Abstract
Sensorineural hearing loss (SNHL) causes an overall deficit in binaural hearing, including the abilities to localize sound sources, discriminate interaural time and level differences (ITDs and ILDs, respectively), and utilize binaural cues to aid signal detection and comprehension in noisy environments. Few studies have examined the effect of SNHL on binaural coding in the central auditory system, and those that have focused on age-related hearing loss. We induced hearing loss in male and female Dutch-belted rabbits via noise overexposure and compared unanesthetized single-unit responses of their inferior colliculi [hearing loss (HL) neurons] with those of unexposed rabbits. Sound-level thresholds of HL neurons to diotic noise were elevated by 75 dB, on average. Sensitivity of firing rates of HL neurons to the azimuth of a broadband noise stimulus was reduced, on average, but was confounded by differences in sound level with respect to detection threshold between groups. We independently manipulated ITD and ILD in virtual acoustic space and found directional sensitivity in binaurally sensitive HL neurons was entirely due to ILD sensitivity and no different than that for unexposed rabbits. However, ITD sensitivity was completely absent in binaurally sensitive HL neurons for noise stimuli both in virtual acoustic space and with ITDs extending to ±3 ms. HL neurons also had weaker spike-timing precision and slightly increased spontaneous rates. Overall, ILD sensitivity was uncompromised, whereas ITD sensitivity was completely lost, implying a specific inability to use information in the timing or correlation of acoustic noise waveforms between the two ears following severe SNHL.
NEW & NOTEWORTHY Sensorineural hearing loss compromises perceptual abilities that arise from hearing with two ears, yet its effects on binaural aspects of neural responses are largely unknown. We found that, following severe hearing loss because of acoustic trauma, auditory midbrain neurons specifically lost the ability to encode time differences between the arrival of a broadband noise stimulus to the two ears, whereas the encoding of sound level differences between the two ears remained uncompromised.
Keywords: acoustic trauma, inferior colliculus, interaural level difference, interaural time difference, sensorineural hearing loss
INTRODUCTION
Sensorineural hearing loss (SNHL) involves damage to the cochlea, auditory nerve, or central auditory areas and is the most common form of permanent hearing loss. It is often acquired by cumulative overexposure to loud sounds; one study estimated that nearly one in four adults in the United States had SNHL due to noise overexposure (Carroll et al. 2017). Its most obvious effect is reduced sensitivity to sound, e.g., individuals must use hearing aids to boost sound to a detectable level. However, even with clearly audible sound, individuals with SNHL are often impaired in various aspects of binaural hearing. For example, sound localization accuracy and precision are degraded in quiet and most dramatically in the presence of background noise (Lorenzi et al. 1999). The association between SNHL and the ability to discriminate either of the two main binaural cues, interaural time and level differences (ITDs and ILDs, respectively), is mixed. Some have reported small increases in ILD discrimination thresholds in individuals with SNHL (Gabriel et al. 1992; Smith-Olinde et al. 1998), while others have not (Häusler et al. 1983; Spencer et al. 2016). All have reported increases in ITD discrimination thresholds with SNHL for at least some stimuli, but effects varied both in magnitude and across stimuli, potentially because of individual differences in hearing loss (Best and Swaminathan 2019; Gabriel et al. 1992; Häusler et al. 1983; Lacher-Fougère and Demany 2005; Smith-Olinde et al. 1998; Smoski and Trahiotis 1986; Spencer et al. 2016). Last, individuals with SNHL have an impaired ability to filter out distracting sound sources based on spatial location while attempting to comprehend a target source (Dai et al. 2018; Marrone et al. 2008).
Despite the above evidence of a perceptual impairment of binaural hearing with SNHL, there have been surprisingly very few studies of the effect of SNHL on binaural coding in the central auditory system. Those studies focused on age-related hearing loss and found some deficits in binaural or spatial coding (Costa et al. 2016; Laumen et al. 2016; McFadden and Willott 1994a, 1994b). However, data in the Laumen et al. (2016) study were evoked potentials at the skin surface, and the Costa et al. (2016) and McFadden and Willott (1994a, 1994b) studies presented stimuli in the free field, preventing individual manipulation of ITD and ILD cues. Therefore, there is a gap in knowledge as to how SNHL, and specifically acoustic trauma, affects the encoding of ITD and ILD by individual neurons. The aim of the present study was to fill this gap, and we accordingly formulated two main research questions. The first was whether or not information about sound source direction in the horizontal plane (azimuth) encoded in neural firing rates decreases following noise-induced hearing loss, similar to the deficit in sound localization ability observed in individuals with SNHL (Lorenzi et al. 1999). The second was whether or not neural sensitivity to ITD or ILD alone was affected by noise-induced hearing loss.
To address the above research questions, we measured single-unit responses from the inferior colliculus (IC) of unanesthetized rabbits before and after a noise exposure that caused permanent hearing loss. The IC was an ideal target because it is the brain area where projections of the primary binaural nuclei in the auditory brain stem converge. Furthermore, rabbits were an appropriate species because they hear over a range that spans ITD- and ILD-dominated frequencies (Heffner and Masterton 1980). We used an overexposure protocol that we recently showed to produce widespread damage of outer hair cells and variable damage of inner hair cells across the extent of the cochlea (Haragopal et al. 2020). Using a broadband noise stimulus, we then measured azimuth, ITD, and ILD tuning curves of IC neurons in rabbits with and without hearing loss and compared between the two groups.
MATERIALS AND METHODS
Data were collected from three female and two male Dutch-belted rabbits (Envigo) at ages spanning 5–32 mo. All experimental procedures were approved by the Ohio University Institutional Animal Care and Use Committee.
Experimental procedures.
Detailed surgical and experimental procedures may be found in Dorkoski et al. (2020). Briefly, rabbits underwent an original surgery to implant a head bar for head fixation during awake neural recording sessions, a subsequent surgery to both drill a hole into the skull dorsal to the IC and implant a tetrode microdrive (Neuralynx 9 Drive), and additional surgeries as needed to replace the microdrive and aim for different regions within the IC (including both sides of the brain). Anesthesia for the first surgery was induced with 6 mg/kg sc xylazine, 44 mg/kg im ketamine, and 1 mg/kg sc acepromazine and maintained with 15 mg/kg im ketamine as needed and for subsequent surgeries was induced with 6 mg/kg sc xylazine and 35 mg/kg im ketamine and maintained via mask with 1.5–2% isoflurane mixed with O2 (1 L/min). Atropine (0.25 mg/kg sc) was administered to reduce mucosal secretions and prevent tracheal blockage. Postoperatively, rabbits were administered 0.3 mg/kg sc dexamethasone to reduce potential brain swelling and 0.025 mg/kg sc buprenorphine every 12 h for at least 36 h for analgesia.
Each microdrive was loaded with four independently drivable tetrodes (0.1–0.5 MΩ impedance). Data were collected during daily 2.5-h awake recording sessions within a double-walled sound-attenuated chamber (ETS-Lindgren Acoustic Systems). Rabbits were loosely secured in a custom-made “chair,” head-fixed via head bar, fitted with ear molds for sound presentation (Reprosil; Dentsply), and monitored by video throughout the session. Before the first session, rabbits were gradually acclimated to the set-up over 1.5 wk.
Stimulus presentation and neural data acquisition.
Detailed methods for presentation of acoustic stimuli and acquisition of neural data may be found in Dorkoski et al. (2020). Acoustic stimuli were created digitally at 50-kHz sampling rate separately for each ear, converted to analog signals, and amplified to drive insert earphones (Etymotic ER-2) whose sound tubes were embedded in ear molds. The ear molds sealed in the ears to create a closed field. The in-ear acoustic assembly was calibrated (Etymotic ER-7C) for level and phase at the beginning of every recording session using a chirp stimulus.
Electrical potentials measured with the tetrode microdrive were digitized at 25-kHz sampling rate, filtered between 0.4 Hz and 11.25 kHz, then stored on disk. Spike sorting for reported data was performed offline (Plexon Offline Sorter). Sorting was based on identifying single-unit clusters of data points within a 2D or 3D scatterplot, where the axes were action potential peak amplitude simultaneously measured from two or three electrodes within a tetrode, respectively. Details of spike sorting may be found in Dorkoski et al. (2020). Spike data were considered single unit if they 1) formed a complete cluster separate from others and 2) had less than 2% submillisecond interspike intervals. Only single-unit data are reported.
Auditory brain stem response measurement.
Auditory brain stem responses (ABRs) were measured both to quantify hearing loss in rabbits overexposed to noise and to ensure that control unexposed rabbits had normal hearing. Detailed methods for ABR measurement may be found in Haragopal et al. (2020). Briefly, ABRs were measured under isoflurane anesthesia with platinum subdermal needle electrodes (Grass Instruments) positioned at the vertex, mastoid (of the ear presented with sound), and back (common). ABRs were measured in response to monaural unipolar clicks (100 µs) and tone pips (5-ms duration, 0.5-ms sin2 on/off ramps) at frequencies of 0.5–16 kHz in octave steps at 30 repetitions/s. Stimuli were presented at multiple sound levels in 5-dB steps. The ABR at each sound level was averaged over 512 stimulus repetitions. For clicks and tone pips with frequencies at and above 2 kHz, stimulus polarity was alternated between successive repetitions to prevent contamination of the signal by the cochlear microphonic at high sound levels. Stimulus polarity was not alternated for 0.5- or 1-kHz tones so that the phase-locked auditory nerve signal in wave I was not eliminated. ABR threshold was defined as the lowest sound level at which the average ABR first appeared, determined by visual inspection.
ABRs were measured before neural recordings. For rabbits that underwent noise overexposure, ABRs were remeasured two weeks postexposure, by which time thresholds likely settled to permanent levels (Borg et al. 1995).
Noise overexposure.
Detailed methods for noise overexposure may be found in Haragopal et al. (2020). Rabbits were exposed to octave-band noise geometrically centered at 750 Hz (530–1,061 Hz) for 75 or 90 min under isoflurane anesthesia. Noise waveforms were presented over two loudspeakers pointed at the left and right ears. Waveforms from left and right speakers were independent, producing binaurally uncorrelated sound. This ensured that overexposure was not restricted to a fixed ITD or ILD since both cues fluctuate moment-to-moment for binaurally uncorrelated sound (e.g., Fig. 1 of Day et al. 2012). The sound levels of noise from the two speakers were equal and combined to 133 dB sound pressure level (SPL; re: 20 μPa) when presented simultaneously, as measured at the end of a probe tube microphone (Etymotic ER-7C) dangled at the approximate location of the center of the rabbit’s head in the absence of the rabbit.
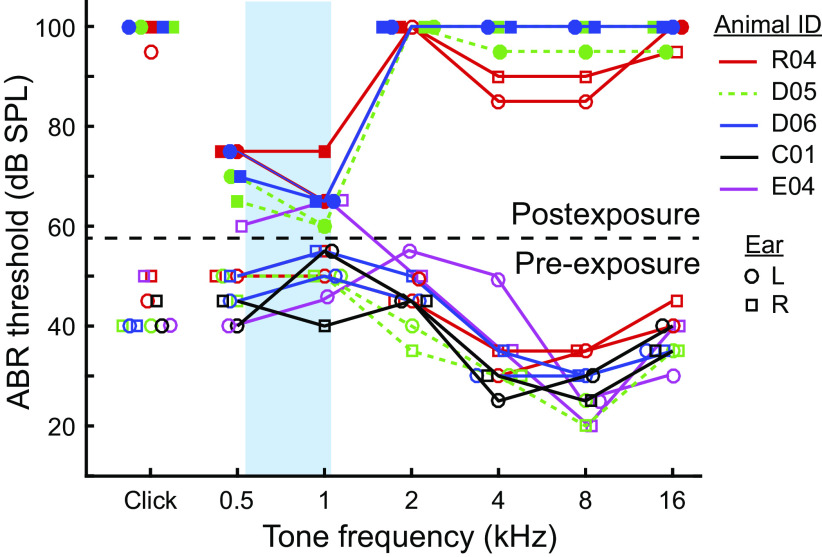
Fig. 1.
Hearing loss following noise overexposure. Click-evoked and tone-evoked auditory brain stem response (ABR) thresholds for all rabbits in the study. Data below and above the broken line were collected before and 2 wk after noise overexposure, respectively. Lines connect data from the same rabbit. Different colored lines indicate different rabbits, indicated to the right of the plot. Responses of left (L) and right (R) ears are indicated by circles and squares, respectively. Blue rectangle indicates frequency passband of the noise overexposure.
Stimuli.
For each neuron the following were measured: 1) the frequency-and-level response area (FRA); 2) the sound level tuning curve in response to diotic broadband (0.1–16 kHz) noise; 3) azimuth tuning curves in response to broadband noise under four different binaural cue conditions; 4) ITD tuning curves in response to broadband and lowpass (0.1–1.5 kHz) noises; and 5) responses to monaural broadband noise in the left and right ears. For each measurement, the order of stimulus parameters was varied randomly across 15 repetitions, except the FRA and monaural noise (2 and 50 repetitions, respectively). Stimuli were either tone pips (100 ms on, 200 ms off, 5-ms on/off sin2 ramps) or frozen noise bursts (300 ms on, 300 ms off, 5-ms on/off sin2 ramps).
The number of stimulus repetitions (usually 15) was chosen to exceed a criterion of accuracy of firing rate estimation. Under the assumption that the distribution of spike counts in response to a repeated stimulus approximately follows a Poisson distribution (Dorkoski et al. 2020), at least 13 stimulus repetitions are necessary to estimate a mean firing rate of 100 spikes (sp)/s over a 300-ms window with a 95% confidence interval of ±10 sp/s. The same repetitions would estimate mean firing rates of 50 and 20 sp/s with 95% confidence intervals of ±7 and ±4 sp/s, respectively.
For FRA measurement, tone pips were presented to the contralateral ear at frequencies from 0.1 to 16 kHz in one-quarter octave steps and at sound levels from 5 to 70 dB SPL [unexposed “normal-hearing” rabbits (NH)] or 35 to 100 dB SPL [overexposed “hearing-loss” rabbits (HL)] in 5-dB steps. For sound level tuning curves, noise bursts were presented at 5 to 70 dB SPL or 35 to 100 dB SPL for NH and HL rabbits, respectively, in 5-dB steps. For ITD tuning curves, ITD was varied over −1,000 to +1,000 μs (ipsilateral to contralateral leading, respectively) or ±800 μs in 50-μs steps. An additional ITD tuning curve in response to broadband noise was measured over ±3 ms in 150-μs steps.
Acoustic waveforms presented over earphones were made directional by filtering with left and right directional transfer functions (DTFs) specific to each azimuth within the front horizontal plane (Day et al. 2012; Dorkoski et al. 2020). For all azimuth tuning curves, stimuli were presented at azimuths between −90° and +90° (ipsilateral and contralateral to the recording site, respectively) in 15° steps where 0° was directly in front of the rabbit. Noise bursts were alternatively filtered with 1) normal DTFs (congruent binaural cues; “ITD + ILD”), 2 and 3) two different DTFs augmented to manipulate ITD and ILD independently (incongruent binaural cues; “ITD only” and “ILD only”), and 4) normal DTFs with sound only in the contralateral ear (monaural; “contra only”). “ITD-only” DTFs had left and right magnitude spectra fixed to one, which fixed ILD to that at 0° while allowing ITD to vary with azimuth. “ILD-only” DTFs had left- and right-phase spectra set to zero, which fixed ITD to that at 0° while allowing ILD to vary with azimuth.
