Abstract
Background
We evaluated whether frailty and multimorbidity predict in-hospital mortality in patients with COVID-19 beyond chronological age.
Method
A total of 165 patients admitted from March 8th to April 17th, 2020, with COVID-19 in an acute geriatric ward in Italy were included. Predisease frailty was assessed with the Clinical Frailty Scale (CFS). Multimorbidity was defined as the co-occurrence of ≥2 diseases in the same patient. The hazard ratio (HR) of in-hospital mortality as a function of CFS score and number of chronic diseases in the whole population and in those aged 70+ years were calculated.
Results
Among the 165 patients, 112 were discharged, 11 were transferred to intensive care units, and 42 died. Patients who died were older (81.0 vs 65.2 years, p < .001), more frequently multimorbid (97.6 vs 52.8%; p < .001), and more likely frail (37.5 vs 4.1%; p < .001). Less than 2.0% of patients without multimorbidity and frailty, 28% of those with multimorbidity only, and 75% of those with both multimorbidity and frailty died. Each unitary increment in the CFS was associated with a higher risk of in-hospital death in the whole sample (HR = 1.3; 95% CI = 1.05–1.62) and in patients aged 70+ years (HR = 1.29; 95% CI = 1.04–1.62), whereas the number of chronic diseases was not significantly associated with higher risk of death. The CFS addition to age and sex increased mortality prediction by 9.4% in those aged 70+ years.
Conclusions
Frailty identifies patients with COVID-19 at risk of in-hospital death independently of age. Multimorbidity contributes to prognosis because of the very low probability of death in its absence.
Keywords: COVID-19, Frailty, In-hospital mortality, Multimorbidity
Clinical manifestations of coronavirus disease 2019 (COVID-19) vary greatly ranging from asymptomatic infection to severe and sometimes fatal respiratory failure (1). Similar to other previously isolated coronaviruses responsible for the severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) (2,3), severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) can cause interstitial pneumonia (4). The case-fatality rate has been reported to steeply increase with age worldwide, from 4.9% in the 0–59 years to 9.5% in the 60–69 years, reaching 25.2% in the 90+ year-old (5). In accordance with the Italian National Institute of Health surveillance system, persons who died by COVID-19 were more frequently older, males, and presented a mean number of 2.7 preexisting chronic diseases. Overall, 74% of the deceased individuals had multimorbidity (ie, 2 or more chronic diseases) (6). Hypertension, chronic obstructive pulmonary disease (COPD), malignancy, chronic kidney disease, cardiovascular disease, and diabetes mellitus were the most common comorbidities among hospitalized persons affected by COVID-19 (7), and patients with preexisting cardiovascular and metabolic conditions have the greatest risk of adverse outcomes (8).
Along with its burden on the health and well-being of affected individuals, the most severe cases of COVID-19 are currently overloading the health care systems of several countries, which are striving to adapt their actions to the new condition. A prompt and reliable identification of patients affected by COVID-19 at higher risk of death may direct clinical management and difficult decision making. Recommendations provided so far by health agencies suggest that patients’ biological age—rather than chronological—must steer clinical decisions (9–11). However, instruments available to assess biological age are not yet ready for clinical applications. As a result, chronological age has been used in decision making, such as intensive care unit (ICU) admissions, without solid evidence supporting such decisions.
Frailty, a state of increased vulnerability caused by reduced homeostasis in several systems (12) and multimorbidity, the simultaneous presence of multiple chronic conditions in the same individuals (13), could be used as surrogate measures of biological aging, as they track the rate of decline in health and function with aging, and, independent of chronological age, their presence has been associated with a number of unfavorable outcomes including hospitalization, disability, and mortality (14–16). In the general population, as well as in individuals admitted to ICUs, the impact of frailty is clear even in persons without multimorbidity (17,18). Frailty predicts mortality in the adult population (19), is associated with lower probability of recovery in elderly persons hospitalized with influenza and acute respiratory illness (increasing the odd of death during the 30 days post-discharge) (20), and is a negative prognostic predictor in patients affected by HIV (21). Although frailty and multimorbidity can overlap, approximately one-fourth of persons with multimorbidity do not have frailty (22), suggesting that frailty and multimorbidity bring complementary information in the patients’ assessment.
The aim of this study was to evaluate whether frailty and multimorbidity predict in-hospital mortality beyond chronological age among patients with COVID-19 admitted to a tertiary care hospital in Italy.
Method
Study Participants and Data Collection
This is a retrospective observational study including 171 consecutive patients with a diagnosis of COVID-19 admitted to the acute geriatric unit of the Civili Hospital in Montichiari (Brescia, Northern Italy) from March 8th to April 17th, 2020. At the onset of the COVID-19 emergency, the Montichiari hospital was designated as COVID-19 special hospital in the Lombardy region and the geriatric medicine unit of the hospital was open to both younger and older adults affected by the disease. Patients were admitted to the geriatric ward if they had signs or symptoms of COVID-19, independently of their age. Of the 171 patients, 6 were not included in this study due to a high amount of missing information in their medical charts. All the remaining 165 patients were followed up for the whole hospitalization in the acute geriatric ward.
