and pim-1. STAT5A contains two lysine residues at 696 and 700, close to tyrosine 694; these amino acids are highly conserved in evolution. SUMO modification of either lysine residue would block tyrosine phosphorylation at 694 for STAT5A. Similar to STAT5A, STAT5B also has two conserved lysine residues adjacent to the tyrosine residue. Thus, in the absence of SENP1, STAT5A and STAT5B remain SUMOylated, but not phosphorylated. Interestingly, STAT5A is also acetylated at lysine 696 (lysine 701 for STAT5B), which competes with SUMOylation at the same lysine residue. Thus SENP1 regulates STAT5 acetylation and phosphorylation and its downstream signaling to affect early T and B cell development.
Deletion of SENP3 in Foxp3+ T cells resulted in dysregulation of immune homeostasis (219). The number of Treg in immune tissues was markedly reduced in mice with SENP3 deletion in Foxp3+ Treg cells. SENP3 regulates the SUMOylation status of BACH2, which promotes the differentiation of Treg cells. SUMOylation of BACH2 is required for its nuclear export; thus SENP3 maintains BACH2 in the nucleus to promote Treg differentiation (219). Furthermore, SENP3 is stabilized by reactive oxygen species generated from T cell receptor activation or CD28 activation. Interestingly, depletion of UBC9 in Fox3p+ T cells also led to reduced cell number and impaired activation of Treg cells (38). This is probably due to the requirement of UBC9 in SUMOylation of IRF4, which regulates a number of suppressor molecules secreted by Tref cells (226). IRF4 is modified by SUMO-2, which prevents IRF4 degradation by the ubiquitin/proteasome pathway. This observation is particularly noteworthy because SUMO modification can also target a protein for degradation, but not with IRF4 (184). Furthermore, a SUMO-conjugating enzyme (UBC9) and a SUMO-deconjugating enzyme (SENP3) can regulate Treg differentiation and activation through different mechanisms through different SUMO substrates.
Retineic-acid-receptor-related orphan nuclear receptor gamma (ROR-γt) is a key transcription factor of Th17 T cells that have been implicated in the development human inflammatory disorders (39). ROR-γt is SUMOylated, which leads to repression of IL-17 transcription (169). SUMOylation of ROR-γt facilitates the recruitment of HDAC2 to the promoter of IL-17 to repress IL-17 transcription. Adoptive transfer to T cells expressing SUMO-mutant of ROR-γt led to development of severe colitis. In this report, the SUMO ligase or SENP involved in ROR-γt regulation has not been identified. Furthermore, it is not known whether the SIM of HDAC2 is involved in its interaction with SUMO-ROR-γt.
Activation of T cell receptor induced SUMO-1 modification of PKC-θ at lysine 325 and 506 (199). When PKC-θ null CD4+ T cells were reconstituted with SUMO-deficient PKC-θ, the transduced cells failed to differentiate into TH2 cells. PKC-θ binds to PIASxβ, a SUMO E3 ligase that catalyze SUMOylation of PKC-θ after T cell receptor activation. SENP1 can reverse PKC-θ SUMOylation; however, this was not demonstrated in animal models. SUMO-less PKC-θ did not localize to central supramolecular activation clusters (cSMAC) after T cell receptor activation and failed to recruit CD28 to cSMAC (42). SUMOylation of PKC-θ was required for binding to CD28 or filamin A and for the binding between CD28 and filamin A in the cSMAC. Thus SUMO is important for forming the immune synapse during T cell activation.
XVI. SUMO, SEIZURE, AND SUDDEN DEATH
Although SUMO modification was thought to occur predominantly in the nucleus, membrane proteins, such as ion channels, can also be SUMO substrates (70). SUMOylation has been shown to regulate ion channel stability, assembly, localization, and activity. The importance of SUMO modification in ion channel physiology was demonstrated in a mouse model with partial deficiency of SENP2 (148). This animal model was generated by retaining the neomycin cassette in the floxed SENP2 allele, which reduced SENP2 gene transcription through the neighborhood effect (150). SENP2 deficiency caused accumulation of SUMOylated proteins in the body, in particular the brain and heart. SENP2-deficient mice developed seizure and died suddenly 8 wk after birth with 100% penetrance (148). Freely moving adult mice implanted with chronic cortical or hippocampal electroencephalogram (EEG) electrodes were monitored. EEG recording revealed frequent interictal spike discharges (10–120/h). Whole cell patch-clamp recordings showed that spontaneous firing and firing evoked by current injection in the hippocampal CA3 pyramidal neurons were much higher in the SENP-deficient neuron than the wild-type neuron. Injection of active SENP2, but not heat-inactivated SENP2, suppressed the increase in neuronal firing. Kv1.1 and Kv7.2/7.3, two voltage-gated potassium channels implicated in epileptic seizures, were selected for further investigation (52, 170–172). Kv1.1, Kv7.2, and SENP2 were shown to colocalize in the cultured hippocampal neurons; SENP2 deficiency did not alter the membrane localization of the Kv1.1 and Kv7.2 channel proteins. Both SUMO-1- and SUMO-2-modified Kv1.1 were highly enriched in the SENP2-deficient brain tissues, whereas only SUMO-2-modified Kv7.2 were increased in the SENP2-deficient brain tissues. Hyper-SUMOylation of the potassium channels in the SENP2-deficient neurons reduced amplitude of whole cell potassium current. With the use of the Kv1.1-specific blocker dendrotoxin K to inhibit the Kv1.1 current in the neuron, the amplitude of the current and firing of the CA3 neuron did not differ significantly between the wild-type and SENP2-deficient mice. Thus, although Kv1.1 is an excellent candidate to explain the neuronal hyperexcitability in the SENP2-deficient mice, the patch-clamp results did not support this hypothesis. However, the amplitude of Kv7.2 current was found to be significantly reduced in the CA3 neuron of the SENP2-deficient mice. Inhibition of the M current, conducted by the Kv7.2/Kv7.3 potassium channels (22), markedly increased the firing of the CA3 neurons in the wild-type mice, but not the SENP2-deficient mice. A M current opener, retigabine (194), increased the M current in the SENP2-deficient neuron to the wild-type level and markedly reduced the firing of the SENP2-deficient neuron. Thus hyper-SUMOylation of the Kv7.2/Kv7.3 potassium channel is the major culprit for seizure causation in the SENP2-deficient mice.
Patients with seizure disorders are 20 times more likely to die suddenly; this is collectively called sudden unexplained death in epilepsy (SUDEP) (107, 191). The seizure and sudden phenotype of the SENP2-deficient mice is a good animal model for SUDEP. Since cardiac arrhythmia is a major cause of sudden death, telemetry recording of the electrocardiogram was used to monitor the cardiac rhythm. The SENP2-deficient mice were shown to have marked bradycardia followed by asystole before death. Interestingly, seizure can be induced acoustically (22.5 kHz for 10 s) in the SENP2-deficient mice. Seizure induction in the SENP2-deficient mice is followed by reduced sinus node firing or atrioventricular (AV) conduction block. Retigabine, the M current opener, can prevent both seizure and AV conduction block. The linkage between seizure and bradycardia and asystole remains to be elucidated. This could be due to vagal hyperactivity caused by M current defect in the SENP2-deficient mice or respirator arrest-induced by seizure or spreading depolarization (2, 98). In any case, the SENP2-deficient mice demonstrate an important role of the SUMO pathway in regulating both cardiac and neuronal physiology that has significant implication for human disease.
XVII. SUMMARY
Since its original report in 1996, SUMO has proven to be a major posttranslational protein modification system that is involved in all facets of cell biology. Despite the simplicity of its biochemistry as compared with the ubiquitination pathway, SUMOylation and de-SUMOylation are capable to carry out many of its biological functions by interacting with other biochemical pathways, such as ubiquitination, phosphorylation, and acetylation (TABLES 1–3). The number of SUMO targets has steadily increased over the year to more than 2,000, and clearly more substrates will be added to the list in the year to come. It should be remembered that the function of SUMO modification or removal depends on the context of the individual substrate and should not be generalized. For example, SUMO modification could enhance transcription or suppress transcription depending on the context of individual substrates. SUMO modification could also affect protein stability, activity, and protein interaction. Thus SUMO is a versatile posttranslational protein modification pathway that is utilized by the cell in different contexts to regulate critical cellular processes.