Azimuth and ITD tuning curves (except the ITD tuning curve over ±3-ms delays) were measured both at an absolute sound level of 70 dB SPL (same between individuals) and a “near-threshold” sound level (different between individuals). The absolute sound level was chosen as the highest level for which NH rabbits would listen to repeated stimuli without indication of agitation. For NH rabbits, the near-threshold level was chosen to be near or equal to the average of the click-evoked ABR thresholds between the two ears of each individual rabbit (in practice, 40–52.5 dB SPL). Click-evoked ABR thresholds in HL rabbits were usually above the limit of our acoustic system (see results); therefore, the same method could not be used to choose the near-threshold level. For HL rabbits, near-threshold level was chosen as either 92.5 or 97.5 dB SPL, which was the highest sound level at which our acoustic system could present noise stimuli without clipping waveforms, and was equal to median postexposure threshold in our previous study using the same overexposure (Haragopal et al. 2020). Monaural noise bursts and ITD tuning curves over ±3-ms delays were only presented at near-threshold level.
Data analysis.
Stimulus trials that contained artifacts resulting from infrequent rabbit movements were omitted from analysis. Stimulus-evoked and spontaneous spike counts were made in the stimulus window and last 100 ms of the 300-ms silent period of each trial, respectively.
The FRA (average firing rate as a function of both log frequency and level) was upsampled by a factor of two then smoothed with a 2D filter made of a Gaussian frequency window (SD = 1/16 oct) and triangular level window (Palmer et al. 2013). For NH rabbits, characteristic frequency (CF) was determined by visual observation as the frequency that elicited an increase or decrease of firing rate from spontaneous rate at the lowest sound level. For single units with complex FRAs, CF was determined as that of the multiunit FRA at the same tetrode site. For single units at ventral recording sites where the low-frequency FRA tail was visible but the high-frequency tip exceeded the range of tone frequencies presented, the CF was assigned a value of 20 kHz.
Multiunit FRAs were recorded at regularly spaced depths along each tetrode track and displayed a tonotopic progression of “V” shapes, with CF systematically decreasing in the ventral-to-dorsal direction (e.g., Fig. 2A, left), consistent with the central nucleus of the IC (ICC; Aitkin et al. 1972; Hind et al. 1963; Merzenich and Reid 1974). For NH rabbits, only single units recorded within this region are reported. For HL rabbits, all single units are reported because these rabbits lacked a clear tonotopic region (see results). Furthermore, CF was not determined for single units from HL rabbits.
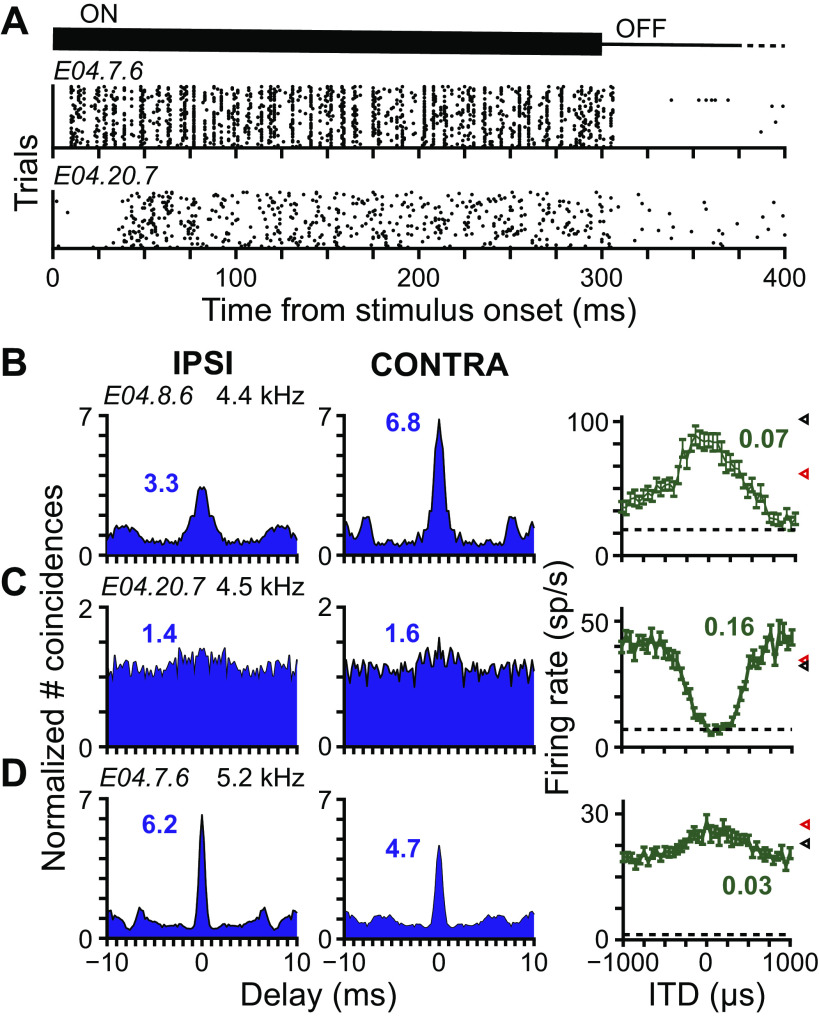
Fig. 2.
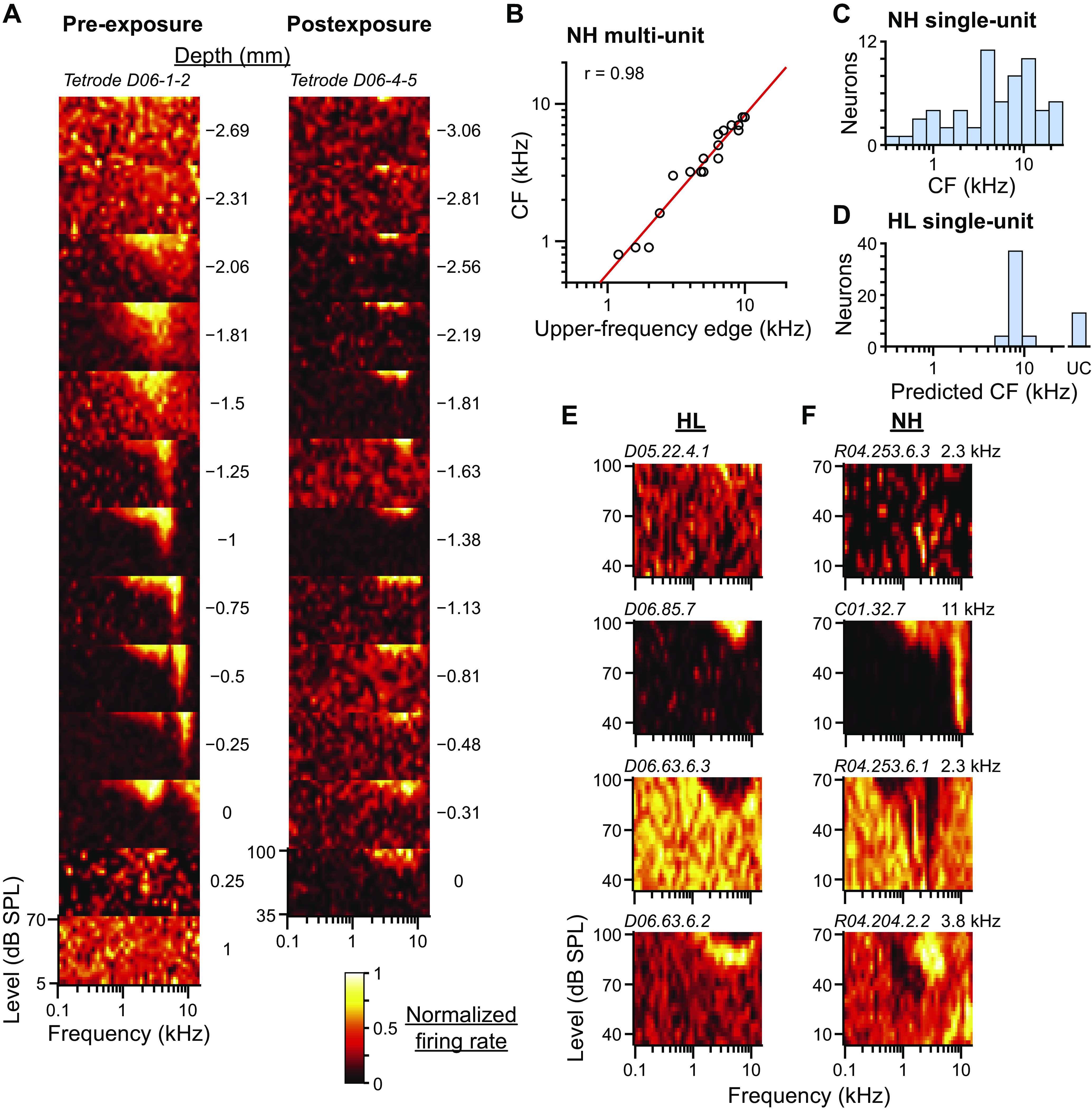
Frequency tuning following noise overexposure. A: multiunit frequency-and-level response areas (FRAs) recorded from two different tetrodes (tetrode IDs at top of each column) at 12–13 different depths (plots within each column). Depth relative to the ventral border of auditory-evoked activity is indicated to the right of each plot. Tetrodes were from the same rabbit before (left) and after (right) noise overexposure. In each FRA, the color associated with firing rate is normalized to highest (white) and lowest (black) rates. Before overexposure, tuning to frequency within the central nucleus of the inferior colliculus (ICC) clearly increases with depth. B: scatterplot of CF versus upper-frequency edge of multiunit FRAs measured at several depths along 5 different tetrodes in normal-hearing (NH) rabbits (N = 29 sites; several data points overlap). Red line is fit line with correlation coefficient indicated in top left corner. C: histogram of the CFs of neurons from NH rabbits (N = 60). D: histogram of the CFs of neurons from hearing loss (HL) rabbits predicted from the upper-frequency edge of the FRA measured from either the neuron or multiunit data at the same site (N = 58). Prediction made using the fit line in B. Neurons with no upper-frequency edges in their FRAs were unclassified (UC). E: example neurons from HL rabbits with FRAs with no tuning, or excitatory, inhibitory, or mixed excitatory and inhibitory regions (top to bottom). Neuron ID indicated in top left corner of each FRA. F: example neurons from NH rabbits demonstrating similar FRA subtypes to those in E. CF additionally indicated in top right corner of each FRA.
The threshold of the level tuning curve in response to diotic broadband noise was defined as the lowest sound level at which the firing rate deviated from spontaneous rate at a significance level of 0.01 (Wald test) at that level and at least 5 dB above.
For azimuth and ITD tuning curves, an estimate of the mutual information (MI; Cover and Thomas 2006) between spike count, n, and stimulus azimuth or ITD, θ, was computed: . The conditional spike count distribution, P(n|θ), was assumed to be a Poisson distribution (Dorkoski et al. 2020) with mean equal to the mean spike count at azimuth or ITD θ. The marginal distribution, P(θ), was a uniform distribution of the M stimulus azimuths or ITDs used to measure the tuning curve, The other marginal distribution, P(n), was computed as . A bootstrap procedure was used to correct for bias in the MI estimate (Chase and Young 2005). Spike count data were resampled with replacement, then MI was computed on the resampled data (MIboot). The bias was estimated as , where was the average over 500 bootstrap-resampled data sets. Debiased MI was . Finally, normalized MI (MInorm) was computed by dividing MI by the entropy of the stimulus azimuth or ITD, log2 M, which is the largest value MI may take.
To quantify spike-timing precision, a normalized shuffled autocorrelogram (SAC) was computed for each neuron from its responses to 50 presentations of the same monaural broadband noise waveform (Joris 2003; Louage et al. 2004). The SAC was a histogram of all-order interspike intervals computed between spikes from all possible pairs of nonidentical trials. Bin size (Δt) of the SAC was set at 200 µs, which was small enough to resolve the temporal structure of the SAC. SACs were normalized by dividing by , where N was the number of stimulus presentations, r was the average firing rate, and D was the stimulus duration. A SAC value at an interspike delay of zero (termed the “correlation index”; Joris et al. 2006) of one indicates random spike timing across trials while that above or below one indicates correlated or anticorrelated spike timing, respectively. Accuracy of the estimation of the SAC was limited by the total number of spikes. To set a criterion of accuracy, we implemented a statistical test similar to that in Joris et al. (2006). For each data set, which consisted of spike trains for each of 50 trials, a resampled data set was created in which 1) the first spike latency of each trial was drawn randomly from a uniform distribution between stimulus onset and the original latency, and 2) the remaining spike times within the trial were created by a random permutation of the interspike intervals within the same trial. The resampled data set therefore had the same number of spikes and same set of first-order interspike intervals as the original data set, yet spike times were scrambled across trials. A correlation index was then computed on the resampled data. The resampling procedure was repeated 2,000 times to create a distribution of correlation indexes based on scrambled spike times. As our criterion for estimation accuracy, we only report correlation indexes for which the upper bound of the 95% confidence interval of correlation indexes computed on scrambled spike times was less than two, meaning data were sufficient to at least detect a correlation index of two or greater at a significance level of 0.05. In practice, this criterion was usually met for firing rates of 10 sp/s or greater.
Experimental design.
Data from NH rabbits came from a total of 76 ICC neurons, including 59 neurons in three female rabbits (rabbit ID–number of neurons: R04–29, C01–28, D05–2) and 17 ICC neurons in two male rabbits (D06–2, E04–15). Of these, 10 neurons (13%) came from the left side of the brain. Data from HL rabbits came from a total of 73 ICC neurons, including 22 neurons in two female rabbits (R04–8, D05–14) and 51 neurons in one male rabbit (D06). Of these, eight neurons (11%) came from the left side of the brain. Data were recorded from all three of the HL rabbits before noise overexposure; 43% of NH data (33/76 neurons) were from rabbits that subsequently underwent noise overexposure. Single-unit isolation was not always maintained for every stimulus listed above. Furthermore, some stimuli were implemented later on during the period of data collection. Therefore, sample sizes are not the same for every stimulus. No significant differences of the normalized MI of azimuth tuning curves were found based on individual rabbit (P > 0.05, Kruskal-Wallis test) or sex or side of the brain (P > 0.05, Wilcoxon rank-sum test) in either group; therefore, data from azimuth tuning curves were pooled across these variables. No significant differences of the normalized MI of ITD tuning curves were found based on sex or side of the brain in either group, or based on individual rabbit within the HL group; however, there was an effect of individual rabbit within the NH group (P = 0.039 and 0.0013 for absolute and near-threshold sound levels, respectively; Kruskal-Wallis test). Differences between ITD data from HL rabbits and individual NH rabbits are addressed in results.
Each tetrode microdrive was constructed to aim the tetrodes emerging from the drive tip to pass through the skull at a certain location on the skull surface. The location at which the tetrodes emerged from the drive tip could be varied for different microdrives. In a typical NH rabbit, several microdrives, each with tetrodes emerging from a different place on the drive tip, were implanted before the tonotopic region of ICC was found. Subsequent microdrives were then constructed and implanted to aim tetrodes at or near the same region. To increase the chances of recording from ICC neurons in HL rabbits, tetrodes were aimed at locations that, in neural recordings in the same rabbit before noise overexposure, yielded tetrode tracks that penetrated a clear tonotopic region (e.g., Fig. 2A).
Statistical analyses.
All statistical tests were performed in MATLAB (Mathworks). Deviation of the firing rate from spontaneous rate was assessed with a Wald test at a significance level of 0.01. The Wald statistic was , where r and rs are the average firing rates over the stimulus duration and last 100 ms of the 300-ms interstimulus silent period, respectively, s2 and are the sample variances of the rates over the same windows, and m and ms are the number of repetitions of the stimulus and silent periods, respectively. Stimuli used in azimuth or ITD tuning curve measurement were considered “above threshold” if the firing rate at any azimuth or ITD deviated significantly from spontaneous rate, as assessed with multiple Wald tests at a significance level of 0.01 and corrected for multiple comparisons using the Benjamini-Hochberg procedure (Wasserman 2004).