By the 17th of April, all patients were either discharged (N = 112%–67.9%), transferred to the ICU (ICUs across the whole Lombardy or other regions) (N = 11%– 6.7%), or dead (N = 42–25.4%).
Patients were discharged if they displayed peripheral oxygen saturation >94% in ambient air, a respiratory frequency lower than 22 times per minute, and had no fever for 48 hours. The decision to discharge a patient was not affected by the paucity of resources, but always in keeping of the clinical conditions of the individual patient. The decision upon ICU transferal was made by critical care physicians, who evaluated the vital parameters, blood exams, and respiratory distress twice daily or upon request of the geriatric ward physician. Patients transferred to the ICU were younger (59.5 years, SD = 16.1); all of them were nonfrail, 5 (45.4%) had multimorbidity and the median number of drugs prescribed at home was 0 (IQR 3).
Nasal and pharyngeal swab samples were collected at hospital admission by trained nurses and total SARS-Cov-2 RNA was extracted for testing. COVID-19 cases were identified by reverse transcriptase-polymerase chain reaction (RT-PCR) assay for SARS-Cov-2 RNA. A small number of cases with a negative or undetermined test (N = 11) were considered to be infected by SARS-Cov-2 based on the computed tomography of the chest and the highly suggestive clinical characteristics (23). A recent systematic review of the literature showed that false-negative test results may occur in up to 20% to 67% of patients affected by COVID-19 (24).
The study was approved by the Ethical Committee of the Brescia County (Italy).
Data Collection
Information on age, sex, education, and living arrangement were collected. Education was categorized into primary (5 years of schooling or less) and secondary education (≥6 years). Clinical and laboratory characteristics and outcome data were obtained from medical records and anonymously aggregated. The following data were collected: smoking history, number of chronic diseases among a predefined and validated list of 60 diseases, (13) multimorbidity (defined as 2+ co-occurring diseases in the same patient), number of drugs before hospital admission, and symptoms and signs of infection (fever, cough, headache, dyspnea, weakness, ageusia, olfactory impairment, gastrointestinal problems).
Frailty was assessed at hospital admission by a geriatrician who collected information about the preinfection health status of the patient. Such information was collected directly from the patient if he/she was not cognitively impaired and affected by moderate disease. If the patient was cognitively impaired or severely ill, this information was collected from a proxy/caregiver. The Clinical Frailty Scale (CFS) was employed to assess frailty: the CFS is an ordinal 9-point scale in which the assessor makes decisions about the degree of frailty from clinical data (25). The patients are scored from 1 “very fit” to 9 “terminally ill,” with a score of 6 or more being indicative of moderate-to-severe frailty. According to the CFS, a patient is non- to mildly frail for scores <6 (1 being robust and physically active to 5 being dependent in instrumental activities of daily living such as shopping and managing medications), moderately frail from score 6 to 7 (6 being dependent in all the outdoor activities and bathing and 7 being dependent in personal care), and severely frail from scores 8 to 9 (8 being dependent in all basic activities of daily living and 9 being terminally ill). In the case of cognitive impairment or severe COVID-19 at admission, information regarding frailty was asked to a proxy/caregiver.
Laboratory data comprised of a complete blood count, white blood cells count, O2, CO2, pH blood gas test, and a C-reactive protein (CRP) test. Pharmacological treatments during hospitalization were prescribed in accordance with the Guidelines of the Lombardy section of the Italian Society of Infectious and Tropical diseases (26), and were informed by the patient’s clinical features and availability of drugs.
Statistical Analysis
Data were described as count and proportion, mean and SD, or median and interquartile range, as appropriate. Differences in the characteristics between survived and deceased patients were assessed using a chi-squared test, Fisher’s exact test, t test, or Mann–Whitney U test, as appropriate. In the Kaplan–Meier survival analysis and plot (Figure 1), the sample was stratified into 3 groups: nonfrail with 0 or 1 chronic conditions, nonfrail with multimorbidity, and frail with multimorbidity. In our sample, all the patients with frailty were also affected by multimorbidity. Cox proportional hazard regression models were employed to investigate the independent association of frailty (as CFS) and multimorbidity (as the count of chronic conditions) with survival. The analyses were run in the whole population and in the subsample of patients aged ≥70. In the survival analyses, the date of hospital admission was used as time 0. The date of discharge, ICU transferal, or death in the acute geriatric ward was used to set time of the right censoring or outcome. The proportional hazard assumption was satisfied in both cases (p for the Schoenfeld test against residuals for the analysis in the whole study population = .890 and among patients ≥70 years old = .900). The ROC curves (Figure 2) evaluate the accuracy in the prediction of mortality according to frailty and multimorbidity. Areas under the curve (AUC) were obtained through nonparametric ROC analyses and their 95% confidence intervals (95% CIs) were estimated by bootstrapping with replacement (N = 2000). Diagnostic tests reported in the table in Figure 2 were based on the cutoffs for moderate–severe frailty (CFS ≥ 6) and multimorbidity, tested in univariate analyses. C-statistics (Table 3) were obtained by calculating the AUCs of the values predicted by Cox proportional hazard models. Their 95% CIs and the comparison between models were estimated by bootstrapping with replacement (N = 2000). All analyses were conducted with R 3.6.3(27), with an alpha-level of .05.