XVIII. FUTURE DIRECTIONS
Important topics for future research are to study how SUMO enzymes function in different cellular compartments and how they are regulated by the external environment. For example, SENP1 is translocated to the mitochondria in fasting to regulate the SUMOylation status of Sirt3 (198). The mechanism whereby fasting regulates translocation of SENP1 to the mitochondria is unknown. Furthermore, insights into genetic polymorphisms that alter function of the SUMO enzymes would also provide valuable linkage of the SUMO pathways to human physiology. For example, the SENP1 C/G or C/C SNP (rs7963934) is associated with more severe chronic mountain sickness (75), consistent with the role of SENP1 in the regulation of HIF1α stability during hypoxia (32). In the coming decade, the SUMO pathway will be increasingly linked to human diseases, and either activation or inhibition of this pathway will be used to treat human diseases (TABLES 4–6).
Table 4.
SENP, physiology, and disease association
| Physiology | Disease Association | |
|---|---|---|
| SENP1 | HIF1α stability in hypoxic condition (32); immune-mediated destruction of β cells (163); insulin secretion (44) | Cancer (7, 10); type I diabetes mellitus (163); type II diabetes mellitus (44) |
| SENP2 | Regulate Kv7.2/Kv7.3 channels (148); p90RSK mediated phosphorylation of SENP2 (72); regulate SUMOylation of Setdb1 (225) | Sudden unexplained death in epilepsy (148); atherosclerosis (72); adipocyte lipid storage (225) |
| SENP3 | Regulate p300 interaction with HIF1α (78); regulate Top2α (203); regulate BACH2 SUMOylation (219) | Cancer (151); genomic instability (203); Treg stability and function (219) |
| SENP5 | Regulate mitochondrial fission through Drp1 (227) | Cardiomyopathy (95) |
| SENP6 | Regulate RPA70 SUMOylation (40); regulate osteochondroprogenitors (108) | Cancer (40); premature aging (108) |
| SENP7 | Endothelial mesenchymal transition (9) | Breast cancer (9) |
HIF1α, hypoxia-inducible factor 1α; SENP, Sentrin-specific protease; SUMO, small ubiquitin-like modifier.
Table 6.
Alterations in SUMO enzyme or substrate in heart failure
| Heart Failure | Alterations in SUMO Enzyme or Substrate |
|---|---|
| Diabetic cardiomyopathy | ↑SUMO-2/3 modified ERK5 (119, 168) |
| Dilated cardiomyopathy | ↓SUMO-1 (93); ↓SUMO-1 modified SERCA2a (93); ↑SUMO-2 (96); ↑SUMO-2 modified Calpain 2 (96); ↑SUMO-2 modified Calpastatin (96); ↑SENP5 (95) |
SUMO, small ubiquitin-like modifier.
GRANTS
This review was supported by grants from the National Institutes of Health.
DISCLOSURES
E. T. H. Yeh is the Frances T. McAndrew Chair in the University of Missouri. No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Correspondence: E. T. H. Yeh (e-mail: dredyeh@gmail.com).
REFERENCES
- 1.Abe JI, Ko KA, Kotla S, Wang Y, Paez-Mayorga J, Shin IJ, Imanishi M, Vu HT, Tao Y, Leiva-Juarez MM, Thomas TN, Medina JL, Won JH, Fujii Y, Giancursio CJ, McBeath E, Shin JH, Guzman L, Abe RJ, Taunton J, Mochizuki N, Faubion W, Cooke JP, Fujiwara K, Evans SE, Le NT. MAGI1 as a link between endothelial activation and ER stress drives atherosclerosis. JCI Insight 4: e125570, 2019. doi: 10.1172/jci.insight.125570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 7: 282ra46, 2015. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313: 1751, 2006. doi: 10.1126/science.1128406. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312: 446–448, 1984. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 5.Attie AD. How do reducing equivalents increase insulin secretion? J Clin Invest 125: 3754–3756, 2015. doi: 10.1172/JCI84011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey D, O’Hare P. Characterization of the localization and proteolytic activity of the SUMO-specific protease, SENP1. J Biol Chem 279: 692–703, 2004. doi: 10.1074/jbc.M306195200. [DOI] [PubMed] [Google Scholar]
- 7.Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM, Yeh ET. SENP1 induces prostatic intraepithelial neoplasia through multiple mechanisms. J Biol Chem 285: 25859–25866, 2010. doi: 10.1074/jbc.M110.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bawa-Khalfe T, Cheng J, Wang Z, Yeh ET. Induction of the SUMO-specific protease 1 transcription by the androgen receptor in prostate cancer cells. J Biol Chem 282: 37341–37349, 2007. doi: 10.1074/jbc.M706978200. [DOI] [PubMed] [Google Scholar]
- 9.Bawa-Khalfe T, Lu LS, Zuo Y, Huang C, Dere R, Lin FM, Yeh ET. Differential expression of SUMO-specific protease 7 variants regulates epithelial-mesenchymal transition. Proc Natl Acad Sci USA 109: 17466–17471, 2012. doi: 10.1073/pnas.1209378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bawa-Khalfe T, Yang FM, Ritho J, Lin HK, Cheng J, Yeh ET. SENP1 regulates PTEN stability to dictate prostate cancer development. Oncotarget 8: 17651–17664, 2017. doi: 10.18632/oncotarget.13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bawa-Khalfe T, Yeh ET. SUMO losing balance: SUMO proteases disrupt SUMO homeostasis to facilitate cancer development and progression. Genes Cancer 1: 748–752, 2010. doi: 10.1177/1947601910382555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374, 2002. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 13.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458: 461–467, 2009. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 14.Bertolotto C, Lesueur F, Giuliano S, Strub T, de Lichy M, Bille K, Dessen P, d’Hayer B, Mohamdi H, Remenieras A, Maubec E, de la Fouchardière A, Molinié V, Vabres P, Dalle S, Poulalhon N, Martin-Denavit T, Thomas L, Andry-Benzaquen P, Dupin N, Boitier F, Rossi A, Perrot JL, Labeille B, Robert C, Escudier B, Caron O, Brugières L, Saule S, Gardie B, Gad S, Richard S, Couturier J, Teh BT, Ghiorzo P, Pastorino L, Puig S, Badenas C, Olsson H, Ingvar C, Rouleau E, Lidereau R, Bahadoran P, Vielh P, Corda E, Blanché H, Zelenika D, Galan P, Aubin F, Bachollet B, Becuwe C, Berthet P, Bignon YJ, Bonadona V, Bonafe JL, Bonnet-Dupeyron MN, Cambazard F, Chevrant-Breton J, Coupier I, Dalac S, Demange L, d’Incan M, Dugast C, Faivre L, Vincent-Fétita L, Gauthier-Villars M, Gilbert B, Grange F, Grob JJ, Humbert P, Janin N, Joly P, Kerob D, Lasset C, Leroux D, Levang J, Limacher JM, Livideanu C, Longy M, Lortholary A, Stoppa-Lyonnet D, Mansard S, Mansuy L, Marrou K, Matéus C, Maugard C, Meyer N, Nogues C, Souteyrand P, Venat-Bouvet L, Zattara H, Chaudru V, Lenoir GM, Lathrop M, Davidson I, Avril MF, Demenais F, Ballotti R, Bressac-de Paillerets B; French Familial Melanoma Study Group . A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. [Correction in Nature 531: 126, 2016.] Nature 480: 94–98, 2011. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 15.Blomster HA, Imanishi SY, Siimes J, Kastu J, Morrice NA, Eriksson JE, Sistonen L. In vivo identification of sumoylation sites by a signature tag and cysteine-targeted affinity purification. J Biol Chem 285: 19324–19329, 2010. doi: 10.1074/jbc.M110.106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boddy MN, Howe K, Etkin LD, Solomon E, Freemont PS. PIC 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene 13: 971–982, 1996. [PubMed] [Google Scholar]
- 17.Boggio R, Colombo R, Hay RT, Draetta GF, Chiocca S. A mechanism for inhibiting the SUMO pathway. Mol Cell 16: 549–561, 2004. doi: 10.1016/j.molcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Boggio R, Passafaro A, Chiocca S. Targeting SUMO E1 to ubiquitin ligases: a viral strategy to counteract sumoylation. J Biol Chem 282: 15376–15382, 2007. doi: 10.1074/jbc.M700889200. [DOI] [PubMed] [Google Scholar]