Differences between azimuth tuning curves from the same neuron measured under different binaural-cue conditions were assessed with multiple Wald tests at a significance level of 0.01 and corrected for multiple comparisons using the Benjamini-Hochberg procedure. In this case the Wald statistic was
Where and are the average firing rates at azimuth θ under binaural-cue conditions 1 and 2, respectively, and are the sample variances of the same firing rates, and m1 and m2 are the number of repetitions. Effect size was defined as the sum of the absolute value of Wald statistics over all azimuths: .
Differences between the medians of values measured from NH versus HL rabbits were assessed with a Wilcoxon rank-sum test at a significance level of 0.05.
RESULTS
Hearing loss following noise overexposure.
We confirmed normal hearing in control NH rabbits (including those that later underwent noise overexposure) by measuring their ABR thresholds and comparing with those of other unexposed rabbits. The ABR is an evoked potential measured at the skin surface whose generators are known to be the auditory nerve and auditory brain stem and midbrain (Melcher et al. 1996). In NH rabbits, mean click-evoked ABR threshold was 43 dB SPL, and mean tone-evoked ABR thresholds were 48, 52.5, 45, 30.5, 28, and 38 dB SPL for 0.5-, 1-, 2-, 4-, 8-, and 16-kHz tones, respectively (Fig. 1). These values were highly consistent with those of a larger sample of Dutch-belted rabbits in a previous study (N = 53 ears, of which 8 ears were from rabbits in the present study; Fig. 2A in Haragopal et al. 2020). The across-ear SD of ABR thresholds (5 dB) was also the same as that in the previous study.
We induced permanent hearing loss in three of the NH rabbits by overexposing them to high-intensity noise under anesthesia using the same exposure parameters as in a previous study (Haragopal et al. 2020). In that study, a 90-min overexposure led to median ABR threshold shifts of ∼55 dB at all frequencies but large variability across ears. A minority of ears had postexposure thresholds that were above the sound-level limit of the acoustic system, meaning only minimum possible threshold shifts could be estimated for those ears. In our HL rabbits, most postexposure thresholds were above the limit of our acoustic system (∼100 dB SPL; filled symbols in Fig. 1). We attempted to decrease postexposure thresholds slightly by lowering the exposure duration for two rabbits (D05 and D06) from 90 to 75 min, but the postexposure thresholds of these rabbits were all above the limit of the acoustic system, whereas those of the rabbit that had a 90-min exposure (R04) were more often below the limit. That our attempt to slightly lower postexposure thresholds failed is not surprising given the known high variability of susceptibility to noise overexposure (Cody and Robertson 1983; Haragopal et al. 2020). Altogether, click-evoked threshold shifts were at least 50 dB and tone-evoked threshold shifts were at least 50, 55, 50, and 50 dB at frequencies of 2, 4, 8, and 16 kHz, respectively. Minimum possible postexposure thresholds at 0.5- and 1-kHz frequencies were lower than the rest not because these thresholds were above the limit of the acoustic system but because the cochlear microphonic began to dominate the signal at a sound level lower than the threshold. The actual threshold shifts at these frequencies were likely similar to those at higher frequencies since, based on a separate set of 60-min exposures in the Haragopal et al. (2020) study following which postexposure thresholds were measurable at all frequencies, average threshold shifts were within ∼10 dB of each other at all frequencies.
The Haragopal et al. (2020) study measured both ABR threshold shifts and cochlear hair cell counts in the same ears and found a “critical” ABR threshold shift of 46 dB; tone-evoked ABR threshold shifts above this value were always associated with frequency-matched regions of the cochlea that had <25% outer hair cell (OHC) survival and variable amounts of inner hair cell (IHC) survival. Because tone-evoked ABR threshold shifts in our HL rabbits were at least 50 dB at frequencies between 2 and 16 kHz, we can infer that the basal and middle regions of their cochleae had very few surviving OHCs and variable amounts of surviving IHCs. The same inference could not be made for the apical region of their cochleae since accurate threshold shifts at low frequencies were not measurable for the reasons listed above, but the Haragopal et al. (2020) study using the same overexposure found that 75% of ears had less than one-half OHC survival in the apical region of the cochlea.
Frequency tuning following noise overexposure.
The left column of Fig. 2A shows multiunit FRAs measured at different depths along a single tetrode track within a NH rabbit. Tetrode descent in the dorsal border of the ICC was indicated by a transition of the FRA from a diffuse pattern, to broad tuning, to a distinctive V shape with frequency tuning tip that increased systematically with depth. At the ventral border, the frequency tuning tip surpassed the range of tone-pip frequencies, leaving only the tail visible, then the FRA often returned to broad tuning followed by a diffuse pattern. The tetrode in Fig. 2A, left, entered the ICC at the level of a CF of 3 kHz and exited at a CF above 16 kHz, but other tetrodes traversed different spans of the cochleotopic axis, such as entering at 300 Hz and exiting at 4 kHz. This indicated that the orientations of tetrode tracks were oblique to the cochleotopic axis of the ICC.
The right column of Fig. 2A shows multiunit FRAs from a tetrode track in the same rabbit following noise overexposure. Frequency tuning tips were completely absent in this tetrode track and all tracks postexposure, with thresholds to tone pips usually not lower than 70 or 80 dB SPL (note the higher range of sound levels). Because frequency tuning tips did not exist following noise overexposure, we could not directly measure CFs nor determine whether single units were within the ICC or shell regions of the IC. Furthermore, because tetrode tracks were oblique to the cochleotopic axis, we could not infer CF from tetrode depth. However, we noted that the upper-frequency edge of FRAs (UFE; highest frequency with a response ∼75% of maximum firing rate: yellow color in FRAs) increased systematically with tetrode depth in HL rabbits similar to that in NH rabbits (Fig. 2A). For multiunit FRAs measured in NH rabbits, log CF was linearly related to log UFE (Fig. 2B) by the equation log10 CF = 1.2 log10 UFE – 0.24, where CF and UFE were in kilohertz. Under the assumption that UFEs remained the same before and after noise overexposure (e.g., Izquierdo et al. 2008), we measured the UFEs of all neurons from HL rabbits from either the single-unit FRA or the multiunit FRA at the same tetrode site, then predicted CF using the above equation. Predicted CFs of neurons from HL rabbits were all high (Fig. 2D), whereas directly measured CFs of neurons from NH rabbits spanned the cochleotopic axis (Fig. 2C).
Approximately one-half of neurons from HL rabbits had diffuse FRAs with no coherent features (Fig. 2E, top), approximately one-third had a single excitatory region at high frequencies and intensities (Fig. 2E, second from top), and the rest had either a single inhibitory region (Fig. 2E, second from bottom) or combinations of excitatory and inhibitory subregions (Fig. 2E, bottom). FRAs of neurons from NH rabbits were very heterogeneous and included those similar to the subtypes found in HL rabbits (Fig. 2F).
Spontaneous rates and diotic noise thresholds following noise overexposure.
Spontaneous rates of neurons in NH and HL rabbits covered a wide overlapping range whose distributions were best represented on a logarithmic axis (Fig. 3A). Median spontaneous rate was slightly greater for HL than for NH rabbits (P = 0.028, Wilcoxon rank-sum test). This result was consistent with many previous reports of increased spontaneous rates of ICC neurons following acoustic trauma (e.g., Longenecker and Galazyuk 2011; Ma et al. 2006; Mulders and Robertson 2009) although the effect size varies widely between studies.
Fig. 3.
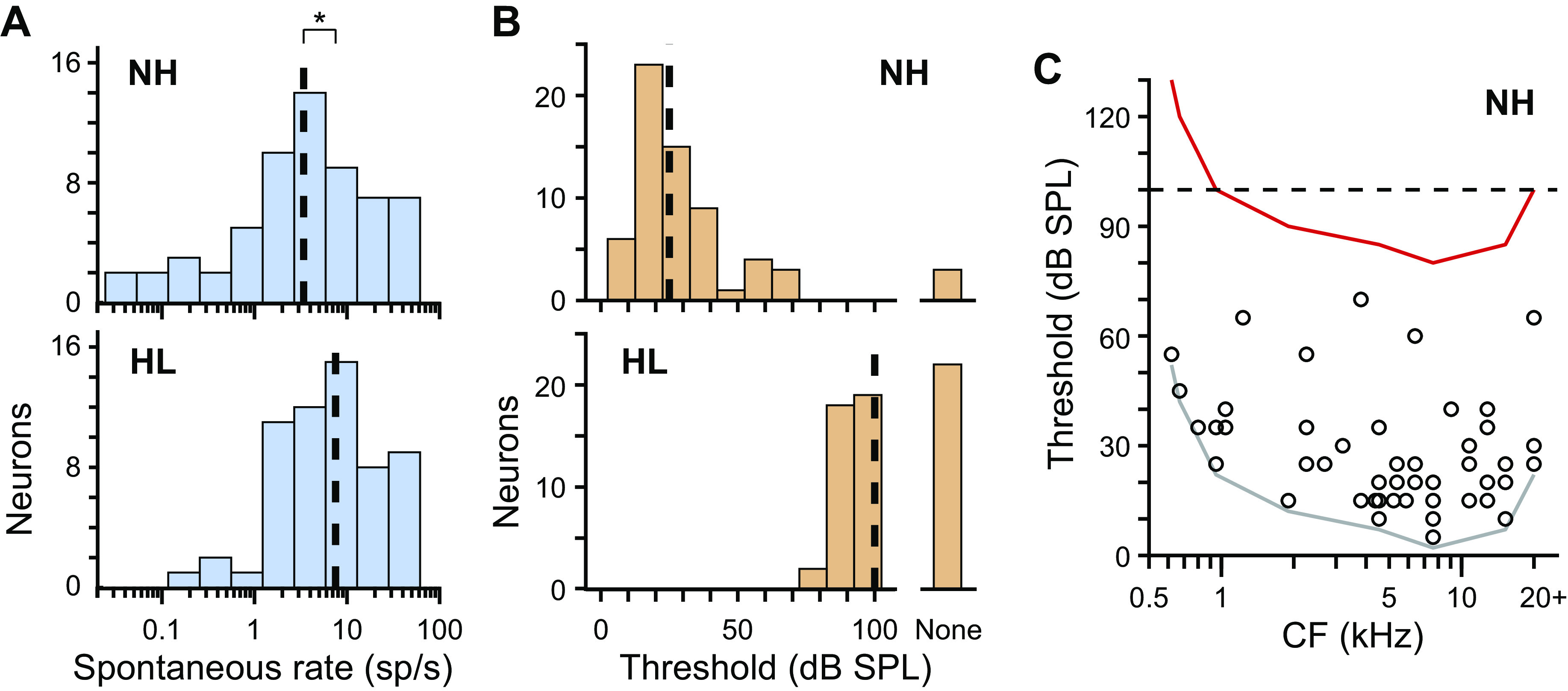
Spontaneous rates and diotic noise thresholds following noise overexposure. A: histograms of spontaneous rates of neurons from normal-hearing (NH, top; N = 61) and hearing loss (HL, bottom; N = 59) rabbits. Broken lines indicate medians. *P < 0.05 (Wilcoxon rank-sum test). B: histograms of sound-level thresholds to diotic broadband noise for neurons from NH (top; N = 64) and HL (bottom; N = 61) rabbits. Broken lines indicate medians computed under the assumption that neurons with no threshold had actual thresholds that were >100 dB SPL. C: scatterplot of sound-level threshold versus CF for neurons from NH rabbits. Gray line marks the lower envelope of the data, and red line marks this same envelope following a hypothetical shift of 75 dB, the same shift in median threshold following overexposure as in B. Broken line indicates approximate sound-level limit of the acoustic system. The hypothetical distribution of CFs of neurons with measurable responses would be biased to high CFs following overexposure.
There was no overlap of distributions of sound-level thresholds to diotic broadband noise between NH and HL rabbits (Fig. 3B). Of neurons from HL rabbits, 64% (39/61) had measurable thresholds, i.e., within the limit of our acoustic system (≤100 dB SPL). Under the assumption that neurons with no measurable thresholds had actual thresholds >100 dB SPL, median threshold was 75 dB greater for HL than for NH rabbits. Therefore, HL rabbits had substantial hearing loss. A greater percentage of neurons in HL than in NH rabbits had firing rates in response to broadband noise at threshold sound level that were suppressed below, as opposed to facilitated above, spontaneous rate [HL: 38% (15/39), NH: 8% (5/61), only neurons with measurable thresholds], suggesting either overrepresentation of the suppressive subtype of neurons or increased strength of inhibition following noise overexposure.
Thresholds of neurons from NH rabbits were lowest at high CFs (Fig. 3C), consistent with previous behavioral and physiological studies in rabbits (Day and Delgutte 2016; Heffner and Masterton 1980). We shifted the lower envelope of the scatterplot in Fig. 3C by 75 dB, the same shift in median threshold shown in Fig. 3B, to predict the hypothetical lower envelope of data following noise overexposure. The hypothetical envelope was cut off at low CFs by the sound-level limit of our acoustic system, suggesting a bias toward high CFs for neurons from HL rabbits, consistent with the predicted CFs in Fig. 2D.
Directional sensitivity following noise overexposure.
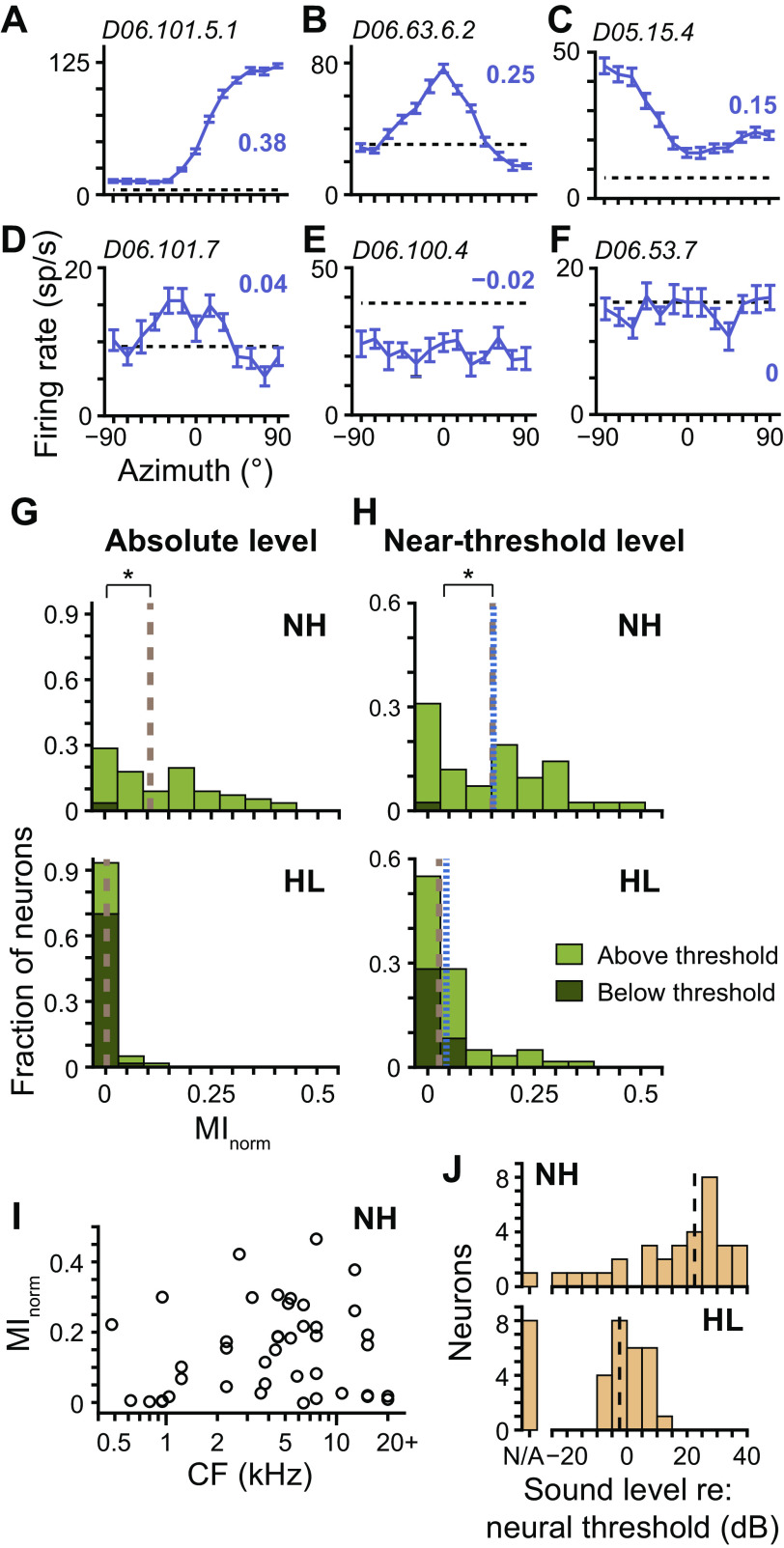
For each neuron, we measured the azimuth tuning curve to broadband noise in the front horizontal plane and then quantified the neuron’s directional sensitivity as the MI between firing rate and stimulus azimuth, normalized by the maximum possible MI (MInorm). An MInorm of one indicates the azimuth of the stimulus can be exactly predicted by observing the firing rate, whereas a MInorm of zero indicates a flat azimuth tuning curve, which provides no information about azimuth. Figure 4, A and B, shows azimuth tuning curves of two neurons from HL rabbits that had relatively strong directional sensitivity, one with a contralaterally maximal sigmoidal shape and the other with a broad bell shape centered at 0°. Tuning curve shapes of neurons from HL rabbits were heterogeneous, including shapes similar to those in Fig. 4A, shapes similar to those in Fig. 4B but centered at different azimuths, shapes that were a hybrid of those in Fig. 4, A and B, and ipsilaterally maximal sigmoidal shapes (Fig. 4C). Such tuning curve heterogeneity was consistent with that of neurons from NH rabbits, both in the present and previous studies (Day and Delgutte 2013; Kuwada et al. 2011). Many neurons from HL rabbits had low (Fig. 4D) or no (Fig. 4, E and F) directional sensitivity; the latter occurred because of the stimulus either evoking a response that had no directional sensitivity (Fig. 4E) or simply not being sufficient to create a deviation of the firing rate from spontaneous rate (Fig. 4F). (Note that the MInorm in Fig. 4E was slightly negative, which could occur because of bias correction.)