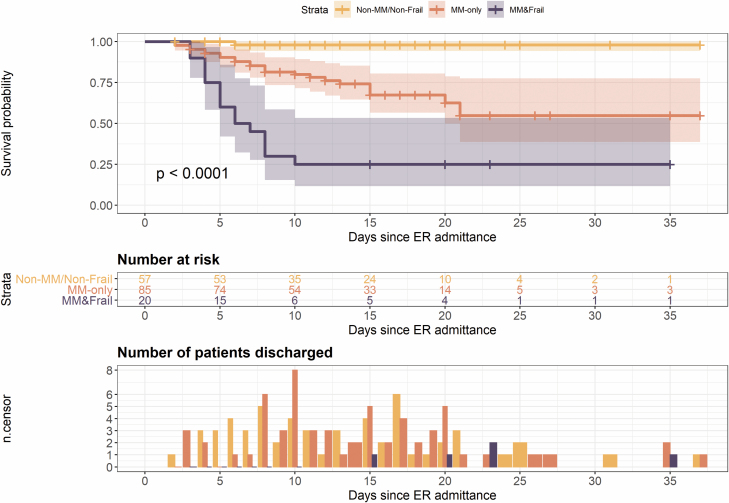
Figure 1.
Kaplan–Meier curve for survival by multimorbidity (MM) and frailty combinations and discharge rate (frailty without multimorbidity was absent).
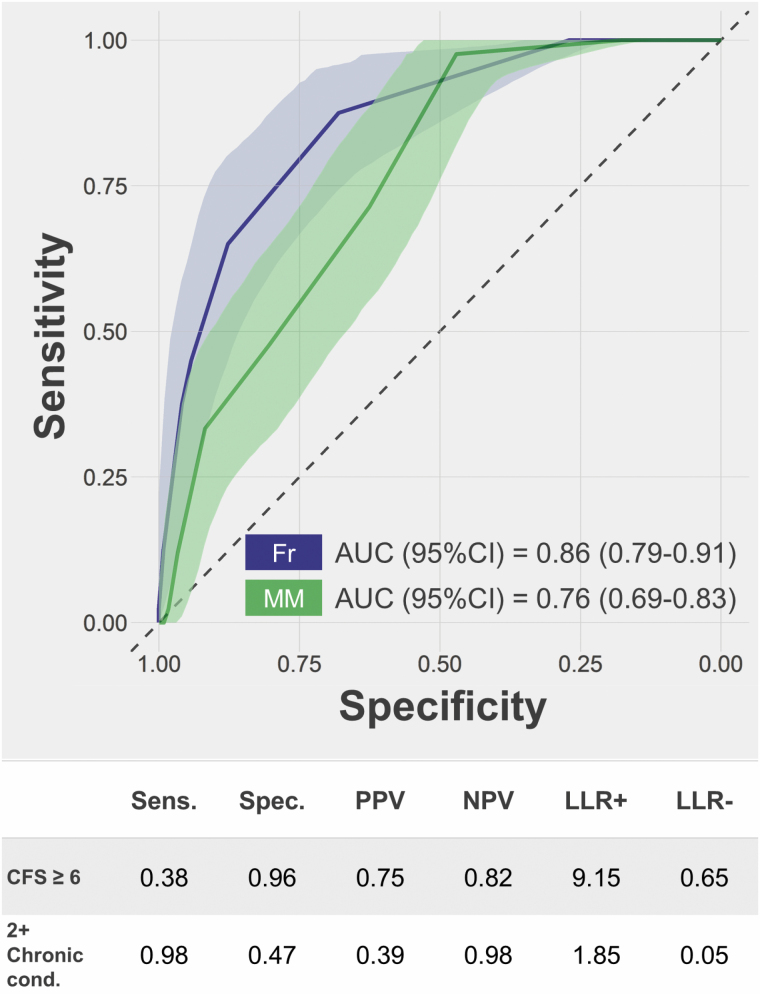
Figure 2.
ROC curves for Clinical Frailty Scale (Fr) score and number of chronic conditions (MM) in the prediction of mortality. AUC and 95% confidence intervals (95% CIs) were estimated through bootstrapping (N = 2000). The table shows sensitivity (Sens.), specificity (Spec.), positive and negative predictive values (PPV and NPV), and positive and negative likelihood ratios (LLR+ and LLR−) for frailty (CFS ≥ 6) and multimorbidity (2+ chronic condition) in the prediction of mortality.
Table 3.