- 19.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21: 349–357, 2006. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10: 748–754, 2009. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brems-Eskildsen AS, Zieger K, Toldbod H, Holcomb C, Higuchi R, Mansilla F, Munksgaard PP, Borre M, Ørntoft TF, Dyrskjøt L. Prediction and diagnosis of bladder cancer recurrence based on urinary content of hTERT, SENP1, PPP1CA, and MCM5 transcripts. BMC Cancer 10: 646, 2010. doi: 10.1186/1471-2407-10-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol 156: 1185–1195, 2009. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdelski C, Menan D, Tsourlakis MC, Kluth M, Hube-Magg C, Melling N, Minner S, Koop C, Graefen M, Heinzer H, Wittmer C, Sauter G, Simon R, Schlomm T, Steurer S, Krech T. The prognostic value of SUMO1/Sentrin specific peptidase 1 (SENP1) in prostate cancer is limited to ERG-fusion positive tumors lacking PTEN deletion. BMC Cancer 15: 538, 2015. doi: 10.1186/s12885-015-1555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai R, Yu T, Huang C, Xia X, Liu X, Gu J, Xue S, Yeh ET, Cheng J. SUMO-specific protease 1 regulates mitochondrial biogenesis through PGC-1α. J Biol Chem 287: 44464–44470, 2012. doi: 10.1074/jbc.M112.422626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capili AD, Lima CD. Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. J Mol Biol 369: 608–618, 2007. doi: 10.1016/j.jmb.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell 131: 309–323, 2007. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 27.Chang CC, Naik MT, Huang YS, Jeng JC, Liao PH, Kuo HY, Ho CC, Hsieh YL, Lin CH, Huang NJ, Naik NM, Kung CC, Lin SY, Chen RH, Chang KS, Huang TH, Shih HM. Structural and functional roles of Daxx SIM phosphorylation in SUMO paralog-selective binding and apoptosis modulation. Mol Cell 42: 62–74, 2011. doi: 10.1016/j.molcel.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Chang CC, Tung CH, Chen CW, Tu CH, Chu YW. SUMOgo: prediction of sumoylation sites on lysines by motif screening models and the effects of various post-translational modifications. Sci Rep 8: 15512, 2018. doi: 10.1038/s41598-018-33951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 88: 1052–1061, 1996. doi: 10.1182/blood.V88.3.1052.1052. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CT, Kuo CY, Ann DK. KAPtain in charge of multiple missions: emerging roles of KAP1. World J Biol Chem 5: 308–320, 2014. doi: 10.4331/wjbc.v5.i3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng J, Bawa T, Lee P, Gong L, Yeh ET. Role of desumoylation in the development of prostate cancer. Neoplasia 8: 667–676, 2006. doi: 10.1593/neo.06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 131: 584–595, 2007. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng J, Wang D, Wang Z, Yeh ET. SENP1 enhances androgen receptor-dependent transcription through desumoylation of histone deacetylase 1. Mol Cell Biol 24: 6021–6028, 2004. doi: 10.1128/MCB.24.13.6021-6028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Best Pract Res Clin Haematol 30: 341–355, 2017. doi: 10.1016/j.beha.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Craig JM, Earle E, Canham P, Wong LH, Anderson M, Choo KH. Analysis of mammalian proteins involved in chromatin modification reveals new metaphase centromeric proteins and distinct chromosomal distribution patterns. Hum Mol Genet 12: 3109–3121, 2003. doi: 10.1093/hmg/ddg330. [DOI] [PubMed] [Google Scholar]
- 36.Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci 122: 775–779, 2009. doi: 10.1242/jcs.036632. [DOI] [PubMed] [Google Scholar]
- 37.Dai XQ, Plummer G, Casimir M, Kang Y, Hajmrle C, Gaisano HY, Manning Fox JE, MacDonald PE. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 60: 838–847, 2011. doi: 10.2337/db10-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding X, Wang A, Ma X, Demarque M, Jin W, Xin H, Dejean A, Dong C. Protein SUMOylation is required for regulatory T cell expansion and function. Cell Rep 16: 1055–1066, 2016. doi: 10.1016/j.celrep.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8: 337–348, 2008. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 40.Dou H, Huang C, Singh M, Carpenter PB, Yeh ET. Regulation of DNA repair through deSUMOylation and SUMOylation of replication protein A complex. Mol Cell 39: 333–345, 2010. doi: 10.1016/j.molcel.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dou H, Huang C, Van Nguyen T, Lu LS, Yeh ET. SUMOylation and de-SUMOylation in response to DNA damage. FEBS Lett 585: 2891–2896, 2011. doi: 10.1016/j.febslet.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Dustin ML. The immunological synapse. Cancer Immunol Res 2: 1023–1033, 2014. doi: 10.1158/2326-6066.CIR-14-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evdokimov E, Sharma P, Lockett SJ, Lualdi M, Kuehn MR. Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J Cell Sci 121: 4106–4113, 2008. doi: 10.1242/jcs.038570. [DOI] [PubMed] [Google Scholar]
- 44.Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J Clin Invest 125: 3847–3860, 2015. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem 82: 357–385, 2013. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 46.Floyd ZE, Stephens JM. Control of peroxisome proliferator-activated receptor gamma2 stability and activity by SUMOylation. Obes Res 12: 921–928, 2004. doi: 10.1038/oby.2004.112. [DOI] [PubMed] [Google Scholar]
- 47.Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462: 935–939, 2009. doi: 10.1038/nature08657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garvin AJ, Densham RM, Blair-Reid SA, Pratt KM, Stone HR, Weekes D, Lawrence KJ, Morris JR. The deSUMOylase SENP7 promotes chromatin relaxation for homologous recombination DNA repair. EMBO Rep 14: 975–983, 2013. doi: 10.1038/embor.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geerts CJ, Jacobsen L, van de Bospoort R, Verhage M, Groffen AJ. Tomosyn interacts with the SUMO E3 ligase PIASγ. PLoS One 9: e91697, 2014. doi: 10.1371/journal.pone.0091697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girach F, Craig TJ, Rocca DL, Henley JM. RIM1α SUMOylation is required for fast synaptic vesicle exocytosis. Cell Rep 5: 1294–1301, 2013. doi: 10.1016/j.celrep.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giraud MF, Desterro JM, Naismith JH. Structure of ubiquitin-conjugating enzyme 9 displays significant differences with other ubiquitin-conjugating enzymes which may reflect its specificity for sumo rather than ubiquitin. Acta Crystallogr D Biol Crystallogr 54: 891–898, 1998. doi: 10.1107/S0907444998002480. [DOI] [PubMed] [Google Scholar]
- 52.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci 30: 5167–5175, 2010. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428, 2002. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 54.Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J Biol Chem 272: 28198–28201, 1997. doi: 10.1074/jbc.272.45.28198. [DOI] [PubMed] [Google Scholar]
- 55.Gong L, Li B, Millas S, Yeh ET. Molecular cloning and characterization of human AOS1 and UBA2, components of the sentrin-activating enzyme complex. FEBS Lett 448: 185–189, 1999. doi: 10.1016/S0014-5793(99)00367-1. [DOI] [PubMed] [Google Scholar]
- 56.Gong L, Millas S, Maul GG, Yeh ET. Differential regulation of sentrinized proteins by a novel sentrin-specific protease. J Biol Chem 275: 3355–3359, 2000. doi: 10.1074/jbc.275.5.3355. [DOI] [PubMed] [Google Scholar]