Fig. 4.
Directional sensitivity following noise overexposure. A–F: azimuth tuning curves of 6 different neurons from hearing loss (HL) rabbits measured at near-threshold level (purple curves; mean ± SE). Normalized mutual information (MInorm) of each curve indicated by bold purple no. adjacent to curve. Positive azimuths indicate sound source azimuths contralateral to the recording site. Broken black lines indicate spontaneous rate. Unit ID is indicated in top left corner of each plot. G: histograms of MInorm for azimuth tuning curves of neurons from normal-hearing (NH, top; N = 56) and HL (bottom; N = 60) rabbits measured at absolute level. Stacked light and dark green bins represent tuning curves that were and were not significantly different from spontaneous rate, respectively (i.e., above and below threshold, respectively; Wald tests with correction for multiple comparisons, P < 0.01). Overall medians indicated by brown broken lines. H: same as G for tuning curves measured at near-threshold level (top: NH, N = 42; bottom: HL, N = 60). Broken blue lines indicate medians computed only for tuning curves that were above threshold. *P < 0.05 (Wilcoxon rank-sum test). I: scatterplot of MInorm measured at near-threshold level versus CF for neurons from NH rabbits. J: histograms of near-threshold sound level with respect to each neuron’s threshold to diotic broadband noise for neurons from NH (top; N = 33) and HL (bottom; N = 33) rabbits. Data only included for neurons with azimuth tuning curves that were above threshold. “N/A” indicates neurons with no measurable thresholds. Black lines indicate medians computed under the assumption that sound levels associated with neurons with no thresholds were at the lowest end of the data set.
At an absolute sound level of 70 dB SPL, neurons from NH rabbits had a broad range of MInorm, whereas those from HL rabbits had MInorm near zero (P = 1 × 10−10, Wilcoxon rank-sum test; Fig. 4G). The firing rates of most neurons from HL rabbits did not deviate from spontaneous rate at 70 dB SPL (dark green bins in Fig. 4G; similar to that in Fig. 4F). Therefore, the lack of directional sensitivity in neurons from HL rabbits could be explained simply because of the stimulus being below threshold. At near-threshold sound levels, the stimulus was above threshold for most neurons from HL rabbits, and the range of MInorm increased, but median MInorm was still less than that for neurons from NH rabbits (P = 9 × 10−5; Fig. 4H). The decrease in median MInorm for neurons from HL rabbits remained statistically significant when computed only on azimuth tuning curves that deviated from spontaneous rate (i.e., above-threshold stimuli; P = 0.015, N = 41 and 38 for NH and HL, respectively; blue dotted lines in Fig. 4H), which indicates that decreased directional sensitivity of neurons from HL rabbits was not solely due to the stimulus being below threshold for some neurons.
Figure 4I shows that MInorm of neurons from NH rabbits could have relatively high values across the cochleotopic axis, indicating that decreased directional sensitivity of neurons from HL rabbits could not be explained by a potential sample bias toward high-CF neurons. Although stimuli presented at near-threshold level were above threshold for most neurons from HL rabbits, it is important to note that they were not at the same relative sound level for NH and HL rabbits. When near-threshold sound level was expressed relative to each neuron’s threshold to diotic (i.e., at 0°) broadband noise, median thresholds were 22.5 dB above and 2.5 dB below neural threshold for NH and HL rabbits, respectively (Fig. 4J). The proximity of sound levels for HL rabbits so close to neural threshold could potentially account for the decrease in directional sensitivity of neurons from HL rabbits.
Binaural versus monaural sensitivity.
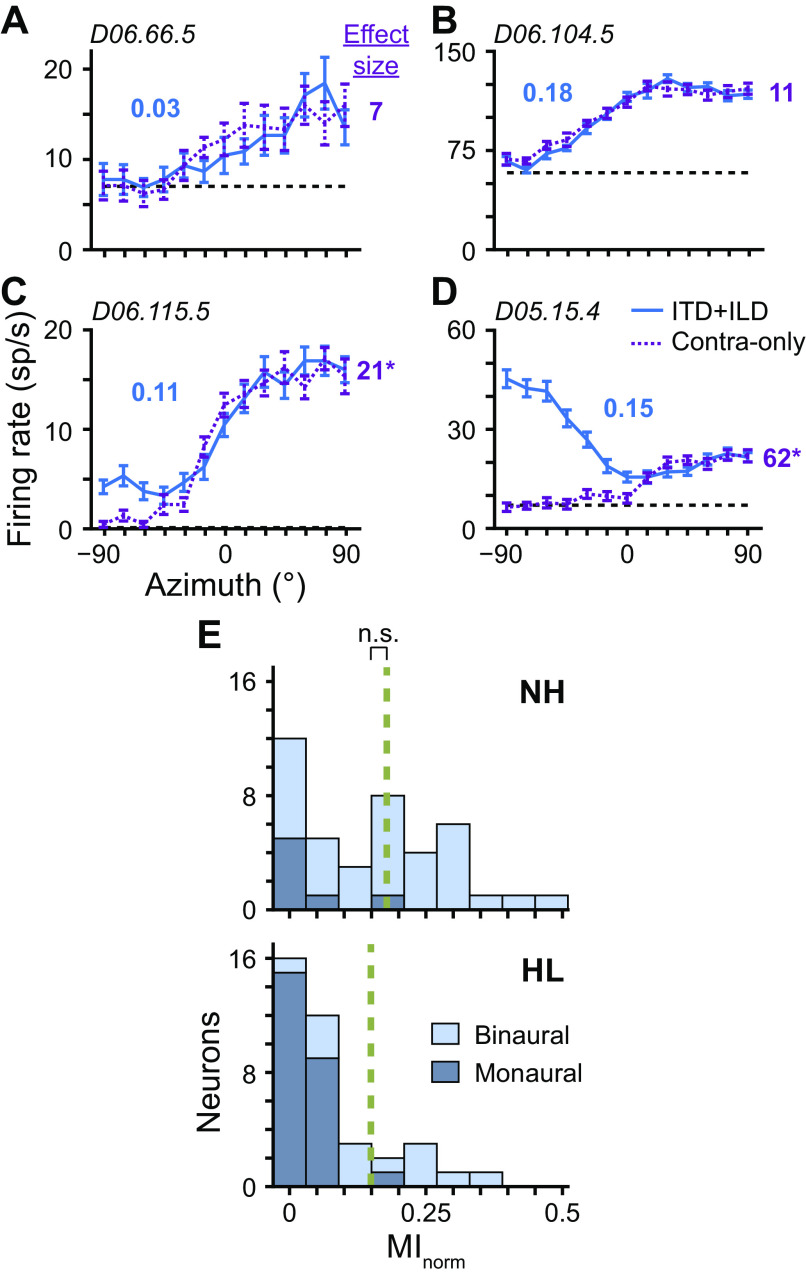
As a sound source moves from ipsilateral to contralateral to one ear, the intensity in that ear attenuates because of the obstructing head. In rabbits, this attenuation is most dramatic above ∼4 kHz (Day et al. 2012; Kim et al. 2010). Neurons that are exclusively sensitive to sound in one ear may therefore have azimuth tuning curves that deviate from spontaneous rate due to purely monaural, not binaural, sensitivity. Responses to monaural sound level are not useful for determining sound source azimuth, which depends on binaural cues. To disambiguate binaural from monaural sensitivity, for each neuron we additionally measured the azimuth tuning curve with sound presented only to the contralateral ear (contra only) and then compared it with the binaural tuning curve (ITD + ILD). (Input to the IC is weighted toward the contralateral ear.) Figure 5, A and B, shows binaural azimuth tuning curves that do not significantly differ from their contra-only curves (P ≥ 0.01, Wald tests with correction for multiple comparisons), indicating monaural sensitivity, whereas Fig. 5, C and D, shows binaural azimuth tuning curves that do significantly differ from their contra-only curves (P < 0.01), indicating binaural sensitivity. All data in Fig. 5, A–D, were from HL rabbits. Most binaural interaction in neurons from HL rabbits was relatively mild, similar to that in Fig. 5C. We quantified binaural interaction by the effect size of the statistical test of difference between binaural and monaural tuning curves (number to right of each panel). Figure 5D shows data from the neuron with greatest binaural interaction in HL rabbits.
Fig. 5.
Binaural versus monaural sensitivity. A–D: azimuth tuning curves of 4 different neurons from hearing loss (HL) rabbits measured at near-threshold level under binaural [interaural time and level differences (ITD + ILD); blue solid lines] and monaural (contra only; purple dotted lines) conditions. In the monaural condition, sound was only presented to the contralateral ear. Broken black lines indicate spontaneous rate. Normalized mutual information (MInorm) of each ITD + ILD curve indicated by bold blue no. adjacent to curve. Effect size of Wald tests for differences between ITD + ILD and contra-only curves are listed to the right of each panel. *P < 0.01. Unit ID indicated in top left corner of each plot. E: histograms of MInorm for azimuth tuning curves (ITD + ILD) of neurons from normal-hearing (NH, top; N = 41) and HL (bottom; N = 38) rabbits measured at near-threshold level. Only neurons with tuning curves significantly different from spontaneous rate were included (i.e., above threshold; P < 0.01, Wald tests with correction for multiple comparisons). Stacked light and dark blue bins represent curves that were and were not significantly different from contra-only curves, respectively (i.e., binaural and monaural sensitivity, respectively; P < 0.01, Wald tests with correction for multiple comparisons). Broken green lines indicate medians computed only for neurons with binaural sensitivity. ns, No statistical significance (P > 0.05, Wilcoxon rank-sum test).
Most monaurally sensitive neurons from NH or HL rabbits had low directional sensitivity (Fig. 5E), similar to that in Fig. 5A; Figure 5B shows data from the monaurally sensitive neuron with greatest directional sensitivity. Neurons from HL rabbits were more often monaurally sensitive than those from NH rabbits [66% (25/38) versus 17% (7/41) for HL and NH rabbits, respectively; only neurons with above-threshold azimuth tuning curves]. The relatively greater proportion of monaurally sensitive neurons in HL rabbits may again potentially be explained by the proximity of near-threshold sound levels to neural thresholds for HL rabbits. Kuwada et al. (2011) showed that azimuth tuning curves of IC neurons measured at 10 dB (re: neural threshold) were similar between binaural and contralateral sound presentation; neurons that were monaurally sensitive at low relative levels were binaurally sensitive at high relative levels. When only azimuth tuning curves of binaurally sensitive neurons were considered, the difference in median MInorm between NH and HL rabbits was no longer significant (P = 0.88, Wilcoxon rank-sum test, N = 34 and 13 for NH and HL, respectively; Fig. 5E). The overall decrease in median MInorm for neurons from HL compared with NH rabbits (Fig. 4H) can therefore be accounted for, first, by stimuli being below threshold for some neurons and, second, because of a greater proportion of monaural neurons, the latter of which may be the result of stimuli being near neural threshold.
There remains the possibility that azimuth tuning curves we deemed binaural may have been because of exclusive sensitivity to the ipsilateral ear, since we only measured monaural responses to the contralateral ear. Of the 13 neurons from HL rabbits whose azimuth tuning curves were significantly different from both spontaneous rate and contra-only curves, 12 had contra-only curves that were also significantly different from spontaneous rate, directly indicating sensitivity to the contralateral ear and indirectly indicating sensitivity to the ipsilateral ear via binaural interaction. Therefore, the neurons were indeed binaural. We were able to additionally measure firing rates in response to broadband noise presented only to the ipsilateral ear at near-threshold level for 8 of the 13 neurons described above. Six of these neurons had firing rates to ipsilateral noise that were significantly different from spontaneous rate, thereby providing direct evidence of sensitivity to each ear separately.
Binaural cues underlying directional sensitivity.
To determine if the directional sensitivity of a binaurally sensitive neuron was the result of underlying sensitivity to ITD, ILD, or both, we additionally measured azimuth tuning curves using filters that preserved either the relationship of ITD and azimuth while fixing ILD to that at 0° (“ITD only”) or the relationship of ILD and azimuth while fixing ITD to that at 0° (“ILD only”) and then compared these curves with the normal ITD + ILD curve. Figure 6A shows data from a neuron from a NH rabbit whose ITD + ILD azimuth tuning curve was significantly different (P < 0.01, Wald tests with correction for multiple comparisons) from the ILD only but not ITD only curve, indicating directional sensitivity due to underlying ITD sensitivity. Conversely, Fig. 6B shows data from a neuron from a HL rabbit whose ITD + ILD azimuth tuning curve was significantly different from the ITD-only but not ILD-only curve, indicating directional sensitivity due to underlying ILD sensitivity. Last, Fig. 6C shows data from a neuron from a NH rabbit whose ITD + ILD azimuth tuning curve was significantly different from both ITD-only and ILD-only curves, indicating directional sensitivity due to the interaction of ITD and ILD sensitivities. Our full data set of azimuth tuning curves of all neurons measured under all conditions may be found in Supplemental Fig. S1 (https://doi.org/10.6084/m9.figshare.12837728.v1).
Fig. 6.