Multiadjusted Hazard Ratios (HRs) and 95% Confidence Intervals (95% CIs) of Death in Patients Aged 70+ Years (N = 91)
| Model | Variables | HR (95% CI) | C-Statistic | % Difference With Model 1 | p (absolute difference with Model 1) |
|---|---|---|---|---|---|
| 1 | Age | 1.10 (1.04–1.17) | 0.709 | Ref. | Ref. |
| Male sex | 1.76 (0.85–3.67) | ||||
| Primary education | 1.51 (0.71–3.24) | ||||
| 2 | Age | 1.10 (1.03–1.16) | 0.704 | −0.7% | .695 |
| Male sex | 1.66 (0.79–3.51) | ||||
| Primary education | 1.40 (0.63–3.09) | ||||
| Number of chronic diseases | 1.06 (0.90–1.23) | ||||
| 3 | Age | 1.08 (1.01–1.15) | 0.776 | +9.4% | .091 |
| Male sex | 2.22 (1.03–4.78) | ||||
| Primary education | 1.45 (0.64–3.24) | ||||
| CFS score | 1.29 (1.04–1.62) | ||||
| 4 | Age | 1.08 (1.00–1.15) | 0.780 | +10.0% | .086 |
| Male sex | 2.17 (0.98–4.81) | ||||
| Primary education | 1.40 (0.59–3.33) | ||||
| Number of chronic diseases | 1.02 (0.86–1.21) | ||||
| CFS score | 1.29 (1.03–1.62) |
Note: CFS = Clinical Frailty Scale. The absolute difference between models was assessed through bootstrapping (N = 2000): p is shown. C-statics and absolute difference with Model 1; Model 1: age, sex, and education. Model 2: Model 1 + number of chronic conditions. Model 3: Model 1 + CFS score; Model 4: Model 1 + CFS score and number of chronic conditions. N at risk = 81, events = 34.
Results
As showed in Table 1, patients were 69.3 years old (SD 14.5) on average and 55.1% were 70 years or older; 60.6% were males and 49.7% had primary education. The median duration of symptoms before hospital admission was 7 days and the median length of hospital stay was 10 days (IQR 7–17). Overall, 28.5% of patients had an arterial oxygen saturation <90% at hospital admission. The median number of chronic diseases was 2 (IQR 1–4) and 64.2% of patients were affected by multimorbidity. The median CFS score was 2 (IQR 2–4) and 87.7% of patients had a CFS score <6. Patients who died were more likely to be older (p < .001) and had lower educational attainment (p = .001). The percentage of patients with arterial oxygen saturation <90% at hospital admission was 60.5% in those who died versus 17.0% in those who did not (p < .001). The median CRP was higher in those who died (p < .001). The median number of chronic coexisting diseases was higher among those who died (3 vs 2, p < .001). Thirty-seven percent of patients who died versus 4.1% of those who did not had a CFS score ≥6.
Table 1.
Demographic and Clinical Characteristics of the Study Population in the Whole Sample and by Outcome
| Whole, N = 165 | Survived, N = 123 (74.5) | Deceased, N = 42 (25.5) | p | |
|---|---|---|---|---|
| Age, y, mean (SD) | 69.3 (14.5) | 65.2 (14.3) | 81.0 (6.5) | <.001 |
| <40 y | 7 (4.2) | 7 (5.7) | 0 (0.0) | |
| 40–49 y | 16 (9.7) | 16 (13.0) | 0 (0.0) | |
| 50–59 y | 14 (8.5) | 14 (11.4) | 0 (0.0) | |
| 60–69 y | 37 (22.4) | 34 (27.6) | 3 (7.1) | |
| 70–79 y | 48 (29.1) | 34 (27.6) | 14 (33.3) | |
| 80–89 y | 38 (23.0) | 17 (13.8) | 21 (50.0) | |
| ≥ 90 y | 5 (3.0) | 1 (0.8) | 4 (9.5) | |
| Male sex | 100 (60.6) | 73 (59.3) | 27 (64.3) | .702 |
| Primary education | 75 (49.7) | 47 (41.6) | 28 (73.7) | .001 |
| Living alone | 25 (18.2) | 17 (16.5) | 8 (23.5) | .507 |
| Clinical Frailty Scale, median (IQR) | 2.0 (2.0, 4.0) | 2.0 (1.0, 3.0) | 4.0 (3.0, 6.0) | <.001 |
| Frailty (categorized) | <.001 | |||
| No/mild frailty | 142 (87.7) | 117 (95.9) | 25 (62.5) | |
| Moderate frailty | 19 (11.7) | 5 (4.1) | 14 (35.0) | |
| Severe frailty | 1 (0.6) | 0 (0.0) | 1 (2.5) | |
| Smoking habit | .363 | |||
| No | 74 (68.5) | 62 (71.3) | 12 (57.1) | |
| Ex/current | 35 (31.5) | 26 (28.7) | 9 (42.9) | |
| Hypertension | 98 (59.4) | 67 (54.5) | 31 (73.8) | .043 |
| Type 2 diabetes mellitus | 51 (30.9) | 36 (29.3) | 15 (35.7) | .557 |
| Obesity/overweight | 27 (16.4) | 24 (19.5) | 3 (7.1) | .089 |
| Cognitive impairment | 14 (8.5) | 5 (4.1) | 9 (21.4) | .002 |
