Abstract
Area CA3 in the hippocampus is traditionally thought to act as a homogeneous neural circuit that is vital for spatial navigation and episodic memories. However, recent studies have revealed that CA3 pyramidal neurons in dorsal hippocampus display marked anatomic and functional heterogeneity along the proximodistal (transverse) axis. The hippocampus is also known to be functionally segregated along the dorsoventral (longitudinal) axis, with dorsal hippocampus strongly involved in spatial navigation and ventral hippocampus associated with emotion and anxiety. Surprisingly, however, relatively little is known about CA3 functional heterogeneity along the dorsoventral axis. Here, we carried out mouse-brain-slice patch-clamp recordings and morphological analyses to examine the heterogeneity of CA3 cellular properties along both proximodistal and dorsoventral axes. We find that CA3 pyramidal neurons exhibit considerable heterogeneity of somatodendritic morphology and intrinsic membrane properties, with ventral CA3 (vCA3) displaying more elaborate somatodendritic morphology, lower intrinsic excitability, smaller input resistance, greater cell capacitance, and more prominent hyperpolarization‐activated current than dorsal CA3 (dCA3). Furthermore, although both dCA3 and vCA3 exhibit proximal-to-distal gradients in intrinsic properties and neuronal morphology, these proximal-to-distal gradients in vCA3 are more moderate than those in dCA3. Taken together, our results extend previous findings on the proximodistal heterogeneity of dCA3 function and uncover a complex, yet orderly, pattern of topographic organization of CA3 neuronal features that extends to multiple anatomic dimensions and may contribute to its in vivo functional diversity.
NEW & NOTEWORTHY Area CA3 is a major hippocampal region that is classically thought to act as a homogeneous neural network vital for spatial navigation and episodic memories. Here, we report that CA3 pyramidal neurons exhibit marked heterogeneity of somatodendritic morphology and cellular electrical properties along both proximodistal and dorsoventral axes. These new results uncover a complex, yet orderly, pattern of topographic organization of CA3 neuronal features that may contribute to its in vivo functional diversity.
Keywords: CA3, dendritic morphology, hippocampus, input resistance, intrinsic excitability, pyramidal neuron
INTRODUCTION
Area CA3, situated in the middle of the classic trisynaptic circuit (entorhinal cortex → dentate gyrus → CA3 → CA1), is a major hippocampal region that is vital for spatial navigation and memory formation (Kesner 2007; Nakashiba et al. 2008; Nakazawa et al. 2002; Rebola et al. 2017; Rolls 2013a, 2013b; Witter 2007). Although Lorente De Nó in his 1934 landmark paper presciently divided CA3 into a, b, and c subregions along the proximodistal (transverse) axis (Lorente De Nó 1934), presumably based on morphological observation using Golgi staining, the functional heterogeneity of CA3 remains relatively understudied for many decades. The early intracellular recordings in guinea pigs have provided the first evidence of proximodistal variations in CA3 dendritic morphology and electrophysiological properties (Bilkey and Schwartzkroin 1990; Masukawa et al. 1982). More recently, a number of studies, including ours, have provided additional evidence that CA3 pyramidal neurons are highly heterogeneous in molecular profiles, dendritic morphology, cellular electrical properties, synaptic connectivity, place cell properties, and behavioral roles (Cembrowski and Spruston 2019; Hunsaker et al. 2008; Hunt et al. 2018; Ishizuka et al. 1995; Kowalski et al. 2016; Lee et al. 2015; Lu et al. 2015; Nakamura et al. 2013; Oliva et al. 2016; Sun et al. 2017; Thompson et al. 2008; Turner et al. 1995). Surprisingly, however, despite the well-known dorsoventral segregation of hippocampal function (Fanselow and Dong 2010; Moser et al. 1993; Strange et al. 2014), little is known about the dorsoventral heterogeneity of intrinsic cellular properties of CA3 pyramidal neurons, compared with the neighboring CA1 (Dougherty et al. 2012; Malik et al. 2016; Masurkar et al. 2020; Papatheodoropoulos 2018).
