Commentary on ‘Endothelial cell infection and endotheliitis in COVID-19’, by Varga et al., Lancet 2020;395:1417–1418.
The COVID-19 pandemic undoubtedly influenced the focus of many scientific fields, including cardiovascular research, and is still a global challenge for healthcare systems. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) predominantly affects the respiratory tract, and in severe cases, also other organs, including the liver, kidney, heart, and intestine.1 The leading cause of mortality in patients with COVID-19 is a hypoxic respiratory failure caused by acute respiratory distress syndrome (ARDS). It is well established that SARS-CoV-2 hijacks angiotensin-converting enzyme 2 (ACE2) receptors to infect host cells. ACE2 receptors are widely expressed in various tissues, suggesting the broad clinical consequences of SARS-CoV-2 infection that make COVID-19 a multiorgan disease.1 Endothelial cells have recently been implicated as the primary cell type involved in the initiation and propagation of ARDS caused by SARS-CoV-2, resulting in severe endothelial injury and widespread thrombosis.2 In fact, the first reports from Wuhan, China reported an increase in D-dimers (reporting thrombosis and/or disseminated intravascular coagulation) as a very early biomarker predicting an adverse outcome in COVID-19 patients, even preceding elevations of troponin or interleukin-6.3 Accordingly, patients with pre-existing conditions such as hypertension, obesity, and diabetes, which are all associated with endothelial dysfunction, are more susceptible to an adverse course of COVID-19. While the exact mechanisms are incompletely resolved, SARS-CoV-2 impinging on endothelial cell function has evolved as a key unifying candidate.2
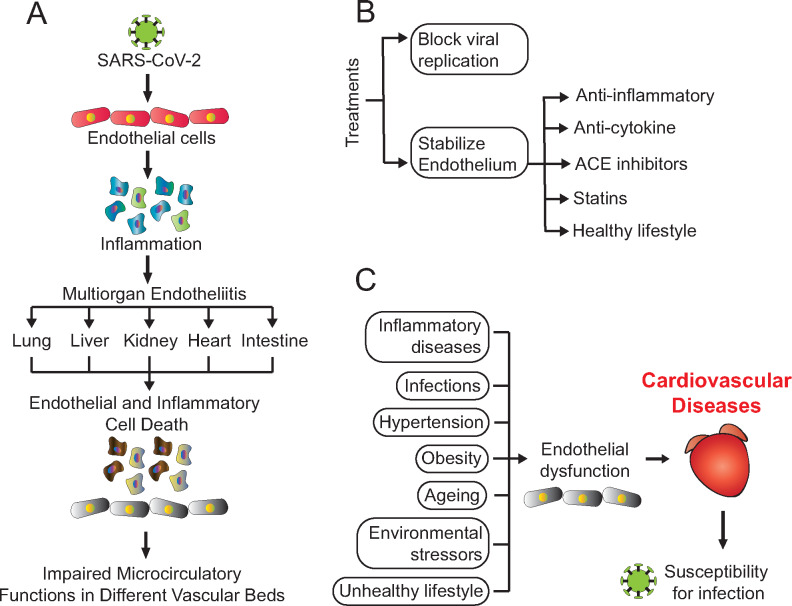
As a proof of concept, Varga et al.4 recently presented three cases that have in common a significant involvement of endothelial cells across vascular beds of different organs in patients with cardiovascular comorbidities infected with SARS-CoV-2. Patients included (two males and one female, 58–71 years old) had different pre-existing conditions, including arterial hypertension, coronary artery disease, or diabetes. Upon infection with SARS-CoV-2, all patients required hospitalization and developed respiratory failure, bilateral pulmonary infiltrates, and mesenteric ischaemia. Two patients died from complications caused by the infection. The first patient (male 71 years old) died from multisystem organ failure 8 days after admission. The second patient (female 58 years old) died due to acute right coronary artery occlusion. Post-mortem analysis of both patients revealed lymphatic endotheliitis with the recruitment of inflammatory cells and an unusually high amount of apoptotic bodies in many organs. Additionally, an accumulation of inflammatory cells in the endothelium led to congestion of small lung vessels. The third patient (male, 69 years old) survived the infection but developed atrial fibrillation, left ventricular dilation, and systolic dysfunction. Histological analysis of the small intestine resection from this patient revealed prominent endotheliitis of the submucosal vessels and the presence of apoptotic bodies. The authors observed that the presence of the virus within endothelial cells, together with altered inflammatory response, caused endotheliitis and cell death in several organs, which led to impaired microcirculatory function (Figure 1A). Therefore, the main take home from this study is that endothelial cells are directly infected with SARS-CoV-2, which might substantially contribute to the progression of the disease.
Figure 1.
(A) Pathophysiology of infection with SARS-CoV-2 in different organs. (B) Therapeutic avenues for COVID-19. (C) Main genetic and environmental risk factors leading to endothelial dysfunction and cardiovascular diseases.