| Heart failure | 5 (3.0) | 3 (2.4) | 2 (4.8) | .602 |
| COPD | 4 (2.4) | 2 (1.6) | 2 (4.8) | .268 |
| Chronic diseases, median (IQR) | 2.0 (1.0, 4.0) | 2.0 (1.0, 3.0) | 3.0 (2.0, 5.0) | <.001 |
| Multimorbidity (2+ chronic conditions) | 106 (64.2) | 65 (52.8) | 41 (97.6) | <.001 |
| No. of drugs, mean (SD) | 3.8 (3.2) | 3.1 (3.2) | 5.5 (2.4) | <.001 |
| No. of days with symptoms before admission, median (IQR) | 7.0 (5.0, 10.0) | 7.0 (5.0, 10.0) | 7.0 (4.0, 10.0) | .283 |
| Length of hospital stay, days, median (IQR) | 10.0 (7.0, 17.0) | 13.0 (8.0, 18.0) | 6.0 (4.0, 8.0) | <.001 |
| COVID-19 clinical features | ||||
| Fever | 147 (89.1) | 110 (89.4) | 37 (88.1) | 1.000 |
| Cough | 83 (50.3) | 64 (52.0) | 19 (45.2) | .561 |
| Dyspnea | 70 (42.4) | 51 (41.5) | 19 (45.2) | .805 |
| Gastrointestinal disturbances | 31 (18.8) | 27 (22.0) | 4 (9.5) | .109 |
| Headache | 7 (4.2) | 6 (4.9) | 1 (2.4) | .680 |
| Arterial oxygen saturation < 90% | 41 (28.5) | 18 (17.0) | 23 (60.5) | <.001 |
| Arterial oxygen concentration, mm Hg, median (IQR) | 67.0 (53.5, 79.0) | 69.0 (60.0, 81.5) | 53.0 (42.7, 69.2) | <.001 |
| White blood cells, 103/μL, median (IQR) | 7.4 (5.8, 9.7) | 6.8 (5.5, 8.9) | 9.2 (6.4, 10.8) | .003 |
| C-reactive protein, mg/L, median (IQR) | 101.9 (51.1, 151.1) | 86.6 (38.8, 140.0) | 139.9 (91.8, 205.2) | .001 |
Note: COPD = chronic obstructive pulmonary disease; IQR = interquartile range. Missing: 14 for education, 28 for living situation, 3 for Clinical Frailty Scale and its categorization, 3 for number of days with symptoms, 14 for oxygen saturation and concentration, 8 for white blood cell count, and 8 for C-reactive protein.
Tables 2 and 3 show Cox regression models reporting the hazard ratios (HRs) for death and the c-statistics derived from survival analyses associated with CFS score and number of chronic diseases adjusted for age, sex, and education in the whole sample and in patients aged 70+ years (N = 91), respectively. Each unit increase in CFS score was associated with increased in-hospital death both in the whole sample and among older individuals, whereas each unit increase in the number of chronic diseases was not significantly associated with higher risk of in-hospital mortality. The addition of the CFS improved the model’s goodness of fit by 9.4% in the subsample of patients aged 70 years and older: the model containing CFS has a 9% increased probability to assign a higher risk of death to those who actually died during the hospitalization, in comparison to the model based on age, sex, and education only.
Table 2.
Multiadjusted Hazard Ratios (HRs) and 95% Confidence Intervals (95% CIs) of Death in All Patients
| Model | Variables | HR (95% CI) | C-Statistic | % Difference With Model 1 | p (absolute difference with Model 1) |
|---|---|---|---|---|---|
| 1 | Age | 1.13 (1.08–1.18) | 0.856 | Ref. | Ref. |
| Male sex | 1.75 (0.86–3.53) | ||||
| Primary education | 1.47 (0.70–3.07) | ||||
| 2 | Age | 1.12 (1.07–1.17) | 0.857 | +0.1% | .778 |
| Male sex | 1.62 (0.79–3.33) | ||||
| Primary education | 1.34 (0.62–2.87) | ||||
| Number of chronic conditions | 1.07 (0.92–1.24) | ||||
| 3 | Age | 1.10 (1.04–1.16) | 0.888 | +3.7% | .030 |
| Male sex | 2.19 (1.05–4.58) | ||||
| Primary education | 1.40 (0.65–3.04) | ||||
| CFS score | 1.31 (1.05–1.62) | ||||
| 4 | Age | 1.10 (1.04–1.16) | 0.888 | +3.7% | .042 |
| Male sex | 2.11 (0.99–4.52) | ||||
| Primary education | 1.33 (0.58–3.04) | ||||
| Number of chronic conditions | 1.03 (0.87–1.22) | ||||
| CFS score | 1.30 (1.05–1.62) |
Note: CFS = Clinical Frailty Scale. The absolute difference between models was assessed through bootstrapping (N = 2000): p is shown. C-statics and absolute difference with Model 1; Model 1: age, sex, and education. Model 2: Model 1 + number of chronic conditions. Model 3: Model 1 + CFS score; Model 4: Model 1 + CFS score and number of chronic conditions. N at risk = 148, events = 36.
