In recent times, systemic coagulation, fibrinolysis, and cardio-pulmonary injury has been recognized in patients with COVID-19, the clinical disease state caused by infection of the novel coronavirus, SARS-CoV-2. While originally believed to be a primary lower respiratory infection, as more cases are identified, treated, and examined, hematologic complications are being identified as a significant driver of morbidity and mortality associated with the disease. Elevated D-dimer levels (>1 µg/ml) on hospital admission have been identified as being associated with increased mortality [1], and levels greater than 2 µg/ml predict fatal outcomes in patients [2]. Alongside these results, there is also a greater risk of thrombotic events in COVID-19 patients, with risk increasing to as high as 31% [3], and pulmonary embolism risk increases proportionately alongside this [4]. Whilst understanding of the pathophysiology is incomplete, there is clearly a component of coagulative disorder in these patients. D-dimer could prove a valuable tool to identify patients who are likely to have poorer outcomes and allow for prophylactic treatment and monitoring of secondary complications (Fig. 1).
Figure 1.
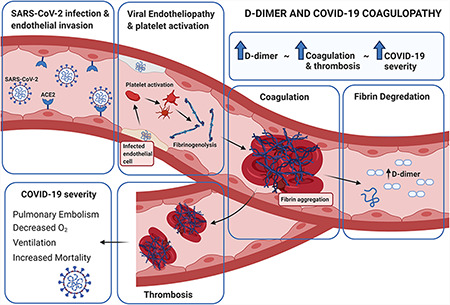
D-dimer, COVID-19 coagulopathy, and disease outcomes.
The D-dimer test is a biomarker-based evaluation which identifies the amount of ongoing coagulation at a given point of time. The D-dimer molecule is a product of the degradation of the fibrin protein. It arises after the cross-linking of two D-fragments of the fibrin protein after lysis by the thrombin, plasmin, and factor XIIIa enzymes [5]. Its clinical elevation is strongly indicative of ongoing coagulation and, as such, is used in the diagnosis of pulmonary embolism. It is, however, a non-specific marker of coagulation, and as such, a positive test must be evaluated by imaging and viewed alongside other clinical signs of acute pulmonary vascular injury [6].
In this study, we assessed the role of D-dimer in relation to clinical course of patients with COVID-19. It was hypothesized that D-dimer levels will predict poorer outcomes of these patients. Participants were recruited on admittance to hospital for moderate-to-severe COVID-19. COVID-19 patients (n = 29) hospitalized in the Clinic for Infective Disease in Skopje or in General Hospital Kumanovo with moderate and severe clinical symptoms were assessed. All tested positive for SARS-CoV-2 RNA via PCR at the Institute for Public Health. D-dimer levels were analyzed by the Dade Behring method at the hemostasis department. The normal value was defined as 500 ng/ml fibrinogen-equivalent units. Independent t-test was used to identify whether the participants who would go on to require mechanical ventilation support had higher baseline D-dimer levels. Alpha level of significance was set at 0.05. This retrospective cohort study was conducted according to the Helsinki declaration.
Demographics of the participants at baseline are described in Table 1.
Table 1.
Demographic information of participants
| Variables | Ventilated | Unventilated |
|---|---|---|
| No. of participants | 11 | 18 |
| Age (mean ± SD) | 53.8 ± 9.3 | 62.5 ± 8.4 |
| Sex | ||
| Male | 7 | 11 |
| Female | 4 | 7 |
| Number of comorbidities (mean) | 1.8 per participant | 2.7 per participant |
| Hypertension | 9 | 12 |
| Diabetes | 8 | 10 |
| Obesity | 9 | 14 |
Significant differences in D-dimer levels at admission were noted in COVID-19 patients who would require artificial ventilation (1250 ± 210 ng/ml) compared to those who did not (650 ± 175 ng/ml), respectively (P < 0.05; Fig. 2). This is particularly noteworthy, given that the unventilated group tended to be older, had a greater number of important comorbidities, and had a greater percentage of males—all risk factors for poorer COVID-19 outcomes.
Figure 2.
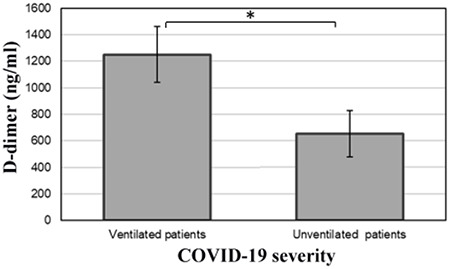
Differences in D-dimer level in COVID-19 patients with different outcomes *P < 0.05.