- 57.González-Prieto R, Cuijpers SA, Kumar R, Hendriks IA, Vertegaal AC. c-Myc is targeted to the proteasome for degradation in a SUMOylation-dependent manner, regulated by PIAS1, SENP7 and RNF4. Cell Cycle 14: 1859–1872, 2015. doi: 10.1080/15384101.2015.1040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo D, Li M, Zhang Y, Yang P, Eckenrode S, Hopkins D, Zheng W, Purohit S, Podolsky RH, Muir A, Wang J, Dong Z, Brusko T, Atkinson M, Pozzilli P, Zeidler A, Raffel LJ, Jacob CO, Park Y, Serrano-Rios M, Larrad MT, Zhang Z, Garchon HJ, Bach JF, Rotter JI, She JX, Wang CY. A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. [Correction in Nat Genet 36: 1024, 2004.] Nat Genet 36: 837–841, 2004. doi: 10.1038/ng1391. [DOI] [PubMed] [Google Scholar]
- 59.Häkli M, Karvonen U, Jänne OA, Palvimo JJ. SUMO-1 promotes association of SNURF (RNF4) with PML nuclear bodies. Exp Cell Res 304: 224–233, 2005. doi: 10.1016/j.yexcr.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 60.Hammer E, Heilbronn R, Weger S. The E3 ligase Topors induces the accumulation of polysumoylated forms of DNA topoisomerase I in vitro and in vivo. FEBS Lett 581: 5418–5424, 2007. doi: 10.1016/j.febslet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 61.Han Y, Huang C, Sun X, Xiang B, Wang M, Yeh ET, Chen Y, Li H, Shi G, Cang H, Sun Y, Wang J, Wang W, Gao F, Yi J. SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress. J Biol Chem 285: 12906–12915, 2010. doi: 10.1074/jbc.M109.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hang J, Dasso M. Association of the human SUMO-1 protease SENP2 with the nuclear pore. J Biol Chem 277: 19961–19966, 2002. doi: 10.1074/jbc.M201799200. [DOI] [PubMed] [Google Scholar]
- 63.Hannoun Z, Maarifi G, Chelbi-Alix MK. The implication of SUMO in intrinsic and innate immunity. Cytokine Growth Factor Rev 29: 3–16, 2016. doi: 10.1016/j.cytogfr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14: 340–345, 2004. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hattori T, Eberspaecher H, Lu J, Zhang R, Nishida T, Kahyo T, Yasuda H, de Crombrugghe B. Interactions between PIAS proteins and SOX9 result in an increase in the cellular concentrations of SOX9. J Biol Chem 281: 14417–14428, 2006. doi: 10.1074/jbc.M511330200. [DOI] [PubMed] [Google Scholar]
- 67.He X, Lai Q, Chen C, Li N, Sun F, Huang W, Zhang S, Yu Q, Yang P, Xiong F, Chen Z, Gong Q, Ren B, Weng J, Eizirik DL, Zhou Z, Wang CY. Both conditional ablation and overexpression of E2 SUMO-conjugating enzyme (UBC9) in mouse pancreatic beta cells result in impaired beta cell function. Diabetologia 61: 881–895, 2018. doi: 10.1007/s00125-017-4523-9. [DOI] [PubMed] [Google Scholar]
- 68.He X, Riceberg J, Soucy T, Koenig E, Minissale J, Gallery M, Bernard H, Yang X, Liao H, Rabino C, Shah P, Xega K, Yan ZH, Sintchak M, Bradley J, Xu H, Duffey M, England D, Mizutani H, Hu Z, Guo J, Chau R, Dick LR, Brownell JE, Newcomb J, Langston S, Lightcap ES, Bence N, Pulukuri SM. Probing the roles of SUMOylation in cancer cell biology by using a selective SAE inhibitor. Nat Chem Biol 13: 1164–1171, 2017. doi: 10.1038/nchembio.2463. [DOI] [PubMed] [Google Scholar]
- 69.Heaton PR, Deyrieux AF, Bian XL, Wilson VG. HPV E6 proteins target Ubc9, the SUMO conjugating enzyme. Virus Res 158: 199–208, 2011. doi: 10.1016/j.virusres.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henley JM, Craig TJ, Wilkinson KA. Neuronal SUMOylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiol Rev 94: 1249–1285, 2014. doi: 10.1152/physrev.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heo KS, Chang E, Le NT, Cushman H, Yeh ET, Fujiwara K, Abe J. De-SUMOylation enzyme of sentrin/SUMO-specific protease 2 regulates disturbed flow-induced SUMOylation of ERK5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ Res 112: 911–923, 2013. doi: 10.1161/CIRCRESAHA.111.300179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heo KS, Le NT, Cushman HJ, Giancursio CJ, Chang E, Woo CH, Sullivan MA, Taunton J, Yeh ET, Fujiwara K, Abe J. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J Clin Invest 125: 1299–1310, 2015. doi: 10.1172/JCI76453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 13: 755–766, 2012. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA 103: 45–50, 2006. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsieh MM, Callacondo D, Rojas-Camayo J, Quesada-Olarte J, Wang X, Uchida N, Maric I, Remaley AT, Leon-Velarde F, Villafuerte FC, Tisdale JF. SENP1, but not fetal hemoglobin, differentiates Andean highlanders with chronic mountain sickness from healthy individuals among Andean highlanders. Exp Hematol 44: 484–490.e2, 2016. doi: 10.1016/j.exphem.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsieh YL, Kuo HY, Chang CC, Naik MT, Liao PH, Ho CC, Huang TC, Jeng JC, Hsu PH, Tsai MD, Huang TH, Shih HM. Ubc9 acetylation modulates distinct SUMO target modification and hypoxia response. EMBO J 32: 791–804, 2013. doi: 10.1038/emboj.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang C, Cheng J, Bawa-Khalfe T, Yao X, Chin YE, Yeh ETH. SUMOylated ORC2 recruits a histone demethylase to regulate centromeric histone modification and genomic stability. Cell Rep 15: 147–157, 2016. doi: 10.1016/j.celrep.2016.02.091. [DOI] [PubMed] [Google Scholar]
- 78.Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, Chen Y, Cang H, Li H, Shi G, Cheng J, Tang X, Yi J. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J 28: 2748–2762, 2009. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itahana Y, Yeh ET, Zhang Y. Nucleocytoplasmic shuttling modulates activity and ubiquitination-dependent turnover of SUMO-specific protease 2. Mol Cell Biol 26: 4675–4689, 2006. doi: 10.1128/MCB.01830-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 81.Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell 49: 795–807, 2013. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 82.Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106: 735–744, 2001. doi: 10.1016/S0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 83.Johnson ES, Schwienhorst I, Dohmen RJ, Blobel G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J 16: 5509–5519, 1997. doi: 10.1093/emboj/16.18.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell 113: 127–137, 2003. doi: 10.1016/S0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 85.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383: 1068–1083, 2014. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kahyo T, Nishida T, Yasuda H. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol Cell 8: 713–718, 2001. doi: 10.1016/S1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 87.Kamitani T, Kito K, Nguyen HP, Fukuda-Kamitani T, Yeh ET. Characterization of a second member of the sentrin family of ubiquitin-like proteins. J Biol Chem 273: 11349–11353, 1998. doi: 10.1074/jbc.273.18.11349. [DOI] [PubMed] [Google Scholar]
- 88.Kamitani T, Kito K, Nguyen HP, Wada H, Fukuda-Kamitani T, Yeh ET. Identification of three major sentrinization sites in PML. J Biol Chem 273: 26675–26682, 1998. doi: 10.1074/jbc.273.41.26675. [DOI] [PubMed] [Google Scholar]
- 89.Kamitani T, Nguyen HP, Yeh ET. Preferential modification of nuclear proteins by a novel ubiquitin-like molecule. J Biol Chem 272: 14001–14004, 1997. doi: 10.1074/jbc.272.22.14001. [DOI] [PubMed] [Google Scholar]
- 90.Kang X, Li J, Zou Y, Yi J, Zhang H, Cao M, Yeh ET, Cheng J. PIASy stimulates HIF1α SUMOylation and negatively regulates HIF1α activity in response to hypoxia. Oncogene 29: 5568–5578, 2010. doi: 10.1038/onc.2010.297. [DOI] [PubMed] [Google Scholar]