Figure 1 displays Kaplan–Meier survival plots for patients with no multimorbidity and no frailty as the reference group (n = 57 – median hospital length of stay = 11), those with multimorbidity only (n = 85 – median hospital length of stay = 11), and those with multimorbidity and frailty (n = 20 – median hospital length of stay = 6.5). In our study, frailty always co-occurred with multimorbidity. 1.8% of patients without multimorbidity and frailty, 28% of those with multimorbidity only, and 75% of those with both multimorbidity and frailty died during hospitalization.
The AUC for the prediction of in-hospital mortality was 0.86 (0.79–0.91) for the CFS and 0.76 (0.69–0.83) for the number of chronic diseases (Figure 2). The positive predictive value (PPV) of moderate-to-severe frailty (CFS ≥ 6) was 0.75 and the positive likelihood ratio was 9.15. The negative predictive value (NPV) of multimorbidity was 0.98 and the negative likelihood ratio was 0.05.
Discussion
In hospitalized patients with COVID-19, higher CFS scores were associated with mortality, independently of chronological age. Moreover, the CFS appeared to improve the prediction of death beyond chronological age among those aged 70+ years.
We used data collected during this emergency period to test the hypothesis that patients admitted to the hospital had some clinical characteristics that differentiated those who survived to the study’s end from those who died. Studies in China have reported that patients with COVID-19 who suffered adverse outcomes were older than those who recovered, and that some specific chronic conditions were more prevalent in those who died (1,8,28,29). The findings of our study align with such previous reports, also adding new insight into the role of multimorbidity and frailty, independent of age as risk factors for mortality in those with COVID-19.
In our study, patients with CFS ≥ 6 were 9 times more likely to die during the hospitalization than those with lower scores. Of note, the CFS alone predicted in-hospital death with a discriminative ability of 88%. Currently, there is no internationally recognized standard definition and operationalization of frailty given its complex pathophysiology. The main reason for that is that the underlying mechanisms of frailty are still under investigation. At the same time, due to the urgency to have tool spendable in clinical practice, a high number of frailty definitions have been proposed in the last 2 decades, showing fair-to-good validity (30). We found that 12% of patients could be defined as moderately or severely frail, according to the CFS, which is the clinical tool suggested by the NICE guidelines for the assessment of older patients affected by COVID-19 infection (11). The CFS has shown a discriminative ability comparable to the Frailty Index (25) which is a model of accumulation of deficits, such as diseases and disability. Further, in patients admitted to a geriatric ward, the CFS and the Fried frailty phenotype (31) were equally suitable for differentiating between patients who died due to any cause from those who survived during follow-up (32). Compared to other frailty scales, the CFS has the specific strength that it has been validated as an adverse outcome predictor in hospitalized older people (33) but, on the other hand, it is strongly tangled with the presence of disability, especially for higher CFS scores. Finally, the CFS is timesaving, especially when applied by a trained physician such as a geriatrician. The latter characteristic was very important during the emergency of COVID-19. A recent scoping review showed that 5 out of 6 studies on frailty assessment in COVID-19 used the CFS to assess frailty (34). Subsequent to this review a large study mostly based on data from the UK was published showing that frailty measured by the CFS was strongly associated with mortality in hospitalized patients aged 61 years and older (35).
Central to the frailty concept is the multisystem involvement, including dysregulation of neuromuscular, endocrine, and immune systems. The age-related decline in immune function is well documented and it can contribute to frailty as well as to an increased susceptibility to acute and chronic diseases; aging and frailty lead to an imbalance between stressors and stress-buffering mechanisms that causes loss of compensatory reserve. In particular, changes in the innate immunity could enhance a proinflammatory state which is a fundamental component of frailty (36,37). In a systematic review of the literature, frailty and prefrailty were associated with higher inflammatory parameters, such as CRP, interleukin-6, elevated white blood cell, and fibrinogen levels (38). Further, in older adults, a reduced chemotaxis with an inefficient neutrophil migration may induced greater tissue damage and secondary systemic inflammation (39) that, among changes in T-cell function, may impair the overall immune response of the organism. Thus, in COVID-19, frailty is likely to be a good clinical marker of a substrate sensitive to infections, and to enhance the multiorgan involvement of the infection itself (40,41) as well as the dysregulated inflammatory response (1,42). All the reasons above may explain the strong association between frailty and mortality found in our and other studies. Whereas inflammatory dysregulation may well account for the link between frailty and COVID-19 mortality in older persons, other hypotheses may apply to middle-aged adults. In fact, frailty has a strong relationship with sarcopenia and malnutrition (36) which are not exclusive of older people and both conditions have been associated with poorer outcomes in adult affected by COVID-19 (43). However, due to the low number of deaths in people aged less than 65 years in this study, conclusions about the utility of the CFS in middle-aged persons cannot be drawn from our results and we cannot recommend its use in this part of the population.