Intrinsic excitability of individual neurons, determined by neuronal morphology and the expression of various ion conductances, can crucially influence action potential (AP) output (Zhang and Linden 2003). Persistent changes in intrinsic excitability are thought to play an important role in memory storage (Zhang and Linden 2003), stabilize network activity in response to prolonged perturbation of activity (Desai et al. 1999; Turrigiano 2011), and are involved in a number of brain disorders (Beck and Yaari 2008). In fact, long-term plasticity or modification in CA3 intrinsic excitability has been observed under both normal and diseased conditions (Cellot et al. 2016; Hyun et al. 2013; Milshtein-Parush et al. 2017; Simkin et al. 2015; Soldado-Magraner et al. 2020; Tamir et al. 2017). However, less is known about variations of intrinsic excitability and dendritic morphology of CA3 pyramidal neurons at different geometric positions.
We (Sun et al. 2017) have previously uncovered the marked proximal-to-distal decreasing gradient in intrinsic excitability in dorsal CA3 (dCA3) pyramidal neurons. In this study, we carried out whole cell patch-clamp recordings and Golgi-staining morphological analysis to examine the proximodistal and dorsoventral topographic heterogeneity of neuronal morphology and intrinsic properties of CA3 pyramidal neurons. We demonstrated that intrinsic membrane properties of CA3 pyramidal neurons vary substantially along both dorsoventral and proximodistal axes. Specifically, we found that, in sharp contrast to a dorsal-to-ventral increasing gradient of intrinsic excitability in CA1 (Dougherty et al. 2012; Malik et al. 2016; Marcelin et al. 2012a), ventral CA3 (vCA3) pyramidal neurons were significantly less excitable and displayed lower input resistance, greater hyperpolarization‐activated current (Ih), and more complex somatodendritic structure compared with dCA3. This dorsoventral difference was particularly striking in proximal CA3 (CA3c) that is adjacent to dentate gyrus (DG). Furthermore, we show that both dCA3 and vCA3 pyramidal neurons exhibited proximal-to-distal gradients of intrinsic properties, but the gradients in vCA3 were more moderate compared with dCA3. Taken together, this study extends our (Sun et al. 2017) previous findings on proximodistal heterogeneity in dCA3 and provides new insights into topographic organization of CA3 cellular properties.
METHODS
Mice.
Eighteen C57BL/6 male and female mice of 2–5 mo of age were used in the electrophysiological experiments. Five C57BL/6 male mice of 3 mo of age were used in Golgi-staining experiments. Mice were housed in an animal facility on a 12:12-h light-dark cycle with ad libitum access to food and water. The procedures described were conducted in accordance with National Institutes of Health regulations and approved by the Institutional Animal Care and Use Committees of Case Western Reserve University and Columbia University.
Hippocampal slice preparation.
Transverse hippocampal slices were prepared from 2- to 5-mo-old C57BL/6 wild-type mice as described previously (Sun et al. 2014). In brief, animals were anesthetized with isoflurane and euthanized by decapitation in accordance with institutional regulations. Both left and right hippocampi were dissected out from the mouse brain, and the entire hippocampus was embedded in a block of premade agar (4%). The transverse hippocampal slices (400 µm thick) were then cut from dorsal to ventral on a Leica VT1200 S vibratome (Leica Biosystems) in ice-cold dissection solution containing (in mM): 10 NaCl, 195 sucrose, 2.5 KCl, 10 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 Na-Pyruvate, 0.5 CaCl2 and 7 MgCl2. The slices were then incubated at 33°C in artificial cerebrospinal fluid (in mM: 125 NaCl, 2.5 KCl, 20 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 Na-pyruvate, 2 CaCl2, and 1 MgCl2) for 20–30 min and then kept at room temperature for ≥1.5 h before transfer to the recording chamber. Cutting and recording solutions were both saturated with 95% O2-5% CO2 (pH 7.4). All electrophysiological recordings were performed at 31–32°C.