- 91.Katafuchi T, Holland WL, Kollipara RK, Kittler R, Mangelsdorf DJ, Kliewer SA. PPARγ-K107 SUMOylation regulates insulin sensitivity but not adiposity in mice. Proc Natl Acad Sci USA 115: 12102–12111, 2018. doi: 10.1073/pnas.1814522115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kessler JD, Kahle KT, Sun T, Meerbrey KL, Schlabach MR, Schmitt EM, Skinner SO, Xu Q, Li MZ, Hartman ZC, Rao M, Yu P, Dominguez-Vidana R, Liang AC, Solimini NL, Bernardi RJ, Yu B, Hsu T, Golding I, Luo J, Osborne CK, Creighton CJ, Hilsenbeck SG, Schiff R, Shaw CA, Elledge SJ, Westbrook TF. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science 335: 348–353, 2012. doi: 10.1126/science.1212728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477: 601–605, 2011. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kho C, Lee A, Jeong D, Oh JG, Gorski PA, Fish K, Sanchez R, DeVita RJ, Christensen G, Dahl R, Hajjar RJ. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat Commun 6: 7229, 2015. doi: 10.1038/ncomms8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim EY, Zhang Y, Beketaev I, Segura AM, Yu W, Xi Y, Chang J, Wang J. SENP5, a SUMO isopeptidase, induces apoptosis and cardiomyopathy. J Mol Cell Cardiol 78: 154–164, 2015. doi: 10.1016/j.yjmcc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 96.Kim EY, Zhang Y, Ye B, Segura AM, Beketaev I, Xi Y, Yu W, Chang J, Li F, Wang J. Involvement of activated SUMO-2 conjugation in cardiomyopathy. Biochim Biophys Acta 1852: 1388–1399, 2015. doi: 10.1016/j.bbadis.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 97.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell 127: 265–275, 2006. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, Laux LC, Zhou X, Nordli DR Jr, Richerson GB. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 128: 1141–1153, 2018. doi: 10.1172/JCI94999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim YH, Sung KS, Lee SJ, Kim YO, Choi CY, Kim Y. Desumoylation of homeodomain-interacting protein kinase 2 (HIPK2) through the cytoplasmic-nuclear shuttling of the SUMO-specific protease SENP1. FEBS Lett 579: 6272–6278, 2005. doi: 10.1016/j.febslet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Kirsh O, Seeler JS, Pichler A, Gast A, Müller S, Miska E, Mathieu M, Harel-Bellan A, Kouzarides T, Melchior F, Dejean A. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J 21: 2682–2691, 2002. doi: 10.1093/emboj/21.11.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knipscheer P, Flotho A, Klug H, Olsen JV, van Dijk WJ, Fish A, Johnson ES, Mann M, Sixma TK, Pichler A. Ubc9 sumoylation regulates SUMO target discrimination. Mol Cell 31: 371–382, 2008. doi: 10.1016/j.molcel.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 102.Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J 26: 2797–2807, 2007. doi: 10.1038/sj.emboj.7601711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuo ML, den Besten W, Thomas MC, Sherr CJ. Arf-induced turnover of the nucleolar nucleophosmin-associated SUMO-2/3 protease Senp3. Cell Cycle 7: 3378–3387, 2008. doi: 10.4161/cc.7.21.6930. [DOI] [PubMed] [Google Scholar]
- 104.Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, Zhou J, Zhu J, Raught B, de Thé H. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol 10: 547–555, 2008. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 105.Lamoliatte F, Caron D, Durette C, Mahrouche L, Maroui MA, Caron-Lizotte O, Bonneil E, Chelbi-Alix MK, Thibault P. Large-scale analysis of lysine SUMOylation by SUMO remnant immunoaffinity profiling. Nat Commun 5: 5409, 2014. doi: 10.1038/ncomms6409. [DOI] [PubMed] [Google Scholar]
- 106.Lee MH, Mabb AM, Gill GB, Yeh ET, Miyamoto S. NF-κB induction of the SUMO protease SENP2: a negative feedback loop to attenuate cell survival response to genotoxic stress. Mol Cell 43: 180–191, 2011. doi: 10.1016/j.molcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lhatoo S, Noebels J, Whittemore V; NINDS Center for SUDEP Research . Sudden unexpected death in epilepsy: identifying risk and preventing mortality. Epilepsia 56: 1700–1706, 2015. doi: 10.1111/epi.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li J, Lu D, Dou H, Liu H, Weaver K, Wang W, Li J, Yeh ETH, Williams BO, Zheng L, Yang T. Desumoylase SENP6 maintains osteochondroprogenitor homeostasis by suppressing the p53 pathway. Nat Commun 9: 143, 2018. doi: 10.1038/s41467-017-02413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li SJ, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature 398: 246–251, 1999. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- 110.Li T, Huang S, Dong M, Gui Y, Wu D. Prognostic impact of SUMO-specific protease 1 (SENP1) in prostate cancer patients undergoing radical prostatectomy. Urol Oncol 31: 1539–1545, 2013. doi: 10.1016/j.urolonc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Li YJ, Du L, Wang J, Vega R, Lee TD, Miao Y, Aldana-Masangkay G, Samuels ER, Li B, Ouyang SX, Colayco SA, Bobkova EV, Divlianska DB, Sergienko E, Chung TDY, Fakih M, Chen Y. Allosteric inhibition of ubiquitin-like modifications by a class of inhibitor of SUMO-activating enzyme. Cell Chem Biol 26: 278–288.e6, 2019. doi: 10.1016/j.chembiol.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang Q, Deng H, Li X, Wu X, Tang Q, Chang TH, Peng H, Rauscher FJ III, Ozato K, Zhu F. Tripartite motif-containing protein 28 is a small ubiquitin-related modifier E3 ligase and negative regulator of IFN regulatory factor 7. J Immunol 187: 4754–4763, 2011. doi: 10.4049/jimmunol.1101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liebelt F, Vertegaal AC. Ubiquitin-dependent and independent roles of SUMO in proteostasis. Am J Physiol Cell Physiol 311: C284–C296, 2016. doi: 10.1152/ajpcell.00091.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin DY, Huang YS, Jeng JC, Kuo HY, Chang CC, Chao TT, Ho CC, Chen YC, Lin TP, Fang HI, Hung CC, Suen CS, Hwang MJ, Chang KS, Maul GG, Shih HM. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol Cell 24: 341–354, 2006. doi: 10.1016/j.molcel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 115.Liu B, Shuai K. Targeting the PIAS1 SUMO ligase pathway to control inflammation. Trends Pharmacol Sci 29: 505–509, 2008. doi: 10.1016/j.tips.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu B, Tahk S, Yee KM, Fan G, Shuai K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science 330: 521–525, 2010. doi: 10.1126/science.1193787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, Loo JA, Shuai K. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell 129: 903–914, 2007. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 118.Liu F, Li L, Li Y, Ma X, Bian X, Liu X, Wang G, Zhang D. Overexpression of SENP1 reduces the stemness capacity of osteosarcoma stem cells and increases their sensitivity to HSVtk/GCV. Int J Oncol 53: 2010–2020, 2018. doi: 10.3892/ijo.2018.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu W, Ruiz-Velasco A, Wang S, Khan S, Zi M, Jungmann A, Dolores Camacho-Muñoz M, Guo J, Du G, Xie L, Oceandy D, Nicolaou A, Galli G, Müller OJ, Cartwright EJ, Ji Y, Wang X. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat Commun 8: 494, 2017. doi: 10.1038/s41467-017-00664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lois LM, Lima CD. Structures of the SUMO E1 provide mechanistic insights into SUMO activation and E2 recruitment to E1. EMBO J 24: 439–451, 2005. doi: 10.1038/sj.emboj.7600552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev 13: 77–83, 2003. doi: 10.1016/S0959-437X(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 122.Lumpkin RJ, Gu H, Zhu Y, Leonard M, Ahmad AS, Clauser KR, Meyer JG, Bennett EJ, Komives EA. Site-specific identification and quantitation of endogenous SUMO modifications under native conditions. Nat Commun 8: 1171, 2017. doi: 10.1038/s41467-017-01271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88: 97–107, 1997. doi: 10.1016/S0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 124.Matic I, Schimmel J, Hendriks IA, van Santen MA, van de Rijke F, van Dam H, Gnad F, Mann M, Vertegaal AC. Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol Cell 39: 641–652, 2010. doi: 10.1016/j.molcel.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 125.Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol 135: 1457–1470, 1996. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miller AJ, Levy C, Davis IJ, Razin E, Fisher DE. Sumoylation of MITF and its related family members TFE3 and TFEB. J Biol Chem 280: 146–155, 2005. doi: 10.1074/jbc.M411757200. [DOI] [PubMed] [Google Scholar]