Since up to date, there is no cure for COVID-19, scientists all over the world are working on the development of effective treatments. Therapies that are under investigation include existing drugs for autoimmune diseases, drugs to treat malaria, antiviral drugs developed for other viruses, and antibodies from individuals who recovered from COVID-19.2 Varga et al.4 provide a rationale that on top of suppressing viral replication, a key focus to prevent severe COVID-19 illness is to stabilize the endothelium using anti-inflammatory and/or anti-cytokine drugs, ACE inhibitors, and/or statins (Figure 1B). Apart from medical options, a healthy lifestyle likely plays a key role in protecting from a severe course of the disease.5
The endothelium is one of the largest, metabolically active organs that interact with nearly every tissue in the human body.2 Therefore, the endothelium is implicated in various systemic illnesses, including cardiovascular diseases. A healthy endothelium controls vascular homeostasis regulates vascular tone, cellular adhesion, thromboresistance, smooth muscle cell proliferation, and vessel wall inflammation.2 Endothelial dysfunction is a consequence of several genetic and environmental risk factors that predisposes to cardiovascular diseases (Figure 1C). Furthermore, the cytokine storm observed in patients with severe COVID-19 contributes to further destruction of the endothelium, leading to ARDS, multiorgan failure, and death.2,6 Therefore, the discovery of new clinical targets to treat endothelial dysfunction is currently an urgent unmet clinical need.7
What can each individual do to avoid a severe course of COVID-19? Considering that, as suggested by the discussed study,4 the endothelium is a key target for SARS-CoV-2, keeping this organ healthy may effectively ameliorate the consequences of infection. In fact, obesity, a well-characterized risk factor for diabetes, hypertension, and cardiovascular disease, is a main risk factor for severe SARS-CoV-2 infection, especially in young patients in whom other comorbidities may still be absent.8 Along similar lines, physical inactivity and smoking also increase the risk of severe COVID-19, and altogether, unhealthy behaviours in combination accounted for more than 50% of the population-attributable fraction of severe COVID-19 in the UK.9 Hence, these data together with the recent report by Varga et al.4 make a strong case that COVID-19 is a systemic cardiovascular disease that threatens especially those with unhealthy lifestyle and/or the (ensuing) non-communicable (and often age-related) diseases. An important dilemma, however, is that global measures to restrict the spread of the pandemic may reduce just this physical activity and thereby predispose to a sedentary lifestyle and unhealthy nutrition especially in children,10 but also adults. Therefore, governmental decision-makers should include clear exceptions for physical activity in local or nationwide lockdowns,5 which—considering the recent rise of a second infection wave—may be unavoidable in the months to come.
Conflict of interest: C.M. received speaker honoraria from AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Novartis, Servier and served as an advisor to Amgen, Boehringer Ingelheim, NovoNordisk, and Servier.
Funding
C.M. is supported by the German Research Foundation (DFG; Ma 2528/7-1; SFB 894, TRR 219), the Federal Ministry of Education and Research (BMBF; 01EO1504), and the Barth Syndrome Foundation.
Authors

Biography: Dr Monika Gladka received her PhD in Molecular Cardiology from the Maastricht University in the Netherlands, where she learned the basics of molecular cardiology and developed an interest in transcriptional and post-transcriptional gene regulation during heart failure. She is currently a senior researcher at the Hubrecht Institute in Utrecht, which is a part of the Royal Netherlands Academy of Arts and Sciences (KNAW). Her current research focuses on understanding the molecular mechanisms that regulate cardiac repair, intending to identify new players to develop novel, improved gene therapies. She uses several state-of-the-art techniques such as single-cell sequencing, enabling an in-depth mechanistic understanding of the biological processes in injured cardiomyocytes. In 2016 and 2020, she received two prestigious Dr E. Dekker personal grants from the Dutch Heart Foundation for heart repair research. She is also a board member of Young@Heart from the Netherlands Heart Institute and nucleus member of the Scientists of Tomorrow from the European Society of Cardiology.

Biography: Prof. Christoph Maack received his MD at the University of Cologne (Germany) in 2000. Between 2000 and 2017, he worked at the Department of Cardiology at the University of the Saarland in Homburg, Germany, and from 2002 to 2005 as a post-doctoral researcher in the lab of Brian O’Rourke at the Department of Cardiology at Johns Hopkins University in Baltimore, MD, USA. In 2017, he became Head of Translational Research at the Comprehensive Heart Failure Center (CHFC) at the University Clinic in Würzburg, Germany, where he also serves as the Spokesperson of the CHFC. His work focuses on cellular defects in chronic heart failure, with a special emphasis on the mechanisms of contractile, mitochondrial, and metabolic dysfunction in heart failure. For his work, Maack received support from the German Research Foundation (Emmy Noether Program, Heisenberg Professorship) and several awards, such as the Albert-Fraenkel (2014) and the Arthur-Weber Awards (2015) of the German Cardiac Society and the Keith Reimer Distinguished Lecture of the International Society of Heart Research (ISHR; 2019), respectively. He served on the Board of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) from 2010 to 2016, where he was Coordinator of the Translational Research Committee (2011–2014) and Chair of the Basic Science section (2014–2016). In 2018, he entered the Council of the ISHR, European Section (ES) and since 2020 is president-elect of the ISHR-ES.
References
- 1. Robba C, Battaglini D, Pelosi P, Rocco PRM. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med 2020;14:865–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lippi G, Henry BM, Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19). Eur J Prev Cardiolog 2020;27:906–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020;20:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monteiro JP, Bennett M, Rodor J, Caudrillier A, Ulitsky I, Baker AH. Endothelial function and dysfunction in the cardiovascular system: the long non-coding road. Cardiovasc Res 2019;115:1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 Infection. Circulation 2020;142:4–6. [DOI] [PubMed] [Google Scholar]
- 9. Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun 2020;87:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunton GF, Do B, Wang SD. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health 2020;20:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]