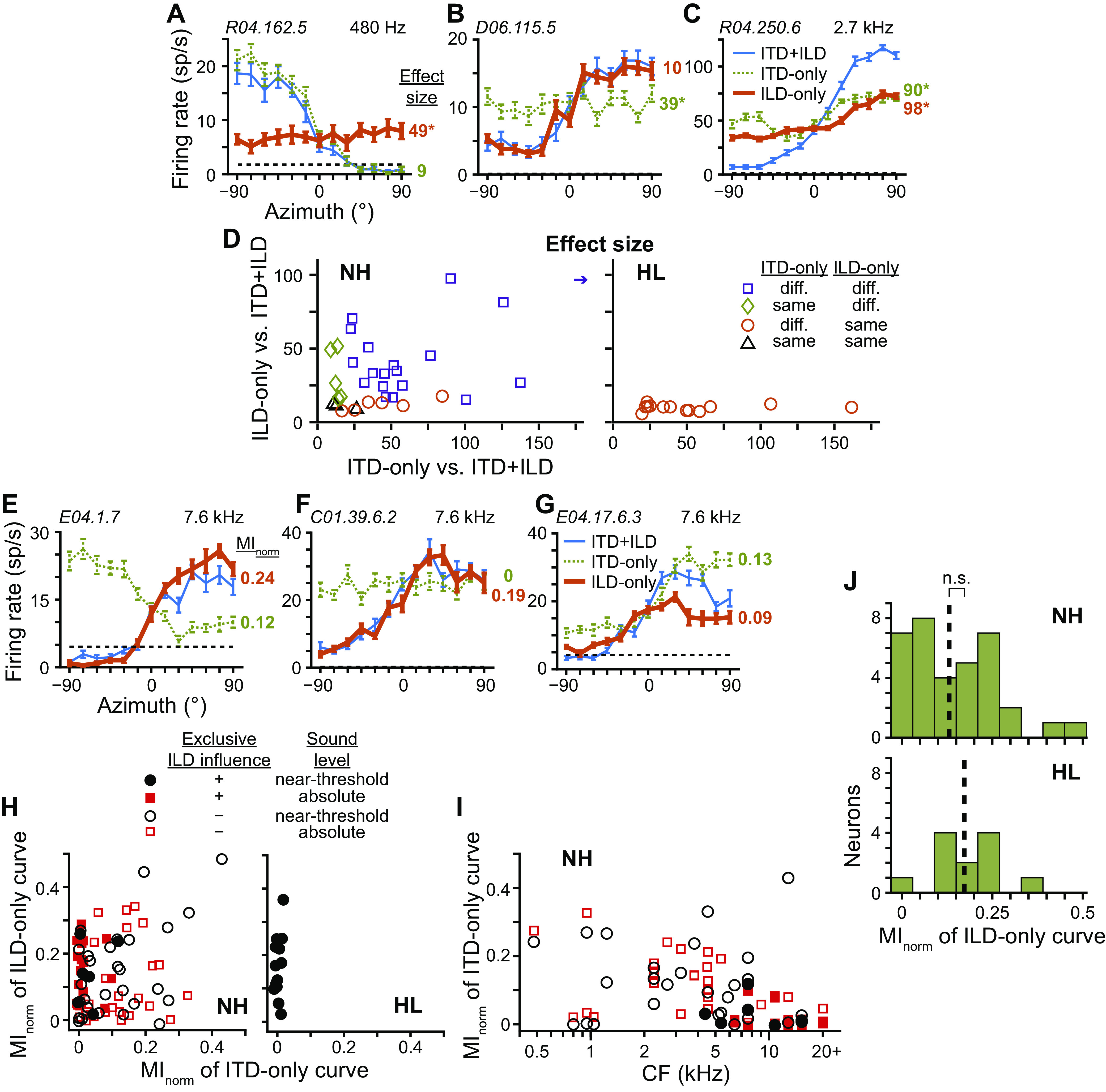
Binaural cues underlying directional sensitivity. A–C: azimuth tuning curves of 3 different neurons measured at near-threshold level under congruent [blue thin lines; interaural time and level differences (“ITD + ILD”)] and 2 incongruent binaural cue conditions, where in the latter cases only either ITD (green dotted lines; “ITD only”) or ILD (orange thick lines; “ILD only”) varied with azimuth while the other cue was fixed to that at 0°. Broken lines indicate spontaneous rate. Data in A and C were from normal-hearing (NH) rabbits with CF indicated in top right corner, whereas data in B were from a hearing loss (HL) rabbit. Unit ID indicated in top left corner. Effect sizes of Wald tests for differences between ITD + ILD and each of ITD-only and ILD-only curves are listed to the right of each panel, color-matched to each incongruent-cue curve. *P < 0.01. D: scatterplots of the effect size between congruent- and incongruent-cue curves of neurons from NH (left; N = 34) and HL (right; N = 13) rabbits measured at near-threshold level. Only neurons with ITD + ILD curves that were significantly different from both spontaneous rate and contra-only curves were included (i.e., above threshold and binaural). Statistically significant differences (P < 0.01, Wald tests with correction for multiple comparisons) between the ITD + ILD and each of the ITD-only and ILD-only curves indicated by symbol shape and color: both different (purple squares), only ILD-only different (green diamonds), only ITD-only different (orange circles), neither different (black triangles). Arrow indicates data point beyond the axis. E–G: azimuth tuning curves of 3 additional neurons from NH rabbits. Format same as in A–C. Data in E and G are measured at near-threshold level and F at absolute level. Normalized mutual information (MInorm) for ITD-only and ILD-only curves listed to the right of each panel, color-matched to each incongruent-cue curve. H: scatterplot of MInorm for ITD-only versus ILD-only curves for each neuron from NH (left) and HL (right) rabbits. Only neurons with ITD + ILD curves that were significantly different from both spontaneous rate and contra-only curves were included. Solid symbols indicate neurons whose ITD + ILD curves were significantly different from their ITD-only but not ILD-only curves while open symbols indicate all other neurons. Black circles and red squares indicate measurement at near-threshold (NH: N = 34; HL: N = 13) and absolute (NH: N = 42) sound levels, respectively. I: scatterplot of MInorm for ITD-only curves versus the CF of each neuron from NH rabbits. Symbols same as in H. J: histograms of MInorm for ILD-only tuning curves of neurons from NH (N = 35) and HL (N = 12) rabbits measured at near-threshold level. Only neurons with ILD-only curves that were significantly different from both spontaneous rate and contra-only curves were included. Broken lines indicate medians. ns, No statistical significance (P > 0.05, Wilcoxon rank-sum test).
Many neurons in our sample from NH rabbits had directional sensitivity due to each of the three types of underlying binaural sensitivities demonstrated in Fig. 6, A–C: exclusive ITD or ILD influence, or the interaction of ITD and ILD sensitivities (Fig. 6D, left). In particular, 82% (28/34) of binaurally sensitive neurons from NH rabbits had directional sensitivity that was influenced by underlying ITD sensitivity. Contrary to this, no binaurally sensitive neurons from HL rabbits had directional sensitivity influenced by ITD sensitivity; all were exclusively influenced by ILD (Fig. 6D, right). These binaurally sensitive neurons were from two different HL rabbits.
That a neuron’s directional sensitivity was exclusively influenced by ILD did not preclude the same neuron from also being sensitive to changes in ITD. For example, Fig. 6E shows data from a neuron from a NH rabbit whose ITD + ILD azimuth tuning curve was significantly different from the ITD-only but not ILD-only curve, indicating directional sensitivity due exclusively to ILD sensitivity even though the neuron’s firing rate was sensitive to changes in ITD when ILD was fixed to that at 0°. For this neuron, the effect of ILD on firing rate obviously outweighed the effect of ITD. To compare sensitivities of firing rates within the same neuron with ITD and ILD varied separately, we plotted MInorm computed from ITD-only curves against MInorm computed from ILD-only curves (Fig. 6H). Neurons from HL rabbits all had MInorm of ITD-only tuning curves near zero, indicating no sensitivity to ITD, similar to the example in Fig. 6B. In the NH data of Fig. 6H, we highlighted those neurons whose directional sensitivity was exclusively influenced by ILD, similar to those of HL rabbits. Most of these neurons had MInorm for ITD-only tuning curves near zero (e.g., Fig. 6F), also similar to neurons from HL rabbits; however, a few had larger MInorm values (e.g., Fig. 6E). The existence of a subpopulation of neurons in NH rabbits so similar to neurons of HL rabbits raised the possibility that sampling in HL rabbits may have been biased toward these neurons, potentially because of a bias in CF. In Fig. 6I, we plot MInorm of ITD-only tuning curves versus each neuron’s CF and again highlight those neurons from NH rabbits whose directional sensitivity was exclusively influenced by ILD. Neurons that both had directional sensitivity exclusively influenced by ILD and had no ITD sensitivity (MInorm near zero) were indeed clustered at high CFs, and overlapped with the predicted CFs of neurons from HL rabbits (Fig. 2D). However, sensitivity to ITD in other neurons existed across the cochleotopic map. Notably, all example neural data from NH rabbits in Fig. 6, E–G, had CFs in the middle of the predicted range for neurons from HL rabbits and demonstrate the range of ITD sensitivities observed. The exclusive influence of ILD on directional sensitivity and lack of ITD sensitivity in neurons from HL rabbits could therefore not be accounted for by a potential bias in CF.
To compare ILD sensitivity between neurons from NH and HL rabbits, we computed the MInorm of ILD-only tuning curves as a way to quantify ILD sensitivity within the physiological range. There was no significant difference of median MInorm of ILD-only curves between binaurally sensitive neurons of NH and HL rabbits (P = 0.26, Wilcoxon rank-sum test; Fig. 6J). Altogether, the remaining directional sensitivity of neurons from HL rabbits was exclusively due to ILD sensitivity, and there was no evidence of a change in ILD sensitivity following noise overexposure.
ITD sensitivity following noise overexposure.
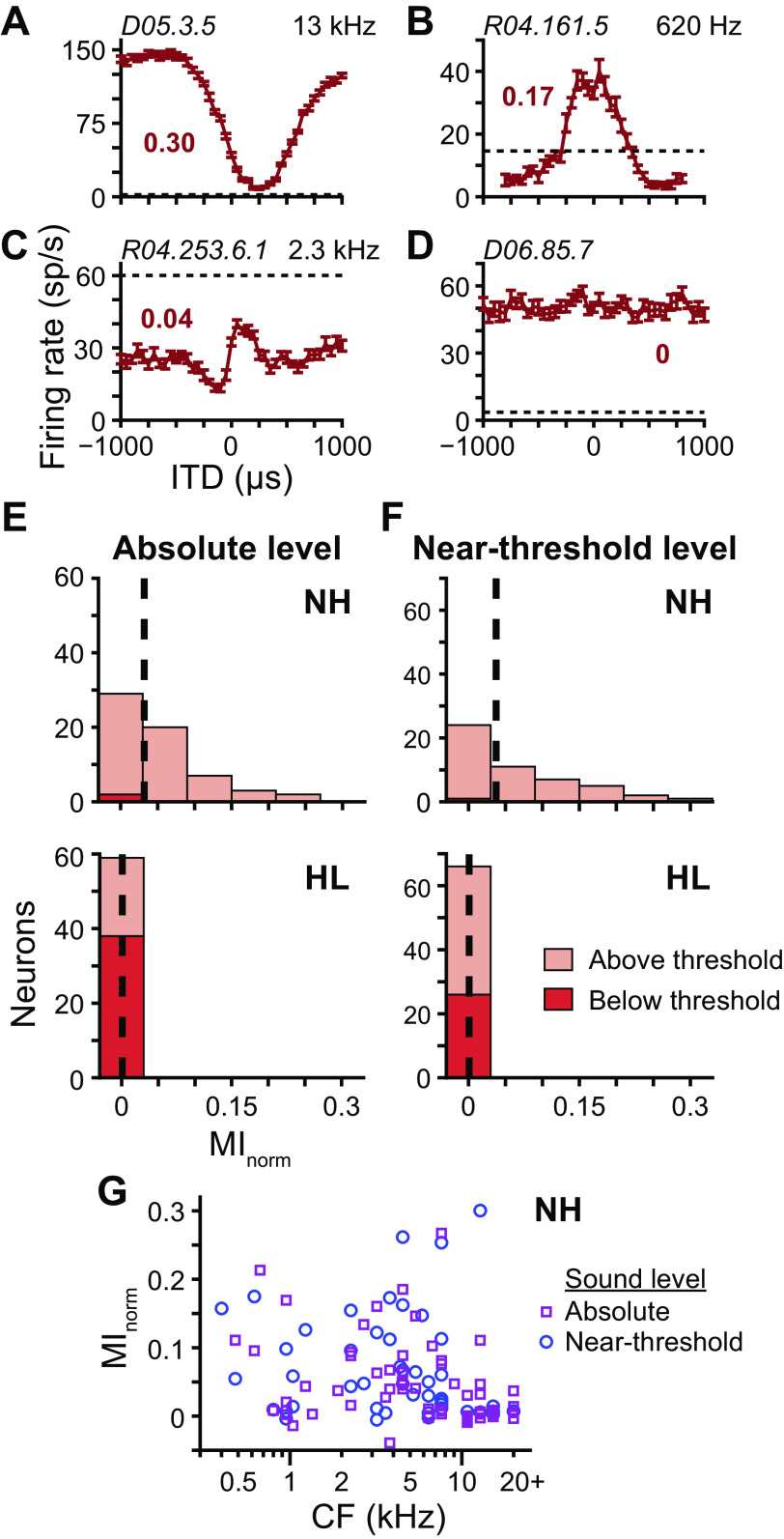
As a virtual sound source moves from −90° to +90° the ITD in the high-frequency limit shifts from −275 to +275 µs. To ensure that we did not miss potential ITD sensitivity outside of the physiological range of ITDs, we measured ITD tuning curves to broadband noise over ±1-ms delays. Figure 7, A–C, shows ITD tuning curves of three different neurons from NH rabbits: the first with a prominent suppression of firing rate compared with that at large ITDs (“trough” type), characteristic of the temporal interaction of excitatory input from one ear and inhibitory input from the other; the second with a prominent facilitation of firing rate compared with that at large ITDs (“peak” type), characteristic of the temporal interaction of excitatory inputs from both ears; and the third of an intermediate response type. Figure 7D shows the ITD tuning curve of a neuron from a HL rabbit that had no ITD sensitivity yet responded to the stimulus with a firing rate well above spontaneous rate. Our full data set of ITD tuning curves of all neurons may be found in Supplemental Fig. S2 (https://doi.org/10.6084/m9.figshare.12838265.v1). Similar to that for directional sensitivity, we quantified the ITD sensitivity of a neuron as the MInorm between firing rate and ITD.
Fig. 7.
Interaural time difference (ITD) sensitivity following noise overexposure. A–D: ITD tuning curves (mean ± SE) of 4 different neurons measured at near-threshold level. Positive ITDs indicate contralateral-leading ITDs. Data in A–C are from normal-hearing (NH) rabbits with CFs indicated in top right corners, and data in D are from a hearing loss (HL) rabbit. Unit IDs indicated in top left corners. Broken lines indicate spontaneous rates. Normalized mutual information (MInorm) of ITD tuning curve indicated by bold no. next to each curve. E: histograms of MInorm for ITD tuning curves of neurons from NH (top; N = 61) and HL (bottom; N = 59) rabbits measured at absolute level. Stacked light and dark red bins represent tuning curves that were and were not significantly different from spontaneous rate, respectively (i.e., above and below threshold, respectively; Wald tests with correction for multiple comparisons, P < 0.01). Medians indicated by dashed lines. F: same as E for tuning curves measured at near-threshold level (top: NH, N = 50; bottom: HL, N = 66). G: scatterplot of MInorm versus CF for neurons from NH rabbits measured at absolute (purple squares) and near-threshold (blue circles) levels.
At an absolute sound level of 70 dB SPL, neurons from NH rabbits had a broad range of MInorm whereas those of neurons from HL rabbits were approximately zero (Fig. 7E). This result again could be explained by most stimuli for HL rabbits being below threshold. However, the same pattern of results persisted for stimuli presented at near-threshold level even though stimuli for the majority of neurons from HL rabbits were above threshold (Fig. 7F). MInorm values equal to or greater than that of the ITD tuning curve in Fig. 7C (0.04) were found at all CFs in NH rabbits (Fig. 7G). Therefore, the lack of ITD sensitivity in neurons from HL rabbits could not be accounted for by potential sample bias toward high-CF neurons. As noted in materials and methods, there was a statistically significant difference of MInorm of ITD tuning curves between individual NH rabbits. To assess consistency of results across individuals, we compared MInorm values of HL rabbits separately with MInorm values of each NH rabbit: values from each NH rabbit were significantly greater than those of HL rabbits at a significance level of 0.05 at both absolute and near-threshold levels (greatest P value was 0.011, Wilcoxon rank-sum tests with correction for multiple comparisons).