- 127.Minty A, Dumont X, Kaghad M, Caput D. Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J Biol Chem 275: 36316–36323, 2000. doi: 10.1074/jbc.M004293200. [DOI] [PubMed] [Google Scholar]
- 128.Mirecka A, Morawiec Z, Wozniak K. Genetic polymorphism of SUMO-specific cysteine proteases - SENP1 and SENP2 in breast cancer. Pathol Oncol Res 22: 817–823, 2016. doi: 10.1007/s12253-016-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miyauchi Y, Yogosawa S, Honda R, Nishida T, Yasuda H. Sumoylation of Mdm2 by protein inhibitor of activated STAT (PIAS) and RanBP2 enzymes. J Biol Chem 277: 50131–50136, 2002. doi: 10.1074/jbc.M208319200. [DOI] [PubMed] [Google Scholar]
- 130.Mohideen F, Capili AD, Bilimoria PM, Yamada T, Bonni A, Lima CD. A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 UBC9. Nat Struct Mol Biol 16: 945–952, 2009. doi: 10.1038/nsmb.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell 5: 865–876, 2000. doi: 10.1016/S1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- 132.Mukhopadhyay D, Ayaydin F, Kolli N, Tan SH, Anan T, Kametaka A, Azuma Y, Wilkinson KD, Dasso M. SUSP1 antagonizes formation of highly SUMO2/3-conjugated species. J Cell Biol 174: 939–949, 2006. doi: 10.1083/jcb.200510103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci 32: 286–295, 2007. doi: 10.1016/j.tibs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell 9: 769–779, 2005. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 135.Nakagawa K, Yokosawa H. PIAS3 induces SUMO-1 modification and transcriptional repression of IRF-1. FEBS Lett 530: 204–208, 2002. doi: 10.1016/S0014-5793(02)03486-5. [DOI] [PubMed] [Google Scholar]
- 136.Oh JG, Watanabe S, Lee A, Gorski PA, Lee P, Jeong D, Liang L, Liang Y, Baccarini A, Sahoo S, Brown BD, Hajjar RJ, Kho C. miR-146a suppresses SUMO1 expression and induces cardiac dysfunction in maladaptive hypertrophy. Circ Res 123: 673–685, 2018. doi: 10.1161/CIRCRESAHA.118.312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei CF, Chang HM, Yeh ET. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol 157: 4277–4281, 1996. [PubMed] [Google Scholar]
- 138.Olsen SK, Capili AD, Lu X, Tan DS, Lima CD. Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature 463: 906–912, 2010. doi: 10.1038/nature08765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Owerbach D, McKay EM, Yeh ET, Gabbay KH, Bohren KM. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem Biophys Res Commun 337: 517–520, 2005. doi: 10.1016/j.bbrc.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 140.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437: 759–763, 2005. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108: 109–120, 2002. doi: 10.1016/S0092-8674(01)00633-X. [DOI] [PubMed] [Google Scholar]
- 142.Pilla E, Möller U, Sauer G, Mattiroli F, Melchior F, Geiss-Friedlander R. A novel SUMO1-specific interacting motif in dipeptidyl peptidase 9 (DPP9) that is important for enzymatic regulation. J Biol Chem 287: 44320–44329, 2012. doi: 10.1074/jbc.M112.397224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Podolsky R, Prasad Linga-Reddy MV, She JX; Type I Diabetes Genetics Consortium . Analyses of multiple single-nucleotide polymorphisms in the SUMO4/IDDM5 region in affected sib-pair families with type I diabetes. Genes Immun 10, Suppl 1: S16–S20, 2009. doi: 10.1038/gene.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Potts PR, Yu H. Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol Cell Biol 25: 7021–7032, 2005. doi: 10.1128/MCB.25.16.7021-7032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab 18: 162–185, 2013. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 146.Preuss KD, Pfreundschuh M, Weigert M, Fadle N, Regitz E, Kubuschok B. Sumoylated HSP90 is a dominantly inherited plasma cell dyscrasias risk factor. J Clin Invest 125: 2179, 2015. doi: 10.1172/JCI82091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prudden J, Pebernard S, Raffa G, Slavin DA, Perry JJ, Tainer JA, McGowan CH, Boddy MN. SUMO-targeted ubiquitin ligases in genome stability. EMBO J 26: 4089–4101, 2007. doi: 10.1038/sj.emboj.7601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qi Y, Wang J, Bomben VC, Li DP, Chen SR, Sun H, Xi Y, Reed JG, Cheng J, Pan HL, Noebels JL, Yeh ET. Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death. Neuron 83: 1159–1171, 2014. doi: 10.1016/j.neuron.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rajendra R, Malegaonkar D, Pungaliya P, Marshall H, Rasheed Z, Brownell J, Liu LF, Lutzker S, Saleem A, Rubin EH. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J Biol Chem 279: 36440–36444, 2004. doi: 10.1074/jbc.C400300200. [DOI] [PubMed] [Google Scholar]
- 150.Ren SY, Angrand PO, Rijli FM. Targeted insertion results in a rhombomere 2-specific Hoxa2 knockdown and ectopic activation of Hoxa1 expression. Dev Dyn 225: 305–315, 2002. doi: 10.1002/dvdy.10171. [DOI] [PubMed] [Google Scholar]
- 151.Ren YH, Liu KJ, Wang M, Yu YN, Yang K, Chen Q, Yu B, Wang W, Li QW, Wang J, Hou ZY, Fang JY, Yeh ET, Yang J, Yi J. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget 5: 7093–7104, 2014. doi: 10.18632/oncotarget.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ribet D, Hamon M, Gouin E, Nahori MA, Impens F, Neyret-Kahn H, Gevaert K, Vandekerckhove J, Dejean A, Cossart P. Listeria monocytogenes impairs SUMOylation for efficient infection. [Correction in Nature 580: E20, 2020.] Nature 464: 1192–1195, 2010. doi: 10.1038/nature08963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ritho J, Arold ST, Yeh ET. A critical SUMO1 modification of LKB1 regulates AMPK activity during energy stress. Cell Rep 12: 734–742, 2015. doi: 10.1016/j.celrep.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 154.Ritterhoff T, Das H, Hofhaus G, Schröder RR, Flotho A, Melchior F. The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes. Nat Commun 7: 11482, 2016. doi: 10.1038/ncomms11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roth W, Sustmann C, Kieslinger M, Gilmozzi A, Irmer D, Kremmer E, Turck C, Grosschedl R. PIASy-deficient mice display modest defects in IFN and Wnt signaling. J Immunol 173: 6189–6199, 2004. doi: 10.4049/jimmunol.173.10.6189. [DOI] [PubMed] [Google Scholar]
- 156.Rytinki MM, Palvimo JJ. SUMOylation attenuates the function of PGC-1alpha. J Biol Chem 284: 26184–26193, 2009. doi: 10.1074/jbc.M109.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem 275: 6252–6258, 2000. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- 158.Sampson DA, Wang M, Matunis MJ. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem 276: 21664–21669, 2001. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- 159.Santti H, Mikkonen L, Anand A, Hirvonen-Santti S, Toppari J, Panhuysen M, Vauti F, Perera M, Corte G, Wurst W, Jänne OA, Palvimo JJ. Disruption of the murine PIASx gene results in reduced testis weight. J Mol Endocrinol 34: 645–654, 2005. doi: 10.1677/jme.1.01666. [DOI] [PubMed] [Google Scholar]