Our study adds to the previous literature as we elucidated both the role of frailty measured by the CFS and multimorbidity. As demonstrated in an Italian case series by Onder et al., multimorbidity was found in 3 quarters of the individuals who died from COVID-19 (6). Indeed, the proportion of persons with multimorbidity increases with age (13), as well as the case-fatality rate of COVID-19, reported thus far (5). However, given the high prevalence of multimorbidity in the older population, this would not directly imply an effect of multimorbidity on poor prognosis in COVID-19. Interestingly, in our study, those without multimorbidity were 20 times more likely to survive during hospitalization than those affected by 2+ chronic diseases. In previous studies conducted in the community, it was estimated that about 1 quarter of persons affected by multimorbidity are not frail (22), suggesting that frailty and multimorbidity capture different aspects of health. Previous studies carried out before the COVID-19 pandemic showed that frailty indices and multimorbidity have different accuracy in predicting mortality in older adults (44). Our findings on the different specificity and sensitivity of frailty and multimorbidity in predicting mortality in hospitalized patients with COVID-19 reinforce this assumption.
Identifying those who will require specific interventions early in the progression of the disease might help to avoid adverse outcomes. Moreover, the identification of those who are likely to recover without intensive care might be useful in planning resource allocation. This is particularly true in older patients; while chronological age is still used as a marker of health and a decision-making tool, there is strong evidence that frailty, multimorbidity, and other geriatric syndromes such as sarcopenia are better predictors of health-related outcomes in older patients (15,16,45). For example, decisions about specific treatments are still often based on chronological age and the presence of 1 or 2 chronic diseases, especially in the ICUs triage. Yet, literature indicates that “high-quality care cannot be accomplished by looking only at age and diagnoses” (46,47). In our study, patients who were transferred to the ICU were younger and nonfrail. Notably, the Italian Society of Anaesthesia, Analgesia and Intensive Care (SIAARTI) released some recommendations for exceptional resource-limited situations (48). Although the document mentions that “it might be needed to set an age limit for the admission to intensive care,” it also suggests that “the presence of comorbidity and functional status must be carefully evaluated in addition to age.” Our study suggests that frailty assessment should be considered.
Limitations
First, data were collected retrospectively using medical charts and electronic records and a complete comprehensive assessment of patients was not performed. Future studies should include a multidimensional evaluation including the assessment of predisease mobility, functional and cognitive abilities, and the availability of social support. Indeed, despite the CFS is a scale widely used as a measure of frailty, it is mainly based on a functional evaluation and, as such, it may partially describe some aspects of the disability process. Thus, other frailty tools based on more objective measures should be tested in the future in order to confirm our findings. Secondly, we cannot exclude that during the severe and unexpected public health emergency, the most severely frail older patients were not even admitted to hospitals (in our sample, the percentage of patients with moderate-to-severe frailty was 12%). Under this assumption, our results are underestimating the strength of the association, but this hypothesis should be tested in future studies. Third, we were not able to retrieve the vital status of patients transferred to the ICU, because patients needed of intensive care were transferred to other hospitals within the same or to other regions according to the availability of resources. However, sensitivity analyses, first excluding patients transferred to the ICU, and second treating transferred patients as deaths, led to comparable results. Fourth, CFS intra- or interobserver variability was not evaluated due to limited time and resources. Fifth, the aim of our study was to investigate only in-hospital mortality and we lack data about early mortality after discharge. So, we cannot exclude the possibility that some patients may have died right after the discharge. Lastly, during the emergency, blood samples were not stored and, thus, they are not available for future analyses of biomarkers of interest, as for example inflammatory cytokines.
On the other hand, the major strengths of the study are the few missing data and the novelty of the clinical characteristics analyzed; despite the small sample size, the results are robust.
Conclusions
Our findings indicated that frailty was independently associated with mortality in patients affected by COVID-19 and added prognostic information beyond chronological age in those aged 70 years or older. Furthermore, the absence of multimorbidity appeared to be a relevant positive prognostic feature. Assessing for frailty and multimorbidity and embedding these 2 conditions in the decision-making process and clinical management of COVID-19 patients should be considered.
Acknowledgments
We would like to thank all the residents in geriatrics of the University of Brescia for their courageous and tireless contribution to the care of patients with COVID-19 and data collection. We also thank all the other health care professionals of the Montichiari Hospital that with great skills and humanity took care of the patients. We would also express our affection to patients and families suffering for the disease. Finally, this work is dedicated to GZ who lost his battle but left us his strength. We are grateful to Clare Tazzeo, from Karolinska Institutet, Stockholm, Sweden, for the editing of the present manuscript.
Funding
This work was supported in part by the Intramural Research Program of the National Institute on Aging, NIH, Baltimore, MD, USA.
Conflict of Interest
None declared.