Whole cell patch-clamp recording.
Whole cell recordings were obtained from pyramidal neurons using the “blind” patch-clamp technique, as described previously (Sun et al. 2017). The patch pipettes were pulled from borosilicate capillary glass using a P-97 Flaming/Brown Micropipette Puller (Sutter Instrument, Novato, CA) and had resistances ranging from 4 to 6 MΩ. The pipettes were filled with an intracellular solution of the following chemicals (in mM): 135 K-gluconate, 5 KCl, 0.1 EGTA-Na, 10 HEPES, 2 NaCl, 5 ATP, 0.4 GTP, and 10 phosphocreatine (pH 7.2; 280–290 mOsm). 0.2–0.5% Biocytin was routinely included in the intracellular solution. Neurons were held at resting potential or around –72 mV for current-clamp recordings. Resting membrane potentials were measured immediately on break-in. Liquid junction potential was not corrected. Series resistance was monitored throughout each experiment; neurons with a series resistance >25 MΩ or resting potential more depolarized than −60 mV were excluded from analysis. We only included CA3a neurons that could be clearly identified using biocytin staining to determine the presence of thorny excrescences.
The input resistance was calculated based on the slope of the linear fit of the voltage–current plot from the steady-state voltage response to 10-pA step current injections (−40 to 30 pA). The voltage threshold of AP was determined as the voltage where the value of dV/dt exceeded 50 mV/ms. The voltage sag was determined by hyperpolarizing the membrane potential from −70 to −100 mV by injecting a negative current. The injected negative currents were adjusted for each neuron based on their input resistance. The membrane time constant was calculated using a single exponential fit of the voltage change in response to a −100-pA hyperpolarizing current injection with 500-ms duration. The cell capacitance was calculated under current-clamp mode using the formula c = r/τ, where c is membrane capacitance, r is cell membrane resistance, and τ is membrane time constant. r And τ were determined by injecting a −100-pA, 500-ms hyperpolarizing current into the cell. AP rise time (20–80%) and AP half-width were analyzed using in-house functions in AxoGraph software.
Biocytin staining.
Biocytin staining was performed as described previously (Sun et al. 2017). Briefly, slices that underwent whole cell recordings were fixed at 4°C for ≥24 h in 4% paraformaldehyde in phosphate buffer (PBS), pH 7.3. The fixed slices were subsequently treated with PBS containing normal goat serum (10%) and 0.5% Triton X-100 for 2 h at room temperature. Slices were then incubated in PBS containing streptavidin, Alexa Fluor 594 conjugate (1:500), and 0.1% Triton X-100 for 2 days at 4°C. Subsequently, slices were rinsed in PBS several times and processed through increasing concentrations of glycerol and then embedded in mounting media (Fluoro-Gel; Electron Microscopy Sciences).
Golgi staining.
To visualize the somatodendritic morphology of CA3 pyramidal neurons, we performed Golgi staining using FD Rapid GolgiStain Kit (cat. no. PK401; FD Neurotechnologies, Columbia, MD). We followed the protocol recommended by the manufacturer with small modification. Briefly, 3-mo-old male mice were deeply anesthetized with isoflurane and euthanized by decapitation in accordance with institutional regulations. Bilateral hippocampi were then dissected from the brain and incubated in the mixture of equal amount of FD Solutions A and B for 5 days, followed by 3-day incubation in Solution C. The hippocampi were then embedded in 4% agarose, and 150-μm-thick transverse slices from the entire hippocampus were cut from dorsal to ventral using VT1000 S vibratome (Leica Biosystems). Sections were subsequently mounted on Superfrost glass slides and let dry overnight before staining. Better visualization of individual pyramidal neurons was achieved by 8-min incubation in the staining solution, composed of solutions D and E and double-distilled water at a ratio of 1:1:2. Stained sections were then dehydrated sequentially in ethanol solutions with increasing concentration and cleared in xylene. Finally, slides were covered with Eukitt quick-hardening mounting medium for microscopic imaging.
Neuron morphology analysis.