To ensure that lack of ITD sensitivity was not simply a result of neurons from HL rabbits being monaurally sensitive, we measured firing rates to broadband noise presented separately to the ipsi- and contralateral ears at near-threshold level. We found 12 neurons in two different HL rabbits that had firing rates significantly different from spontaneous rate (P < 0.01, Wald test) for both ipsi- and contralateral sound presentation. All had firing rates that were facilitated above spontaneous rate for sound presented to either ear. These 12 binaural neurons only partially overlapped with the previous 12 ILD-sensitive neurons that had evidence of binaural interaction in their azimuth tuning curves when compared between binaural and monaural conditions. Combining these together, 20 different neurons from HL rabbits had evidence of responsiveness to binaural input at near-threshold level, and all had MInorm of their ITD tuning curves approximately equal to zero.
There remains the possibility that stimulation of a neuron near its sound-level threshold may not be sufficient to produce an ITD-sensitive response, even though that stimulation may be sufficient to produce a response to separate ipsi- and contralateral stimulation. In our sample of neurons from NH rabbits, it was difficult to assess the existence of a potential dependence of ITD sensitivity on relative sound level because 1) most ITD tuning curves were measured at a sound level >10 dB above neural threshold (Fig. 4J), and 2) thresholds measured from level tuning curves were not always an accurate measurement of overall sound-level threshold because the stimulus was fixed at zero ITD (diotic). The dependence of estimated sound level threshold on the ITD of the stimulus can be illustrated by the ITD tuning curve in Fig. 7A: a level tuning curve measured with a stimulus fixed at 250 µs would yield a much higher threshold than a level tuning curve measured with a stimulus fixed at −500 µs. We found one neuron from a NH rabbit that avoided the above limitations: its ITD tuning curve was measured at −2.5 dB (re: neural threshold, similar to that for neurons from HL rabbits; see Fig. 4J) and it had an ITD tuning peak at zero ITD, meaning its level tuning curve measured with a stimulus at zero ITD yielded an accurate estimate of sound-level threshold. The ITD tuning curve of this neuron, which was measured at 52.5 dB SPL, is shown in Fig. 7B. Its corresponding level tuning curve (data not shown), which was measured in response to the same stimulus fixed at zero ITD and in level steps of 5 dB, had firing rates equal to spontaneous rate at 50 dB SPL and 12 sp/s above spontaneous rate at 55 dB SPL, meaning the 52.5-dB-SPL stimulus used to measure the ITD tuning curve was either at or within a few decibels of threshold. Furthermore, its MInorm value (0.17) was one of the highest in the sample. Therefore, stimulation near the sound-level threshold of the neuron did not preclude it from being sensitive to the ITD of the stimulus.
Last, we measured additional ITD tuning curves at near-threshold level in response to broadband noise over ±3-ms delays (HL: N = 51) and lowpass noise (0.1–1.5 kHz) over ±1-ms delays (HL: N = 56) to specifically search for sensitivity to very large ITDs and to ITDs in the fine structure of sound waveforms, respectively. In all neurons from HL rabbits, MInorm was approximately zero (data not shown). However, in response to lowpass noise, most ITD tuning curves did not deviate significantly from spontaneous rate (P > 0.01, Wald tests with correction for multiple comparisons), meaning the stimulus was below threshold. Overall, the complete lack of ITD sensitivity in neurons from HL rabbits could not be accounted for by subthreshold stimuli, monaural sensitivity, stimulation near level threshold, potential sample bias in CF, range of delays tested, or broadband versus lowpass stimulus spectrum.
Spike-timing precision following noise overexposure.
ITD sensitivity arises in neurons of the superior olivary complex (SOC) via the detection of coincidence between precisely timed neural inputs originating from the ipsi- and contralateral ears. One way ITD sensitivity may be decreased is by a reduction in temporal precision of the inputs to the SOC. Because the ICC is one synapse away from the SOC, we entertained the notion that the temporal precision of ipsi- and contralateral inputs to the SOC may be reflected in the spike-timing precision of ICC neurons to ipsi- and contralateral sound presentation.
Figure 8A , top and bottom, shows raster plots of spike trains from two different neurons from NH rabbits in response to 50 trials of the same broadband noise waveform presented to the contralateral ear. Spike trains of the neuron Fig. 8A, top, clearly aligned vertically at various time points, indicating high spike-timing precision and, therefore, locking of the neuron’s response to particular features in the ongoing noise waveform. Spike trains of the neuron in Fig. 8A, bottom, showed no apparent vertical alignment of spikes, indicating poor spike-timing precision. We quantified the degree of spike-timing precision by computing the SAC, which is a histogram of time intervals between spikes from different trials. For example, the second panels of Fig. 8, D and C, show the SACs of the spike trains in Fig. 8A, top and bottom, respectively. The value of the SAC at zero delay, henceforth called the correlation index, indicates the number of spikes that occurred at the same time across trials, normalized so that a value of one indicates the number of coincidences that would occur by chance for random spike times at the same mean rate (no spike-timing precision) and values greater than one indicate coincidences more often than by chance (some degree of spike-timing precision).
Fig. 8.
No relationship between spike-timing precision and interaural time difference (ITD) sensitivity. A: raster plots of spike times relative to stimulus onset in response to the same broadband noise waveform presented to the contralateral ear at near-threshold level. Top and bottom, data from 2 different neurons from normal-hearing (NH) rabbits, with unit ID indicated in top left corner. Each dot indicates one action potential. Each row is 1 of 50 trials. Thick and thin lines at top indicate times when the stimulus was on and off, respectively. Silent period extended past the axis limit to 600 ms. B–D: data from 3 different neurons from NH rabbits. Unit ID and CF indicated above panels in first column. Left and middle columns contain shuffled autocorrelograms (SACs) of responses to broadband noise at near-threshold level presented to the ipsi- and contralateral ears, respectively. Correlation index indicated next to each correlogram. Column on right contains ITD tuning curves measured at near-threshold level. Normalized mutual information (MInorm) indicated next to each curve. Firing rates to monaural presentation of the stimulus to the ipsi- and contralateral ears indicated by respective red and black triangles to the right of each plot. Broken lines indicate spontaneous rate.
The SACs in Fig. 8B for responses to ipsi- and contralateral noise both had relatively high correlation indexes. That this ICC neuron had spikes that were precisely timed to features within the noise waveform presented to the ipsi- or contralateral ear implies that its input from all areas upstream, including the ipsi- and contralateral inputs to the SOC, also encoded temporal features of the noise waveform in their spike times. The same neuron was also sensitive to ITD (last panel of Fig. 8B). ITD sensitivity in ICC neurons is likely inherited from their inputs from the SOC, since there has been no evidence of de novo ITD sensitivity in the ICC. Furthermore, because ITD sensitivity arises from the coincidence of precisely timed inputs to the SOC, the existence of ITD-sensitive responses in an ICC neuron implies precisely timed inputs to the SOC. Because both temporal coding and ITD sensitivity in ICC neurons are inherited from upstream brain areas, we hypothesized that ITD sensitivity in ICC neurons would correlate with correlation indexes to ipsi- and contralateral noise, similar to the pattern of that in Fig. 8B, and that this potential relationship could be exploited to identify degradation of spike timing as a mechanism for loss of ITD sensitivity in HL rabbits.
Although data from some neurons were consistent with the pattern in Fig. 8B, data of other neurons were not. Figure 8C shows data from a neuron from a NH rabbit that had poor spike-timing precision but strong ITD sensitivity. Firing rates to monaural presentation were well above spontaneous rate, meaning the lack of spike-timing precision was not the result of stimuli being below threshold. As mentioned above, the existence of ITD sensitivity in this neuron implies that the upstream inputs to the SOC were precisely timed, yet this precision was not reflected in the spiking output of the ICC neuron. As is demonstrated by these data, temporal precision in inputs upstream to a neuron does not necessarily imply precision in the neuron’s spiking output. Figure 8D shows data from another neuron from a NH rabbit that had high spike-timing precision but weak ITD sensitivity. As mentioned above, high spike-timing precision in the ICC implies temporal precision upstream, yet this precision did not correlate with ITD sensitivity. The weak ITD sensitivity in this ICC neuron could have been inherited from its inputs from the SOC or a result of the input/output function of the neuron on inputs with strong ITD sensitivity. As is demonstrated by these data, weak ITD sensitivity does not necessarily imply poor temporal precision in the inputs upstream to a neuron. Overall, we found no relationship between spike-timing precision and ITD sensitivity in ICC neurons of NH rabbits.
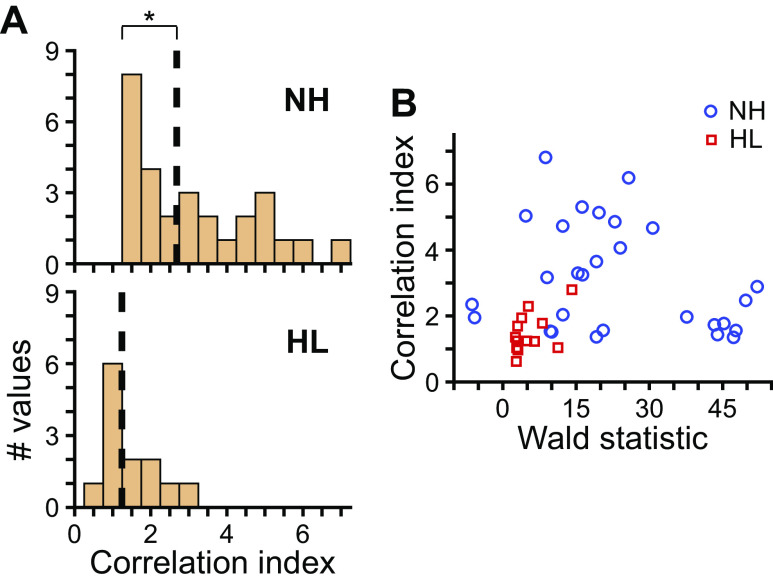
Although we could not test degradation of spike timing as a potential mechanism for the loss of ITD sensitivity in HL rabbits, the autocorrelogram analysis did allow us to simply compare spike-timing precision between neurons from NH and HL rabbits. Correlation indexes for neurons from NH rabbits spanned a range of values corresponding to high or low spike-timing precision, whereas those of neurons from HL rabbits mostly took on values corresponding to low spike-timing precision (P = 3 × 10−4, Wilcoxon rank-sum test; Fig. 9A). The correlation index of a neuron may potentially change based on the extent to which the neuron is driven by a stimulus. For example, if a stimulus weakly drives a neuron, a greater proportion of total spikes may occur spontaneously as opposed to in response to the stimulus than in the case of a stimulus that strongly drives the neuron; the former and latter cases could potentially lead to lower and higher correlation indexes, respectively. Because stimuli at near-threshold level were at lower sound levels with respect to neural threshold for neurons from HL rabbits (Fig. 4J), we considered the possibility that lower correlation indexes in neurons of HL rabbits may be because of weaker stimuli. To quantify the extent to which a neuron was driven by the monaural noise stimulus, we computed the Wald statistic between driven rate and spontaneous rate (see materials and methods). The Wald statistic is similar to a difference of firing rates but additionally accounts for the fact that firing rate variability across trials increases with mean firing rate. The greater the Wald statistic is from zero (in either direction), the greater the difference between driven and spontaneous rates. Wald statistics for neurons from HL rabbits all had relatively low values compared with those for neurons from NH rabbits (Fig. 9B), consistent with the discrepancy in sound level (re: neural threshold). However, neurons from NH rabbits that had Wald statistics that overlapped with those of neurons from HL rabbits had correlation indexes that spanned the range from low to high spike-timing precision. Therefore, the low spike-timing precision observed in neurons of HL rabbits could not be accounted for by neurons simply being driven less by the stimulus and was consistent with a previous study of IC in mice following near-complete cochlear denervation (Chambers et al. 2016).
Fig. 9.
Spike-timing precision following noise overexposure. A: histograms of correlation indexes of responses to monaural broadband noise at near-threshold level for neurons from normal-hearing (NH, top; N = 28 values from 15 neurons) and hearing loss (HL, bottom; N = 13 values from 11 neurons) rabbits. Data pooled across ipsi- and contralateral presentation. Values only included for responses that both significantly deviated from spontaneous rate (i.e., above threshold; P < 0.01, Wald test) and had sufficient total number of spikes to accurately estimate correlation index (see materials and methods). Broken lines indicate medians. *P < 0.05 (Wilcoxon rank-sum test). B: scatterplot of correlation index versus Wald statistic computed on firing rate to monaural noise and spontaneous rate (see materials and methods). The Wald statistic is a difference of firing rates scaled by a factor that accounts for the increase in firing rate variability with mean firing rate. Data from NH and HL rabbits indicated by blue circles and red squares, respectively.
DISCUSSION
We exposed rabbits to a high-intensity octave-band noise stimulus centered at 750 Hz, which caused a shift in ABR thresholds of 50 dB or greater at frequencies from 2 to 16 kHz and caused median neural sound-level threshold to increase by 75 dB for IC neurons from HL compared with NH rabbits. Directional sensitivity to broadband noise of IC neurons from HL rabbits was reduced compared with that from NH rabbits and could be accounted for by stimuli being subthreshold for some neurons and by HL rabbits having a greater proportion of monaurally sensitive neurons, which tended to have lower MInorm. Remaining directional sensitivity in binaurally sensitive neurons of HL rabbits was not significantly different from that of NH rabbits and was entirely the result of underlying sensitivity to ILD. We found no evidence for a decrease in neural sensitivity to ILD of broadband noise in neurons from HL rabbits but found a lack of neural sensitivity to ITD, which could not be accounted for by subthreshold stimuli, monaural sensitivity, stimulation near sound-level threshold, potential sample bias in CF, range of delays tested, or use of a broadband versus lowpass stimulus. Last, compared with NH rabbits, we found neurons from HL rabbits to have weaker spike-timing precision to broadband noise, slightly increased spontaneous rates, and to have lost the low-level tips of their FRAs, which in NH rabbits define the tonotopic map in the IC.
At two weeks following noise overexposure, ABR thresholds of all overexposed rabbits exceeded a critical shift at frequencies from 2 to 16 kHz, which according to our previous study (Haragopal et al. 2020), indicates that the basal and middle regions of their cochleae had very few surviving OHCs and variable loss of IHCs. Furthermore, it is likely that these rabbits had damage to the stereocilia of their IHCs and loss of synapses between IHCs and auditory nerve fibers because such damage is known to occur at exposure levels less than that which causes OHC loss (Borg et al. 1995; Kujawa and Liberman 2009). Our noise overexposure therefore succeeded in producing widespread damage of the cochlea. A consequence of the death of most OHCs is the elimination of the medial olivocochlear reflex, a feedback circuit that relies on the effect of medial olivocochlear neurons on OHCs mostly in the ear contralateral to the neurons. Therefore, binaural sensitivity in IC neurons of our HL rabbits cannot be explained by the medial olivocochlear reflex. In our previous study (Haragopal et al. 2020), the same noise overexposure as in the present study caused a 50-dB increase in median click-evoked ABR threshold, but there was high variability in threshold shifts across ears and rabbits. The 75-dB shift in neural sound-level thresholds from the three HL rabbits in the present study was therefore greater than that expected from average ABR threshold shifts of the previous study and was likely the result of the rabbits having higher-than-average susceptibility to overexposure.