- 160.Sapetschnig A, Rischitor G, Braun H, Doll A, Schergaut M, Melchior F, Suske G. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J 21: 5206–5215, 2002. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schellenberg MJ, Lieberman JA, Herrero-Ruiz A, Butler LR, Williams JG, Muñoz-Cabello AM, Mueller GA, London RE, Cortés-Ledesma F, Williams RS. ZATT (ZNF451)-mediated resolution of topoisomerase 2 DNA-protein cross-links. Science 357: 1412–1416, 2017. doi: 10.1126/science.aam6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schulz S, Chachami G, Kozaczkiewicz L, Winter U, Stankovic-Valentin N, Haas P, Hofmann K, Urlaub H, Ovaa H, Wittbrodt J, Meulmeester E, Melchior F. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep 13: 930–938, 2012. doi: 10.1038/embor.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shao L, Zhou HJ, Zhang H, Qin L, Hwa J, Yun Z, Ji W, Min W. SENP1-mediated NEMO deSUMOylation in adipocytes limits inflammatory responses and type-1 diabetes progression. Nat Commun 6: 8917, 2015. doi: 10.1038/ncomms9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shen L, Tatham MH, Dong C, Zagórska A, Naismith JH, Hay RT. SUMO protease SENP1 induces isomerization of the scissile peptide bond. Nat Struct Mol Biol 13: 1069–1077, 2006. doi: 10.1038/nsmb1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shen LN, Dong C, Liu H, Naismith JH, Hay RT. The structure of SENP1-SUMO-2 complex suggests a structural basis for discrimination between SUMO paralogues during processing. Biochem J 397: 279–288, 2006. doi: 10.1042/BJ20052030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY; Clinical Efficacy and Pharmacokinetics in Relapsed Patients . Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89: 3354–3360, 1997. doi: 10.1182/blood.V89.9.3354. [DOI] [PubMed] [Google Scholar]
- 167.Shin EJ, Shin HM, Nam E, Kim WS, Kim JH, Oh BH, Yun Y. DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO Rep 13: 339–346, 2012. doi: 10.1038/embor.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shishido T, Woo CH, Ding B, McClain C, Molina CA, Yan C, Yang J, Abe J. Effects of MEK5/ERK5 association on small ubiquitin-related modification of ERK5: implications for diabetic ventricular dysfunction after myocardial infarction. Circ Res 102: 1416–1425, 2008. doi: 10.1161/CIRCRESAHA.107.168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh AK, Khare P, Obaid A, Conlon KP, Basrur V, DePinho RA, Venuprasad K. SUMOylation of ROR-γt inhibits IL-17 expression and inflammation via HDAC2. Nat Commun 9: 4515, 2018. doi: 10.1038/s41467-018-06924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet 18: 25–29, 1998. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 171.Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, Anderson VE, Sanguinetti MC, Leppert MF; BFNC Physician Consortium . KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain 126: 2726–2737, 2003. doi: 10.1093/brain/awg286. [DOI] [PubMed] [Google Scholar]
- 172.Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron 20: 809–819, 1998. doi: 10.1016/S0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 173.Song GG, Choi SJ, Ji JD, Lee YH. Association between the SUMO4 M55V (A163G) polymorphism and susceptibility to type 1 diabetes: a meta-analysis. Hum Immunol 73: 1055–1059, 2012. doi: 10.1016/j.humimm.2012.07.341. [DOI] [PubMed] [Google Scholar]
- 174.Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci USA 101: 14373–14378, 2004. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song J, Zhang Z, Hu W, Chen Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: a reversal of the bound orientation. J Biol Chem 280: 40122–40129, 2005. doi: 10.1074/jbc.M507059200. [DOI] [PubMed] [Google Scholar]
- 176.Sriramachandran AM, Meyer-Teschendorf K, Pabst S, Ulrich HD, Gehring NH, Hofmann K, Praefcke GJK, Dohmen RJ. Arkadia/RNF111 is a SUMO-targeted ubiquitin ligase with preference for substrates marked with SUMO1-capped SUMO2/3 chain. Nat Commun 10: 3678, 2019. doi: 10.1038/s41467-019-11549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stehmeier P, Muller S. Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Mol Cell 33: 400–409, 2009. doi: 10.1016/j.molcel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 178.Su YF, Yang T, Huang H, Liu LF, Hwang J. Phosphorylation of Ubc9 by Cdk1 enhances SUMOylation activity. PLoS One 7: e34250, 2012. doi: 10.1371/journal.pone.0034250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science 324: 1327–1330, 2009. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Suh HY, Kim JH, Woo JS, Ku B, Shin EJ, Yun Y, Oh BH. Crystal structure of DeSI-1, a novel deSUMOylase belonging to a putative isopeptidase superfamily. Proteins 80: 2099–2104, 2012. doi: 10.1002/prot.24093. [DOI] [PubMed] [Google Scholar]
- 181.Sun H, Leverson JD, Hunter T. Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J 26: 4102–4112, 2007. doi: 10.1038/sj.emboj.7601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tahk S, Liu B, Chernishof V, Wong KA, Wu H, Shuai K. Control of specificity and magnitude of NF-kappa B and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc Natl Acad Sci USA 104: 11643–11648, 2007. doi: 10.1073/pnas.0701877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang ST, Peng WJ, Wang CJ, Tang HQ, Zhang Q. Polymorphism M55V in gene encoding small ubiquitin-like modifier 4 (SUMO4) protein associates with susceptibility to type 1 (and type 2) diabetes. Diabetes Metab Res Rev 28: 679–687, 2012. doi: 10.1002/dmrr.2335. [DOI] [PubMed] [Google Scholar]
- 184.Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol 10: 538–546, 2008. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 185.Tatham MH, Jaffray E, Vaughan OA, Desterro JM, Botting CH, Naismith JH, Hay RT. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J Biol Chem 276: 35368–35374, 2001. doi: 10.1074/jbc.M104214200. [DOI] [PubMed] [Google Scholar]
- 186.Tatham MH, Kim S, Jaffray E, Song J, Chen Y, Hay RT. Unique binding interactions among Ubc9, SUMO and RanBP2 reveal a mechanism for SUMO paralog selection. Nat Struct Mol Biol 12: 67–74, 2005. doi: 10.1038/nsmb878. [DOI] [PubMed] [Google Scholar]
- 187.Tatham MH, Kim S, Yu B, Jaffray E, Song J, Zheng J, Rodriguez MS, Hay RT, Chen Y. Role of an N-terminal site of Ubc9 in SUMO-1, -2, and -3 binding and conjugation. Biochemistry 42: 9959–9969, 2003. doi: 10.1021/bi0345283. [DOI] [PubMed] [Google Scholar]
- 188.Ullmann R, Chien CD, Avantaggiati ML, Muller S. An acetylation switch regulates SUMO-dependent protein interaction networks. Mol Cell 46: 759–770, 2012. doi: 10.1016/j.molcel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Van Nguyen T, Angkasekwinai P, Dou H, Lin FM, Lu LS, Cheng J, Chin YE, Dong C, Yeh ET. SUMO-specific protease 1 is critical for early lymphoid development through regulation of STAT5 activation. Mol Cell 45: 210–221, 2012. doi: 10.1016/j.molcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Van Wijk SJ, Timmers HT. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J 24: 981–993, 2010. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- 191.Velagapudi P, Turagam M, Laurence T, Kocheril A. Cardiac arrhythmias and sudden unexpected death in epilepsy (SUDEP). Pacing Clin Electrophysiol 35: 363–370, 2012. doi: 10.1111/j.1540-8159.2011.03276.x. [DOI] [PubMed] [Google Scholar]
- 192.Vertegaal AC. SUMO chains: polymeric signals. Biochem Soc Trans 38: 46–49, 2010. doi: 10.1042/BST0380046. [DOI] [PubMed] [Google Scholar]