Z-stack tile Golgi-staining images of individual CA3 pyramidal neurons from each CA3 subarea were captured using the bright-field imaging acquisition function under the Zeiss LSM 800 confocal microscope (Carl Zeiss Microscopy, White Plains, NY) and were subsequently analyzed using Neurolucida software (MBF Bioscience, Williston, VT). We drew contours along the soma of pyramidal neurons at individual planes and then traced apical and basal dendrites using neuronal tracing tools. Based on the tracing data, surface area of pyramidal neuronal soma and length of dendrites were obtained using the neuron summary tool in Neurolucida Explorer (MBF Bioscience). The diameters of proximal apical dendrites and proximal basal dendrites were measured at 30 and 20 µm from the soma center using ImageJ, respectively.
Data analysis and statistics.
Fifteen mice used in electrophysiological experiments were 2–3 mo old, and three mice were 5 mo old. We found no statistical differences in intrinsic properties between these two groups. Thus the data were combined. In addition, we observed that a very small fraction of CA3 neurons (2 out of 84 cells, or 2.4%) displayed bursting firing patterns that appear to match the description of the athorny cells (lack of thorny excrescences) reported in a recent study (Hunt et al. 2018). These two cells were located in deep pyramidal cell layer of vCA3a. The extremely small number of these cells in our current data set does not allow for detailed quantitative assessment of this cell type. Therefore, we have excluded these two cells from our analyses.
Two-tailed Student’s t test or two-way ANOVA test followed by a Bonferroni test for multiple comparisons was used for statistical analysis. Pearson correlation test was used to determine the correlations between the passive and active parameters and normalized cell transverse positions and between AP frequency and input resistance. Statistics were analyzed using Excel 2016 (Microsoft, Redmond, WA), Igor Pro 8 software (WaveMetrics, Lake Oswego, OR), or Origin 2019b (OriginLab, Northampton, MA). Somatodendritic morphological analyses were performed using Neurolucida Explorer. All data are expressed as means ± SE. P < 0.05 was considered statistically different.
RESULTS
Gross anatomic structures of mouse hippocampal transverse slices from dorsal to ventral pole.
To compare CA3 pyramidal neuron cellular properties along proximodistal and dorsoventral axes, we dissected the hippocampi out from mouse brain and cut a series of the transverse slices continuously from dorsal to ventral pole of the entire hippocampi, as described previously (see methods; Fig. 1A; Sun et al. 2014, 2017). We noted that the most visible and reliable anatomic marker for the dorsal hippocampal slices was a relatively sharp triangular-shaped DG granule cell (GC) layer, whereas ventral slices displayed a U-shaped DG GC layer, with intermediate slices somewhere in between (Fig. 1, B and F), as reported previously (Malik et al. 2016). We measured and quantified several gross anatomic parameters to provide a more quantitative estimate of the longitudinal positions of hippocampal transverse slices that underwent whole cell recordings. Approximately 13–15 400-µm-thick transverse slices can be obtained from each hippocampus. We then generated the reference coordinates that were used to estimate the longitudinal positions of the recorded hippocampal slices (Fig. 1).
Fig. 1.