The first of our two main research questions was whether or not directional sensitivity of IC neurons decreases with noise-induced hearing loss, the idea being that such a neural deficit may be a correlate of the deficit in sound localization of human subjects with SNHL (Lorenzi et al. 1999). We did find a decrease in directional sensitivity to broadband noise in neurons from HL rabbits, but the effect could be accounted for by two dependent variables: proportion of neurons for which the stimulus was subthreshold and proportion of monaurally sensitive neurons. The first of these dependent variables may seem trivial; however, if a stimulus is presented at a fixed sound level with respect to the perceptual threshold of detection (e.g., similar to amplification performed by a hearing aid), the proportion of neurons for which that stimulus is suprathreshold may differ between NH and HL listeners and may cause differences in localization ability. Our intention in choosing the near-threshold levels for NH and HL rabbits was to present sound at similar levels with respect to detection threshold. We assumed median pre- and postexposure ABR thresholds measured in our previous study (Haragopal et al. 2020) would be a sufficient means to select approximately equal sound levels with respect to detection threshold in NH and HL rabbits. However, our data proved this assumption to be incorrect. If we assume that detection threshold is equal to the lowest individual sound-level threshold across the neural sample, then detection thresholds were 5 and 75 dB SPL for NH and HL rabbits, respectively (Fig. 3B). Accordingly, selected near-threshold levels were 35–47.5 dB and 17.5–22.5 dB (re: detection threshold for NH and HL rabbits, respectively). Because these relative sound levels did not overlap, our data are inconclusive as to whether the greater proportion of neurons for which the stimulus was subthreshold and the greater proportion of monaurally sensitive neurons in HL rabbits were due to hearing loss or due to a lower sound level with respect to detectability. Furthermore, a previous study reported azimuth tuning curves measured at 10 dB (re: neural threshold) to be similar between binaural and contralateral-only sound presentation (see Kuwada et al. 2011), which suggests the greater proportion of monaurally sensitive neurons in HL rabbits may have been because of sound levels being near thresholds of individual neurons (Fig. 4J).
The second of our two main research questions was whether or not coding of ITD or ILD was affected by noise-induced hearing loss. Surprisingly, sensitivity to ILD of broadband noise remained unaltered at the population level, whereas sensitivity to ITD was absent in neurons from HL rabbits. Our result regarding ILD sensitivity was tempered by a smaller sample size (N = 13) after omitting data from neurons that were either monaurally sensitive or for which the stimulus was subthreshold. Nonetheless, the difference in median MInorm between neurons from NH and HL rabbits was small (Fig. 6J), which suggests any potentially significant difference for larger sample sizes would also be small. Our finding that ILD sensitivity remained stable after noise overexposure is consistent with reports that impairment of ILD discrimination in individuals with SNHL is either absent or small at best (Gabriel et al. 1992; Häusler et al. 1983; Smith-Olinde et al. 1998; Spencer et al. 2016). Our finding that ITD sensitivity to broadband noise was completely lost after noise overexposure suggests that behavioral ITD discrimination, at least for broadband noise stimuli, would be near impossible, but previous reports show that ITD discrimination is only impaired, not abolished (Best and Swaminathan 2019; Gabriel et al. 1992; Häusler et al. 1983; Lacher-Fougère and Demany 2005; Smith-Olinde et al. 1998; Smoski and Trahiotis 1986; Spencer et al. 2016). However, some studies showed that individuals with the greatest hearing loss had extremely large ITD discrimination thresholds (Best and Swaminathan 2019; Lacher-Fougère and Demany 2005), so the extreme loss of neural ITD sensitivity in the present study may potentially be because of the severity of the hearing loss.
In neurons from NH rabbits, many azimuth tuning curves were influenced by sensitivity to both ITD and ILD and fewer by ILD alone (Fig. 6D). ITD influenced directional sensitivity across the tonotopic map (Fig. 6I, open symbols). Therefore, some of the neurons from HL rabbits likely had azimuth tuning curves that were shaped by both cues before overexposure, and after overexposure only sensitivity to ILD remained, causing a change in shape of the azimuth tuning curve. On the one hand, downstream areas of the brain may depend on consistency of the shapes of azimuth tuning curves of ICC neurons over time and, therefore, a potential change in tuning curve shape with hearing loss may contribute to a deficit in sound localization. On the other hand, downstream areas may be adaptable to changes in tuning curve shapes over time and only depend on the ability of neurons to transmit information about azimuth in their firing rates. In the latter case, noise-induced hearing loss would not affect the localization of high-frequency sounds, which primarily depend upon ILD cues (Dorkoski et al. 2020; Macpherson and Middlebrooks 2002; Strutt 1907). Localization of low-frequency sounds primarily depends on ITD cues (Dorkoski et al. 2020; Macpherson and Middlebrooks 2002; Strutt 1907). In a recent study, we showed that, at a sound level of 70 dB SPL, the firing rates of high-CF neurons contribute substantial information about the azimuth of low-frequency noise stimuli, and this information was based on sensitivity to ITD (Dorkoski et al. 2020). Because our sample of neurons from HL rabbits was likely biased toward high CFs, our results suggest that with noise-induced hearing loss the contribution of information from high-CF neurons regarding the direction of low-frequency noise stimuli is absent.
We predicted that CFs in our neural sample from HL rabbits were high and narrowly distributed around 8 kHz (Fig. 2D). Previous studies have shown that ITD sensitivity to broadband noise in ICC neurons with CFs above 4 kHz is exclusive to the temporal envelope, as opposed to the temporal fine structure, of the cochlear-filtered acoustic waveform (Devore and Delgutte 2010; Joris 2003). It is likely that any ITD sensitivity to broadband noise that existed in our neurons from HL rabbits preexposure was specifically envelope ITD sensitivity. However, auditory filters are known to widen after acoustic trauma. Henry et al. (2016) found that high-CF auditory nerve fibers switched from exclusively encoding high-frequency temporal envelope structure to both temporal envelope and fine structure at low or high frequencies following acoustic trauma. Therefore, it is possible that neurons from our HL rabbits additionally encoded fine structure postexposure. Whereas we attempted to find evidence of fine-structure ITD sensitivity using lowpass noise, this stimulus was below threshold for most neurons. A Wiener-kernel analysis of responses to broadband noise, like that performed in Henry et al. (2016), would be necessary to determine if the loss of ITD sensitivity observed in our data was specific to envelope or both envelope and fine structure.
The main limitations of our study were the presentation of stimuli at different relative sound levels for NH and HL rabbits, which confounded the effect of hearing loss with that of level differences, and the sample bias of neurons from HL rabbits toward high CFs. These limitations arose both because the degree of hearing loss was more severe than expected and because we were limited by the output ceiling of our earphones. The output ceiling is a technical limitation that can theoretically be surmounted, but testing with sound levels >100 dB SPL daily for multiple months runs the risk of creating further damage to the cochleae. Furthermore, humans with SNHL have an abnormally steep relationship between stimulus intensity and perceived loudness, a phenomenon termed loudness recruitment. Whereas our overexposed rabbits showed no signs of discomfort with sound levels near 100 dB SPL, more intense stimuli may be perceived by the rabbit as uncomfortably loud. In future studies, it may be advantageous to induce a lesser degree of hearing loss to see if results for severe hearing loss in the present study hold. Moderate hearing loss would also resolve the limitations of the present study by increasing the range of sound levels between neural threshold and earphone output ceiling. However, control over the degree of hearing loss is not straightforward: susceptibility varies widely across individuals, and we previously found median ABR threshold shift to be only 10 dB for a shortened duration of 60 min at the same exposure level (Haragopal et al. 2020). One way to induce a more moderate hearing loss (e.g., a 30-dB threshold shift) may be to present noise at a slightly lower sound level for a much longer duration.
Comparison with previous studies.
Few studies have investigated the effect of SNHL on binaural coding in the brain; those that have specifically investigated age-related, and not noise-induced, hearing loss. Laumen et al. (2016) found the binaural interaction component of the ABR (difference between response to binaural and monaural presentation) to be smaller in aged gerbils, suggesting weakened binaural interaction in central auditory areas. Our finding of reduced number of ICC neurons with binaural sensitivity in HL rabbits is consistent with this; however it is confounded by the different relative sound levels used for NH and HL rabbits, as described above. Costa et al. (2016) found that azimuth tuning curves of superior colliculus neurons in aged rats were wider than in younger rats. Because directional sensitivity in the superior colliculus likely derives from ILD sensitivity (Slee and Young 2014, 2013), their result suggests a decrease in ILD sensitivity, unlike in the present study. Neural sound-level thresholds in their aged rats were only 8 dB greater than in younger rats; therefore, the difference between the two studies may be because of different effects of age-related versus noise-induced hearing loss, or difference in brain areas.
Most relevant are two studies by McFadden and Willott (1994a, 1994b) of binaural and spatial coding in IC neurons of C57 mice, a strain with early onset hearing loss. They found only a small decrease in directional sensitivity of IC neurons from aged compared with young mice. Interestingly, in a tone-in-noise detection paradigm, they found that the sound-level threshold for detection of a tone based on changes in firing rate was not different between cases where the masking noise was spatially colocated or separated from the tone. Normally a tone can be detected at a lesser threshold when the masker is spatially separated, a phenomenon known as “spatial release from masking,” which can be explained by neural sensitivity to interaural correlation (Lane and Delgutte 2005). Their result implies a deficit in neural sensitivity to interaural correlation, consistent with the deficit in ITD sensitivity found in our data.
Potential mechanisms for loss of ITD sensitivity.
Sensitivity of firing rates to ITD first arises in neurons of the medial and lateral superior olives (MSO and LSO, respectively) via the detection of coincident excitatory synaptic inputs from the ipsi- and contralateral ventral cochlear nucleus (VCN), whose responses are precisely time locked to the sound waveform (Franken et al. 2015; Joris et al. 1994a, 1994b; Plauška et al. 2016; van der Heijden et al. 2013). [The contralateral VCN input to the LSO is by way of the medial nucleus of the trapezoid body, which retains the spike-timing precision of the VCN. (Paolini et al. 2001)]. ITD sensitivity in the ICC is thought to be inherited from synaptic inputs from these SOC nuclei. Degradation of ITD sensitivity in the ICC may therefore develop in response to any of the following: 1) a decrease in spike-timing precision of VCN neurons; 2) a widening of the effective coincidence detection window of SOC neurons; or 3) a change in the input/output function of ICC neurons such that ITD sensitivity in the synaptic input from SOC is nullified in the output firing rate.
Previous studies in MSO were able to accurately predict ITD tuning from the comparison of spike trains in response to the stimulus presented separately to the ipsi- and contralateral ears (Goldberg and Brown 1969; Yin and Chan 1990). This prediction was accurate presumably because the temporal precision of action potentials of MSO neurons was faithful to the temporal precision of their synaptic inputs. To the best of our knowledge, the same result has not been reported in the ICC. In their paper, Yin and Chan (1990) implied the same test could not be performed in the ICC because “cells in the ICC generally do not respond to monaural stimulation of both ears…” (p. 476). We found that ICC neurons readily responded to stimulation of either ear. Their difficulty in obtaining monaural responses to both ears may have been because of use of an anesthetized preparation. Nonetheless, we found no relationship between spike-timing precision of responses to monaural broadband noise and ITD sensitivity in ICC neurons of NH rabbits. Therefore, we were unable to infer from our data potential changes in spike-timing precision of VCN neurons from HL rabbits. Although spike-timing precision was lower in ICC neurons of HL compared with NH rabbits, the same could not necessarily be inferred for VCN neurons. Previous studies of auditory nerve (AN) fibers found no deficit in temporal precision following either acoustic trauma except in the presence of background noise (Henry and Heinz 2012; Henry et al. 2014; Kale and Heinz 2010), or age-related hearing loss (Heeringa et al. 2020). To the best of our knowledge, the effect of acoustic trauma on temporal precision of VCN neurons in vivo has not been reported. VCN neurons in a strain of mice with early onset hearing loss have been shown to have weaker entrainment to a volley of excitatory synaptic potentials in vitro, but no increase in jitter (Wang and Manis 2006). However, acoustic trauma has been shown to be correlated with degeneration and hyperactivity of VCN neurons (Sekiya et al. 2012; Vogler et al. 2011).
In MSO, the temporal window for detection of coincident synaptic inputs from the ipsi- and contralateral VCN is regulated by several factors, including activation of low-voltage-activated K+ channels (Kv1) and hyperpolarization-activated cation current (Ih), glycinergic inhibition from the medial and lateral nuclei of the trapezoid body, and activation of GABAB receptors (Fischl et al. 2012; Khurana et al. 2011; Mathews et al. 2010; Roberts et al. 2013; Svirskis et al. 2002). As of yet, no studies have examined the effect of acoustic trauma on these factors. In LSO, acoustic trauma in mice led to an increase in the decay time of excitatory postsynaptic currents evoked by shocks to VCN axons, but this effect reversed to normal after two months (Pilati et al. 2016). Kv1 currents also likely regulate the coincidence detection window in principal neurons of the LSO (Barnes-Davies et al. 2004). Changes in ion channel distribution may occur following decreased auditory input. For example, in a study of the avian cochlear nucleus, K+ channels in the axon initial segment redistributed subtypes from fast to slow kinetics following removal of the cochlea, leading to enhanced excitability (Kuba et al. 2015).
Delgutte and colleagues found ITD sensitivity in most IC neurons of deaf animals (induced or congenital) when stimulated bilaterally with electric pulse trains via cochlear implants (Chung et al. 2016; Hancock et al. 2013; Smith and Delgutte 2007). That ITD sensitivity existed in these IC neurons is inconsistent with the third hypothetical mechanism listed above, that following SNHL the function of ICC neurons on their inputs nullifies ITD sensitivity. Furthermore, their neurons were sensitive to submillisecond changes in ITD suggesting the coincidence detection window was uncompromised. Electric pulses directly stimulate the auditory nerve and likely produce higher spike-timing precision in AN fibers than that of acoustic stimuli. Such artificially precise timing could potentially overcome possible increased spike-timing jitter of VCN neurons following SNHL. Although we measured ITD sensitivity only with broadband noise, their results suggest ITD sensitivity may be retained with an acoustic stimulus that causes higher spike-timing precision, such as click trains, pure tones, or amplitude-modulated tones. However, it is important to note that their method of deafening (ototoxic drugs or hypotonic stress) only killed cochlear hair cells, whereas acoustic trauma additionally overstimulates the central auditory system.
A final important question regarding our results is: why would ITD sensitivity be eliminated but ILD sensitivity be spared? The answer may be related to the finding that ILD sensitivity depends on binaural integration within a relatively large temporal window (>3 ms; Brown and Tollin 2016), whereas ITD sensitivity depends on coincidence detection within a submillisecond window. Potential disruption of temporal precision at the level of the SOC (either in the inputs or the coincidence window) would therefore be expected to have a larger effect on ITD than ILD sensitivity. Interestingly, a recent study in LSO showed that principal neurons, the most numerous subtype, integrate excitatory and inhibitory inputs from the ipsi- and contralateral ears, respectively, over a time scale similar to that of MSO neurons, whereas nonprincipal neurons integrate the same inputs over longer time scales (Franken et al. 2018). Disruption of temporal precision would therefore be expected to have a greater effect on principal than nonprincipal LSO neurons. Last, ILD sensitivity of ICC neurons is not only inherited from LSO neurons but additionally arises because of interaction between many excitatory and inhibitory projections to the area [review by Pollak (2012)]. Potential disruption to ILD sensitivity in the LSO caused by acoustic trauma may be masked by de novo ILD sensitivity of the ICC.
GRANTS
This research was supported by the National Institute on Deafness and Other Communication Disorders under award numbers R03-DC-013388 and R15-DC-017616 (M.D.) and by an internal award from Ohio University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.H. and M.L.D. conceived and designed research; H.H., R.D., A.R.P., G.A.W., T.R.W., N.C.S., and M.L.D. performed experiments; H.H., R.D., A.R.P., G.A.W., T.R.W., N.C.S., and M.L.D. analyzed data; H.H. and M.L.D. interpreted results of experiments; H.H. and M.L.D. prepared figures; H.H. and M.L.D. drafted manuscript; H.H. and M.L.D. edited and revised manuscript; H.H. and M.L.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ken Hancock for software support and Kinzie Bailey and Lukas Palmer for assisting with data collection.