Author Contributions
A.M. designed the study, had full access to all data, and takes responsibility for the accuracy and integrity of the data analysis. A.Z. and D.L.V. analyzed the data and contributed to the interpretation, literature search, and writing of the manuscript. A.M., F.N., A.A., E.B., E.G., A.B., and P.G. contributed to data collection and interpretation. L.Fe., L.Fr., R.B., and G.O. critically revised the analyses and the interpretation of the findings and contributed to writing of the manuscript. All the co-authors reviewed the manuscript and approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. A.M. is the guarantor.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506 doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747 [DOI] [PubMed] [Google Scholar]
- 3. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 4. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. doi: 10.3390/pathogens9030231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Italian Institute of Health. https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_15aprile%20ITA.pdf. Accessed May 5, 2020.
- 6. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Health System England. http://prod-upp-image-read.ft.com/765d3430-7a57-11ea-af44-daa3def9ae03. Accessed May 5, 2020.
- 10. Canadian Association of Emergency Physicians. https://caep.ca/wp-content/uploads/2020/04/Clinical-Triage-Protocol-for-Major-Surge-in-COVID-Pandemic-March-28–202.pdf. Accessed May 5, 2020.
- 11. NICE. https://www.nice.org.uk/guidance/ng159. Accessed July, 5 2020.
- 12. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calderón-Larrañaga A, Vetrano DL, Onder G, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci. 2017;72(10):1417–1423. doi: 10.1093/gerona/glw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med. 2009;265(2):288–295. doi: 10.1111/j.1365-2796.2008.02017.x [DOI] [PubMed] [Google Scholar]
- 15. Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2016;67:130–138. doi: 10.1016/j.archger.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 16. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47(2):193–200. doi: 10.1093/ageing/afx162 [DOI] [PubMed] [Google Scholar]
- 17. Zucchelli A, Vetrano DL, Marengoni A, et al. Frailty predicts short-term survival even in older adults without multimorbidity. Eur J Intern Med. 2018;56:53–56. doi: 10.1016/j.ejim.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 18. Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–1122. doi: 10.1007/s00134-017-4867-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dugravot A, Fayosse A, Dumurgier J, et al. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: a 24-year follow-up of the Whitehall II cohort study. Lancet Public Health. 2020;5(1):e42–e50. doi: 10.1016/S2468-2667(19)30226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lees C, Godin J, McElhaney JE, et al. Frailty hinders recovery from influenza and acute respiratory illness in older adults. J Infect Dis. 2020;222(3):428–437. doi: 10.1093/infdis/jiaa092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bloch M. Frailty in people living with HIV. AIDS Res Ther. 2018;15(1):19. doi: 10.1186/s12981-018-0210-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vetrano DL, Palmer K, Marengoni A, et al. ; Joint Action ADVANTAGE WP4 Group . Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(5):659–666. doi: 10.1093/gerona/gly110 [DOI] [PubMed] [Google Scholar]
- 23. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 2020;296(2):E41–E45. doi: 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 25. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–95. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lombardy Section Italian Society Infections and Tropical Diseases. Vademecum for the treatment of people with COVID-19. Ed. 2,0, 13 March 2020. Infex Med. 2020;28(2):143–152. [PubMed] [Google Scholar]
- 27. R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br Med J 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 31. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 32. Ritt M, Schwarz C, Kronawitter V, et al. Analysis of Rockwood et al’s Clinical Frailty Scale and Fried et al.’s Frailty Phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging. 2015;19(10):1043–1048. doi: 10.1007/s12603-015-0667-9 [DOI] [PubMed] [Google Scholar]
- 33. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943–949. doi: 10.1093/qjmed/hcv066 [DOI] [PubMed] [Google Scholar]
- 34. Maltese G, Corsonello A, Di Rosa M, et al. Frailty and COVID-19: a systematic scoping review. J Clin Med. 2020;9(7):E2106. doi: 10.3390/jcm9072106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hewitt J, Carter B, Vilches-Moraga A, et al. ; COPE Study Collaborators . The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 37. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 39. Hazeldine J, Harris P, Chapple IL, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13:690–698. doi: 10.1111/acel.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Renu K, Prasanna PL, Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage—a review. Life Sci. 2020;255:117839. doi: 10.1016/j.lfs.2020.117839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laviano A, Koverech A, Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition. 2020;74:110834. doi: 10.1016/j.nut.2020.110834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zucchelli A, Vetrano DL, Grande G, et al. Comparing the prognostic value of geriatric health indicators: a population-based study. BMC Med. 2019;17(1):185. doi: 10.1186/s12916-019-1418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelley GA, Kelley KS. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp Gerontol. 2017;96:100–103. doi: 10.1016/j.exger.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aronson L. Age, complexity, and crisis—a prescription for progress in pandemic. N Engl J Med. 2020;383(1):4–6. doi: 10.1056/NEJMp2006115 [DOI] [PubMed] [Google Scholar]
- 47. Cesari M, Proietti M. COVID-19 in Italy: ageism and decision making in a pandemic. J Am Med Dir Assoc. 2020;21(5):576–577. doi: 10.1016/j.jamda.2020.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva. http://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/Percorso%20COVID-19%20-%20Sezione%201%20-%20Procedura%20Area%20Critica%20-%20Rev%202.0.pdf. Accessed May 5, 2020.