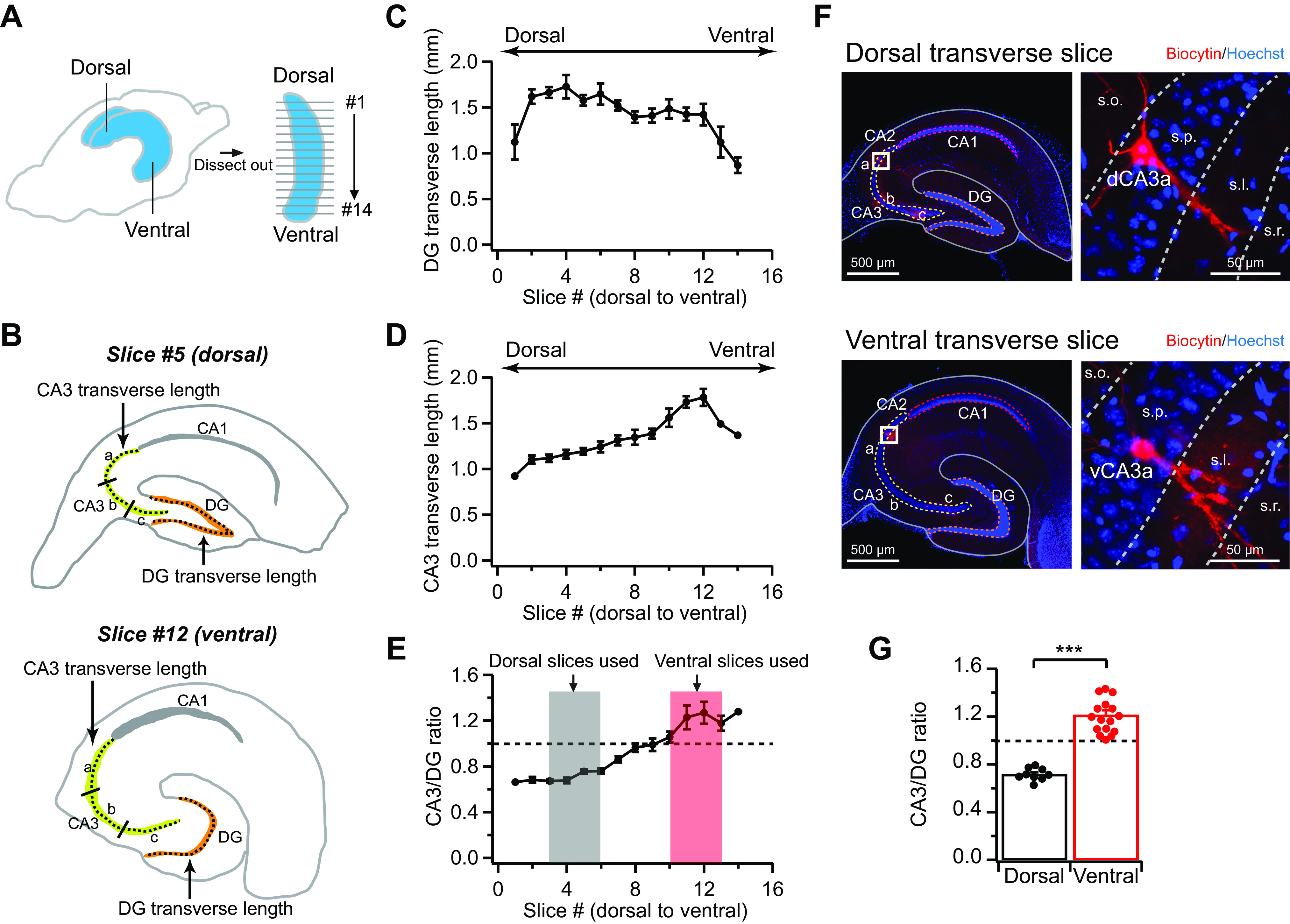
Gross anatomic structures of hippocampal transverse slices along the dorsoventral axis. A: schematic diagram illustrates that the hippocampus was dissected out and 400-µm-thick transverse slices were cut from dorsal (signed as “slice 1”) to ventral pole (“slice 14”). B: schematic drawings of the dorsal (#5) and ventral (#12) hippocampal slices. Both dorsal area CA3 (dCA3) and ventral area CA3 (vCA3) were equally divided into a, b, and c along the proximodistal (transverse) axis for data quantifications. Note the outlines for measurements of transverse lengths of CA3 cell body layer and dentate gyrus (DG) granule cell (GC) layer shown in C–E. C: quantification of transverse length of DG GC layer from dorsal to ventral pole. n = 4 Hippocampi. D: quantification of transverse length of CA3 cell body layer from dorsal to ventral pole. n = 4 Hippocampi. E: the ratio of CA3-to-DG transverse lengths from dorsal to ventral. n = 4 Hippocampi. Shaded areas indicate the slices that were used in the quantification shown in G and in all patch-clamp recording experiments throughout the paper. F, top: representative image of a dorsal transverse slice (left) and high-magnification image of a biocytin-filled dCA3a pyramidal neuron (right). Bottom: representative image of a ventral transverse slice (left) and high-magnification image of a biocytin-filled vCA3a pyramidal neuron (right). s.l., Stratum lucidum; s.o., stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum; G: summary data for CA3-to-DG ratio from a subset of dorsal and ventral slices that underwent whole cell recordings. Error bars show SE. Two-tailed unpaired t test, ***P = 8.3 × 10−10. Dorsal: n = 9 slices (7 mice); ventral: n = 16 slices (10 mice).