The results of this study suggest that D-dimer may be able to predict which COVID-19 patients have poorer outcomes. This could have significant impact on the ability for clinicians to identify which patients are likely to have worse outcomes and act prophylactically, improving the disease burden. D-dimer has been shown to be an indicator for cardiac injury in COVID-19 patients in a setting of prothrombotic state [7]. Some recent studies also suggested that there was a dynamic relation of D-dimer level with prognosis of COVID-19 patients and a need for anticoagulation [8, 9]. However, there has been disagreement in relevant cut-off points for Chinese and Italian patients with COVID-19 [10]. This prognostic ability may be in part due to the previously identified increase in thrombotic events in COVID-19 patients [3]. While useful, major limitations of the present study are its small sample size and its assessment of only one major disease outcome, with these preliminary results informing the appropriate design and powering of a larger trial. Requirement for ventilation is reported to be a very strong predictor of COVID-19 outcomes, with mortality rates greater than 70% in this cohort [11]. Our preliminary results support the hypothesis that D-dimer may have potential as a prognostic tool in patients with severe clinical manifestations of COVID-19. Larger studies should investigate whether D-dimer is able to prognose other critical endpoints such as mortality, stroke, and length of hospitalization and validate clinically meaningful cut points for the biomarker.
Acknowledgements
V.A. would like to thank the Immunology and Translational Research Group and the Institute for Health and Sport, Victoria University, for their support. V.A. would like to thank the Thelma and Paul Constantinou Foundation, and The Pappas Family, whose generous philanthropic support made possible the preparation of this paper. VA was also supported in part by the place based planetary health grant PH098 from VU research, Victoria University, Australia. J.F. was supported by the University of Melbourne Postgraduate Scholarship, as part of the Australian Government research training program. M.B. would like to thank the members of the National Registry on venous thromboembolism.
Contributor Information
Marijan Bosevski, St. Cyril and Methodius Faculty of Medicine, University Cardiology Clinic, Skopje, North Macedonia.
Gorjan Krstevski, St. Cyril and Methodius Faculty of Medicine, University Cardiology Clinic, Skopje, North Macedonia.
Golubinka Bosevska, Institute for Public Health, Skopje, North Macedonia.
Kosta Kapsarov, University Clinic for Infective Diseases, Skopje, North Macedonia.
Emilija Dodic, General Hospital, Kumanovo, North Macedonia.
Jack Feehan, University of Melbourne, Department of Medicine, Western Health, Melbourne, Australia; Institute for Health and Sport, Victoria University, Melbourne, Australia.
Lily Stojanoska, Institute for Health and Sport, Victoria University, Melbourne, Australia; Department of Nutrition and Health, College of Medicine and Health Sciences, United Arab Emirates University Al Ain, UAE.
Vasso Apostolopoulos, Institute for Health and Sport, Victoria University, Melbourne, Australia; Department of Nutrition and Health, College of Medicine and Health Sciences, United Arab Emirates University Al Ain, UAE.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020, 18: 1324–1329. doi: 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers DA, Kant KM, Kaptein FH et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020, 191: 145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation 2020, 142: 184–186. doi: 10.1161/CIRCULATIONAHA.120.047430 [DOI] [PubMed] [Google Scholar]
- 5. Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood 2009, 113: 2878–2887. doi: 10.1182/blood-2008-06-165845 [DOI] [PubMed] [Google Scholar]
- 6. Bosevski M, Krstevski G, Gjorgievski A, Mitevska I, Kostovska ES. Predictors for prognosis in patients with nonfatal pulmonary embolism: a registry-based cohort study. Clin App Thromb/Hemost 2018, 24: S84S-S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA. A current review of COVID-19 for the cardiovascular specialist. Am Heart J 2020, 226: 29–44. doi: 10.1016/j.ahj.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Zhao K, Wei H, Chen W, Wang W, Jia L, Liu Q et al. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol 2020. doi: 10.1111/bjh.16811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haemato 2020, 7: e362–e363. doi: 10.1016/S2352-3026(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gris J-C, Quéré I, Pérez-Martin A, Lefrant J-Y, Sotto A. Uncertainties on the prognostic value of D-dimers in COVID-19 patients. J Thromb Haemost 2020. doi: 10.1111/jth.14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ñamendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med 2020, 8: e18. doi: 10.1016/S2213-2600(20)30110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