REFERENCES
- Aitkin LM, Fryman S, Blake DW, Webster WR. Responses of neurones in the rabbit inferior colliculus. I. Frequency-specificity and topographic arrangement. Brain Res 47: 77–90, 1972. doi: 10.1016/0006-8993(72)90253-3. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci 19: 325–333, 2004. doi: 10.1111/j.0953-816X.2003.03133.x. [DOI] [PubMed] [Google Scholar]
- Best V, Swaminathan J. Revisiting the detection of interaural time differences in listeners with hearing loss. J Acoust Soc Am 145: EL508–EL513, 2019. doi: 10.1121/1.5111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg E, Canlon B, Engström B. Noise-induced hearing loss. Literature review and experiments in rabbits. Morphological and electrophysiological features, exposure parameters and temporal factors, variability and interactions. Scand Audiol Suppl 40: 1–147, 1995. [PubMed] [Google Scholar]
- Brown AD, Tollin DJ. Slow temporal integration enables robust neural coding and perception of a cue to sound source location. J Neurosci 36: 9908–9921, 2016. doi: 10.1523/JNEUROSCI.1421-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll YI, Eichwald J, Scinicariello F, Hoffman HJ, Deitchman S, Radke MS, Themann CL, Breysse P. Vital signs: noise-induced hearing loss among adults—United States 2011–2012. MMWR Morb Mortal Wkly Rep 66: 139–144, 2017. doi: 10.15585/mmwr.mm6605e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89: 867–879, 2016. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase SM, Young ED. Limited segregation of different types of sound localization information among classes of units in the inferior colliculus. J Neurosci 25: 7575–7585, 2005. doi: 10.1523/JNEUROSCI.0915-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Hancock KE, Delgutte B. Neural coding of interaural time differences with bilateral cochlear implants in unanesthetized rabbits. J Neurosci 36: 5520–5531, 2016. doi: 10.1523/JNEUROSCI.3795-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody AR, Robertson D. Variability of noise-induced damage in the guinea pig cochlea: electrophysiological and morphological correlates after strictly controlled exposures. Hear Res 9: 55–70, 1983. doi: 10.1016/0378-5955(83)90134-X. [DOI] [PubMed] [Google Scholar]
- Costa M, Lepore F, Prévost F, Guillemot JP. Effects of aging on peripheral and central auditory processing in rats. Eur J Neurosci 44: 2084–2094, 2016. doi: 10.1111/ejn.13302. [DOI] [PubMed] [Google Scholar]
- Cover TM, Thomas JA. Elements of Information Theory. Hoboken, NJ: Wiley, 2006. [Google Scholar]
- Dai L, Best V, Shinn-Cunningham BG. Sensorineural hearing loss degrades behavioral and physiological measures of human spatial selective auditory attention. Proc Natl Acad Sci USA 115: E3286–E3295, 2018. doi: 10.1073/pnas.1721226115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Delgutte B. Decoding sound source location and separation using neural population activity patterns. J Neurosci 33: 15837–15847, 2013. doi: 10.1523/JNEUROSCI.2034-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Delgutte B. Neural population encoding and decoding of sound source location across sound level in the rabbit inferior colliculus. J Neurophysiol 115: 193–207, 2016. doi: 10.1152/jn.00643.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Koka K, Delgutte B. Neural encoding of sound source location in the presence of a concurrent, spatially separated source. J Neurophysiol 108: 2612–2628, 2012. doi: 10.1152/jn.00303.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore S, Delgutte B. Effects of reverberation on the directional sensitivity of auditory neurons across the tonotopic axis: influences of interaural time and level differences. J Neurosci 30: 7826–7837, 2010. doi: 10.1523/JNEUROSCI.5517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorkoski R, Hancock KE, Whaley GA, Wohl TR, Stroud NC, Day ML. Stimulus-frequency-dependent dominance of sound localization cues across the cochleotopic map of the inferior colliculus. J Neurophysiol 123: 1791–1807, 2020. doi: 10.1152/jn.00713.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl MJ, Combs TD, Klug A, Grothe B, Burger RM. Modulation of synaptic input by GABAB receptors improves coincidence detection for computation of sound location. J Physiol 590: 3047–3066, 2012. doi: 10.1113/jphysiol.2011.226233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken TP, Joris PX, Smith PH. Principal cells of the brainstem’s interaural sound level detector are temporal differentiators rather than integrators. eLife 7: e33854, 2018. doi: 10.7554/eLife.33854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken TP, Roberts MT, Wei L, Golding NL, Joris PX. In vivo coincidence detection in mammalian sound localization generates phase delays. Nat Neurosci 18: 444–452, 2015. doi: 10.1038/nn.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel KJ, Koehnke J, Colburn HS. Frequency dependence of binaural performance in listeners with impaired binaural hearing. J Acoust Soc Am 91: 336–347, 1992. doi: 10.1121/1.402776. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Hancock KE, Chung Y, Delgutte B. Congenital and prolonged adult-onset deafness cause distinct degradations in neural ITD coding with bilateral cochlear implants. J Assoc Res Otolaryngol 14: 393–411, 2013. doi: 10.1007/s10162-013-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haragopal H, Dorkoski R, Johnson HM, Berryman MA, Tanda S, Day ML. Paired measurements of cochlear function and hair cell count in Dutch-belted rabbits with noise-induced hearing loss. Hear Res 385: 107845, 2020. doi: 10.1016/j.heares.2019.107845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler R, Colburn S, Marr E. Sound localization in subjects with impaired hearing. Spatial-discrimination and interaural-discrimination tests. Acta Otolaryngol Suppl 400: 1–62, 1983. doi: 10.3109/00016488309105590. [DOI] [PubMed] [Google Scholar]
- Heeringa AN, Zhang L, Ashida G, Beutelmann R, Steenken F, Köppl C. Temporal coding of single auditory nerve fibers is not degraded in aging gerbils. J Neurosci 40: 343–354, 2020. doi: 10.1523/JNEUROSCI.2784-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner H, Masterton B. Hearing in Glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am 68: 1584–1599, 1980. doi: 10.1121/1.385213. [DOI] [Google Scholar]
- Henry KS, Heinz MG. Diminished temporal coding with sensorineural hearing loss emerges in background noise. Nat Neurosci 15: 1362–1364, 2012. doi: 10.1038/nn.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Kale S, Heinz MG. Noise-induced hearing loss increases the temporal precision of complex envelope coding by auditory-nerve fibers. Front Syst Neurosci 8: 20, 2014. doi: 10.3389/fnsys.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Kale S, Heinz MG. Distorted tonotopic coding of temporal envelope and fine structure with noise-induced hearing loss. J Neurosci 36: 2227–2237, 2016. doi: 10.1523/JNEUROSCI.3944-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind JE, Goldberg JM, Greenwood DD, Rose JE. Some discharge characteristics of single neurons in the inferior colliculus of the cat. II. Timing of the discharges and observations on binaural stimulation. J Neurophysiol 26: 321–341, 1963. doi: 10.1152/jn.1963.26.2.321. [DOI] [PubMed] [Google Scholar]
- Izquierdo MA, Gutiérrez-Conde PM, Merchán MA, Malmierca MS. Non-plastic reorganization of frequency coding in the inferior colliculus of the rat following noise-induced hearing loss. Neuroscience 154: 355–369, 2008. doi: 10.1016/j.neuroscience.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Joris PX. Interaural time sensitivity dominated by cochlea-induced envelope patterns. J Neurosci 23: 6345–6350, 2003. doi: 10.1523/JNEUROSCI.23-15-06345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Carney LH, Smith PH, Yin TC. Enhancement of neural synchronization in the anteroventral cochlear nucleus. I. Responses to tones at the characteristic frequency. J Neurophysiol 71: 1022–1036, 1994a. doi: 10.1152/jn.1994.71.3.1022. [DOI] [PubMed] [Google Scholar]
- Joris PX, Louage DH, Cardoen L, van der Heijden M. Correlation index: a new metric to quantify temporal coding. Hear Res 216-217: 19–30, 2006. doi: 10.1016/j.heares.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TC. Enhancement of neural synchronization in the anteroventral cochlear nucleus. II. Responses in the tuning curve tail. J Neurophysiol 71: 1037–1051, 1994b. doi: 10.1152/jn.1994.71.3.1037. [DOI] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Envelope coding in auditory nerve fibers following noise-induced hearing loss. J Assoc Res Otolaryngol 11: 657–673, 2010. doi: 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Remme MW, Rinzel J, Golding NL. Dynamic interaction of Ih and IK-LVA during trains of synaptic potentials in principal neurons of the medial superior olive. J Neurosci 31: 8936–8947, 2011. doi: 10.1523/JNEUROSCI.1079-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DO, Bishop B, Kuwada S. Acoustic cues for sound source distance and azimuth in rabbits, a racquetball and a rigid spherical model. J Assoc Res Otolaryngol 11: 541–557, 2010. doi: 10.1007/s10162-010-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Yamada R, Ishiguro G, Adachi R. Redistribution of Kv1 and Kv7 enhances neuronal excitability during structural axon initial segment plasticity. Nat Commun 6: 8815, 2015. doi: 10.1038/ncomms9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29: 14077–14085, 2009. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Bishop B, Alex C, Condit DW, Kim DO. Spatial tuning to sound-source azimuth in the inferior colliculus of unanesthetized rabbit. J Neurophysiol 106: 2698–2708, 2011. doi: 10.1152/jn.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacher-Fougère S, Demany L. Consequences of cochlear damage for the detection of interaural phase differences. J Acoust Soc Am 118: 2519–2526, 2005. doi: 10.1121/1.2032747. [DOI] [PubMed] [Google Scholar]
- Lane CC, Delgutte B. Neural correlates and mechanisms of spatial release from masking: single-unit and population responses in the inferior colliculus. J Neurophysiol 94: 1180–1198, 2005. doi: 10.1152/jn.01112.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumen G, Tollin DJ, Beutelmann R, Klump GM. Aging effects on the binaural interaction component of the auditory brainstem response in the Mongolian gerbil: Effects of interaural time and level differences. Hear Res 337: 46–58, 2016. doi: 10.1016/j.heares.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol 12: 647–658, 2011. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi C, Gatehouse S, Lever C. Sound localization in noise in hearing-impaired listeners. J Acoust Soc Am 105: 3454–3463, 1999. doi: 10.1121/1.424672. [DOI] [PubMed] [Google Scholar]
- Louage DH, van der Heijden M, Joris PX. Temporal properties of responses to broadband noise in the auditory nerve. J Neurophysiol 91: 2051–2065, 2004. doi: 10.1152/jn.00816.2003. [DOI] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res 212: 9–21, 2006. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Listener weighting of cues for lateral angle: the duplex theory of sound localization revisited. J Acoust Soc Am 111: 2219–2236, 2002. doi: 10.1121/1.1471898. [DOI] [PubMed] [Google Scholar]
- Marrone N, Mason CR, Kidd G Jr. The effects of hearing loss and age on the benefit of spatial separation between multiple talkers in reverberant rooms. J Acoust Soc Am 124: 3064–3075, 2008. doi: 10.1121/1.2980441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by K(v)1 channels. Nat Neurosci 13: 601–609, 2010. doi: 10.1038/nn.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Willott JF. Responses of inferior colliculus neurons in C57BL/6J mice with and without sensorineural hearing loss: effects of changing the azimuthal location of a continuous noise masker on responses to contralateral tones. Hear Res 78: 132–148, 1994a. doi: 10.1016/0378-5955(94)90019-1. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Willott JF. Responses of inferior colliculus neurons in C57BL/6J mice with and without sensorineural hearing loss: effects of changing the azimuthal location of an unmasked pure-tone stimulus. Hear Res 78: 115–131, 1994b. doi: 10.1016/0378-5955(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Guinan JJ Jr, Knudson IM, Kiang NY. Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hear Res 93: 28–51, 1996. doi: 10.1016/0378-5955(95)00179-4. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain Res 77: 397–415, 1974. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience 164: 733–746, 2009. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Shackleton TM, Sumner CJ, Zobay O, Rees A. Classification of frequency response areas in the inferior colliculus reveals continua not discrete classes. J Physiol 591: 4003–4025, 2013. doi: 10.1113/jphysiol.2013.255943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hear Res 159: 101–116, 2001. doi: 10.1016/S0378-5955(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Pilati N, Linley DM, Selvaskandan H, Uchitel O, Hennig MH, Kopp-Scheinpflug C, Forsythe ID. Acoustic trauma slows AMPA receptor-mediated EPSCs in the auditory brainstem, reducing GluA4 subunit expression as a mechanism to rescue binaural function. J Physiol 594: 3683–3703, 2016. doi: 10.1113/JP271929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plauška A, Borst JG, van der Heijden M. Predicting binaural responses from monaural responses in the gerbil medial superior olive. J Neurophysiol 115: 2950–2963, 2016. doi: 10.1152/jn.01146.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD. Circuits for processing dynamic interaural intensity disparities in the inferior colliculus. Hear Res 288: 47–57, 2012. doi: 10.1016/j.heares.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Seeman SC, Golding NL. A mechanistic understanding of the role of feedforward inhibition in the mammalian sound localization circuitry. Neuron 78: 923–935, 2013. doi: 10.1016/j.neuron.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Viberg A, Kojima K, Sakamoto T, Nakagawa T, Ito J, Canlon B. Trauma-specific insults to the cochlear nucleus in the rat. J Neurosci Res 90: 1924–1931, 2012. doi: 10.1002/jnr.23093. [DOI] [PubMed] [Google Scholar]
- Slee SJ, Young ED. Linear processing of interaural level difference underlies spatial tuning in the nucleus of the brachium of the inferior colliculus. J Neurosci 33: 3891–3904, 2013. doi: 10.1523/JNEUROSCI.3437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee SJ, Young ED. Alignment of sound localization cues in the nucleus of the brachium of the inferior colliculus. J Neurophysiol 111: 2624–2633, 2014. doi: 10.1152/jn.00885.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B. Sensitivity to interaural time differences in the inferior colliculus with bilateral cochlear implants. J Neurosci 27: 6740–6750, 2007. doi: 10.1523/JNEUROSCI.0052-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Olinde L, Koehnke J, Besing J. Effects of sensorineural hearing loss on interaural discrimination and virtual localization. J Acoust Soc Am 103: 2084–2099, 1998. doi: 10.1121/1.421355. [DOI] [PubMed] [Google Scholar]
- Smoski WJ, Trahiotis C. Discrimination of interaural temporal disparities by normal-hearing listeners and listeners with high-frequency sensorineural hearing loss. J Acoust Soc Am 79: 1541–1547, 1986. doi: 10.1121/1.393680. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hawley ML, Colburn HS. Relating interaural difference sensitivities for several parameters measured in normal-hearing and hearing-impaired listeners. J Acoust Soc Am 140: 1783–1799, 2016. doi: 10.1121/1.4962444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt JW. On our perception of sound direction. Philos Mag 13: 214–232, 1907. doi: 10.1080/14786440709463595. [DOI] [Google Scholar]
- Svirskis G, Kotak V, Sanes DH, Rinzel J. Enhancement of signal-to-noise ratio and phase locking for small inputs by a low-threshold outward current in auditory neurons. J Neurosci 22: 11019–11025, 2002. doi: 10.1523/JNEUROSCI.22-24-11019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden M, Lorteije JA, Plauška A, Roberts MT, Golding NL, Borst JG. Directional hearing by linear summation of binaural inputs at the medial superior olive. Neuron 78: 936–948, 2013. doi: 10.1016/j.neuron.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci 31: 6639–6645, 2011. doi: 10.1523/JNEUROSCI.6538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J Assoc Res Otolaryngol 7: 412–424, 2006. doi: 10.1007/s10162-006-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman L. All of Statistics. New York: Springer, 2004. [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]