We measured 2 parameters from each transverse slice, which we found varied relatively consistently from dorsal to ventral pole across different hippocampi: the transverse length of DG GC layer and the transverse length of CA3 cell body layer (Fig. 1, B–D). The transverse length of DG GC layer decreased gradually from dorsal to ventral pole with the notable exception in the extreme dorsal slices (Fig. 1C). By contrast, the transverse length of CA3 cell body layer increased from dorsal to ventral pole with a sharp drop in the extreme ventral slices (Fig. 1D). As a result, the calculated ratios of CA3 to DG provided the most sensitive and reliable measurement that allowed us to estimate the approximate longitudinal position of recorded slices (Fig. 1E). Its values increased nearly linearly from dorsal (∼0.6) to ventral (∼1.2; Fig. 1E).
These parameters were measured in a subset of dorsal and ventral hippocampal slices that underwent whole cell recordings (Fig. 1, F and G). We verified from these recorded slices that the dorsal CA3-to-DG ratio was 0.72 ± 0.02 (ranging from 0.63 to 0.79, n = 9 slices), and the ventral CA3-to-DG ratio was 1.20 ± 0.03 (ranging from 1.01 to 1.43, n = 16 slices; P < 0.001; Fig. 1G). Thus, if the hippocampi were evenly divided into seven segments from dorsal to ventral pole, with the first segment as the most dorsal and the seventh segment as the most ventral, we estimated that the dorsal and ventral slices used in this study were well separated longitudinally and positioned at approximately the second and third segments for dorsal and the fifth and sixth segments for ventral, respectively (Fig. 1E).
To delineate the proximodistal positions of CA3 pyramidal neurons, we performed biocytin-based staining in a subset of recorded neurons from both dCA3 and vCA3, as described previously (Sun et al. 2017). We normalized the relative position of the recorded neurons along the proximodistal axis, with 0 assigned to the most proximal region of CA3 and 1 assigned to the end of mossy fibers near the CA2/CA3 border (Fig. 2A). Similar to our (Sun et al. 2017) previous study, we evenly divided CA3 into three segments along the proximodistal axis in both dorsal and ventral slices (from proximal to distal: CA3c, CA3b, and CA3a; Lorente De Nó 1934). Because CA2 and a subset of CA3a pyramidal neurons are intermingled near CA2 region and share some overlapping electrophysiological properties (Sun et al. 2017), we only included CA3a neurons that can be clearly identified using biocytin staining to determine the presence of thorny excrescences, a hallmark anatomic feature of CA3 pyramidal neurons.
Fig. 2.