- 193.Wang J, Chen L, Wen S, Zhu H, Yu W, Moskowitz IP, Shaw GM, Finnell RH, Schwartz RJ. Defective sumoylation pathway directs congenital heart disease. Birth Defects Res A Clin Mol Teratol 91: 468–476, 2011. doi: 10.1002/bdra.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang L, Qiao GH, Hu HN, Gao ZB, Nan FJ. Discovery of novel retigabine derivatives as potent KCNQ4 and KCNQ5 channel agonists with improved specificity. ACS Med Chem Lett 10: 27–33, 2019. doi: 10.1021/acsmedchemlett.8b00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang L, Wansleeben C, Zhao S, Miao P, Paschen W, Yang W. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep 15: 878–885, 2014. doi: 10.15252/embr.201438534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang R, Liu F, Zhao Y, Wu D, Chen L, Yeh ETH, Huang C. Reversible regulation of ORC2 SUMOylation by PIAS4 and SENP2. Oncotarget 8: 70142–70155, 2017. doi: 10.18632/oncotarget.19594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang RT, Zhi XY, Zhang Y, Zhang J. Inhibition of SENP1 induces radiosensitization in lung cancer cells. Exp Ther Med 6: 1054–1058, 2013. doi: 10.3892/etm.2013.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang T, Cao Y, Zheng Q, Tu J, Zhou W, He J, Zhong J, Chen Y, Wang J, Cai R, Zuo Y, Wei B, Fan Q, Yang J, Wu Y, Yi J, Li D, Liu M, Wang C, Zhou A, Li Y, Wu X, Yang W, Chin YE, Chen G, Cheng J. SENP1-Sirt3 signaling controls mitochondrial protein acetylation and metabolism. Mol Cell 75: 823–834.e5, 2019. doi: 10.1016/j.molcel.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 199.Wang XD, Gong Y, Chen ZL, Gong BN, Xie JJ, Zhong CQ, Wang QL, Diao LH, Xu A, Han J, Altman A, Li Y. TCR-induced sumoylation of the kinase PKC-θ controls T cell synapse organization and T cell activation. Nat Immunol 16: 1195–1203, 2015. doi: 10.1038/ni.3259. [DOI] [PubMed] [Google Scholar]
- 200.Wang Z, Jin J, Zhang J, Wang L, Cao J. Depletion of SENP1 suppresses the proliferation and invasion of triple-negative breast cancer cells. Oncol Rep 36: 2071–2078, 2016. doi: 10.3892/or.2016.5036. [DOI] [PubMed] [Google Scholar]
- 201.Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol 1: 20–26, 1999. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 202.Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett 579: 5007–5012, 2005. doi: 10.1016/j.febslet.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 203.Wei B, Huang C, Liu B, Wang Y, Xia N, Fan Q, Chen GQ, Cheng J. Mitotic phosphorylation of SENP3 regulates DeSUMOylation of chromosome-associated proteins and chromosome stability. Cancer Res 78: 2171–2178, 2018. doi: 10.1158/0008-5472.CAN-17-2288. [DOI] [PubMed] [Google Scholar]
- 204.Wei W, Yang P, Pang J, Zhang S, Wang Y, Wang MH, Dong Z, She JX, Wang CY. A stress-dependent SUMO4 sumoylation of its substrate proteins. Biochem Biophys Res Commun 375: 454–459, 2008. doi: 10.1016/j.bbrc.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 205.Werner A, Flotho A, Melchior F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol Cell 46: 287–298, 2012. doi: 10.1016/j.molcel.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 206.Wong KA, Kim R, Christofk H, Gao J, Lawson G, Wu H. Protein inhibitor of activated STAT Y (PIASy) and a splice variant lacking exon 6 enhance sumoylation but are not essential for embryogenesis and adult life. Mol Cell Biol 24: 5577–5586, 2004. doi: 10.1128/MCB.24.12.5577-5586.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wu J, Lei H, Zhang J, Chen X, Tang C, Wang W, Xu H, Xiao W, Gu W, Wu Y. Momordin Ic, a new natural SENP1 inhibitor, inhibits prostate cancer cell proliferation. Oncotarget 7: 58995–59005, 2016. doi: 10.18632/oncotarget.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xiang-ming Y, Zhi-Qiang X, Ting Z, Jian W, Jian P, Li-Qun Y, Ming-Cui F, Hong-Liang X, Xu C, Yun Z. SENP1 regulates cell migration and invasion in neuroblastoma. Biotechnol Appl Biochem 63: 435–440, 2016. doi: 10.1002/bab.1375. [DOI] [PubMed] [Google Scholar]
- 209.Xu W, Gong L, Haddad MM, Bischof O, Campisi J, Yeh ET, Medrano EE. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp Cell Res 255: 135–143, 2000. doi: 10.1006/excr.2000.4803. [DOI] [PubMed] [Google Scholar]
- 210.Xu Y, Zuo Y, Zhang H, Kang X, Yue F, Yi Z, Liu M, Yeh ET, Chen G, Cheng J. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J Biol Chem 285: 36682–36688, 2010. doi: 10.1074/jbc.M110.164236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yamashita D, Yamaguchi T, Shimizu M, Nakata N, Hirose F, Osumi T. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells 9: 1017–1029, 2004. doi: 10.1111/j.1365-2443.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 212.Yan Y, Ollila S, Wong IPL, Vallenius T, Palvimo JJ, Vaahtomeri K, Mäkelä TP. SUMOylation of AMPKα1 by PIAS4 specifically regulates mTORC1 signalling. Nat Commun 6: 8979, 2015. doi: 10.1038/ncomms9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang SH, Galanis A, Witty J, Sharrocks AD. An extended consensus motif enhances the specificity of substrate modification by SUMO. EMBO J 25: 5083–5093, 2006. doi: 10.1038/sj.emboj.7601383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yeh ET. SUMOylation and de-SUMOylation: wrestling with life’s processes. J Biol Chem 284: 8223–8227, 2009. doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yeh ET, Gong L, Kamitani T. Ubiquitin-like proteins: new wines in new bottles. Gene 248: 1–14, 2000. doi: 10.1016/S0378-1119(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 216.Yeh ETH, Rosse WF. Paroxysmal nocturnal hemoglobinuria and the glycosylphosphatidylinositol anchor. J Clin Invest 93: 2305–2310, 1994. doi: 10.1172/JCI117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yu B, Swatkoski S, Holly A, Lee LC, Giroux V, Lee CS, Hsu D, Smith JL, Yuen G, Yue J, Ann DK, Simpson RM, Creighton CJ, Figg WD, Gucek M, Luo J. Oncogenesis driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9. Proc Natl Acad Sci USA 112: E1724–E1733, 2015. doi: 10.1073/pnas.1415569112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yu L, Ji W, Zhang H, Renda MJ, He Y, Lin S, Cheng EC, Chen H, Krause DS, Min W. SENP1-mediated GATA1 deSUMOylation is critical for definitive erythropoiesis. J Exp Med 207: 1183–1195, 2010. doi: 10.1084/jem.20092215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yu X, Lao Y, Teng XL, Li S, Zhou Y, Wang F, Guo X, Deng S, Chang Y, Wu X, Liu Z, Chen L, Lu LM, Cheng J, Li B, Su B, Jiang J, Li HB, Huang C, Yi J, Zou Q. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat Commun 9: 3157, 2018. doi: 10.1038/s41467-018-05676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yun C, Wang Y, Mukhopadhyay D, Backlund P, Kolli N, Yergey A, Wilkinson KD, Dasso M. Nucleolar protein B23/nucleophosmin regulates the vertebrate SUMO pathway through SENP3 and SENP5 proteases. J Cell Biol 183: 589–595, 2008. doi: 10.1083/jcb.200807185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yunus AA, Lima CD. Structure of the Siz/PIAS SUMO E3 ligase Siz1 and determinants required for SUMO modification of PCNA. Mol Cell 35: 669–682, 2009. doi: 10.1016/j.molcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang FP, Mikkonen L, Toppari J, Palvimo JJ, Thesleff I, Jänne OA. Sumo-1 function is dispensable in normal mouse development. Mol Cell Biol 28: 5381–5390, 2008. doi: 10.1128/MCB.00651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang H, Saitoh H, Matunis MJ. Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex. Mol Cell Biol 22: 6498–6508, 2002. doi: 10.1128/MCB.22.18.6498-6508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de Thé H, Chen SJ, Chen Z. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 328: 240–243, 2010. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]
- 225.Zheng Q, Cao Y, Chen Y, Wang J, Fan Q, Huang X, Wang Y, Wang T, Wang X, Ma J, Cheng J. Senp2 regulates adipose lipid storage by de-SUMOylation of Setdb1. J Mol Cell Biol 10: 258–266, 2018. doi: 10.1093/jmcb/mjx055. [DOI] [PubMed] [Google Scholar]
- 226.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458: 351–356, 2009. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci 120: 1178–1188, 2007. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]