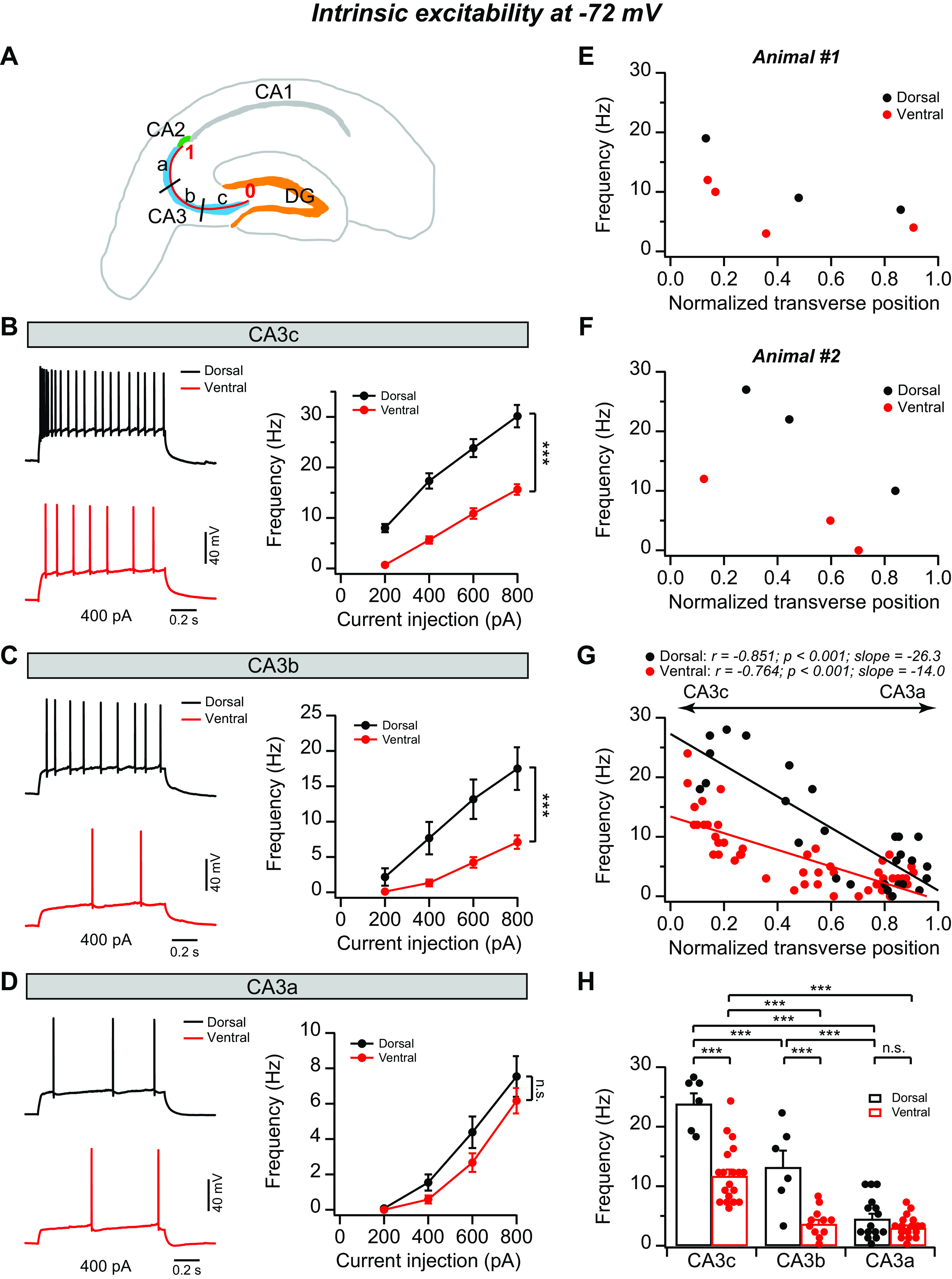
Topographic heterogeneity of area CA3 intrinsic excitability at −72 mV. A: schematic diagram of a transverse hippocampal slice. Both dorsal and ventral CA3 are divided into 3 equal-sized regions along the transverse axis (CA3c, CA3b, and CA3a). The position of each recorded CA3 neuron was normalized from 0 to 1 based on their transverse position. CA3 neurons located proximally near dentate gyrus (DG) were assigned a location of 0, whereas neurons distally located at the end of the mossy fibers were assigned a location of 1. B–D, left: sample action potential (AP) traces of firing patterns in response to indicated constant-current injection from CA3c (B), CA3b (C), and CA3a (D). Right: mean AP frequency during the current step as function of current injection from CA3c (B), CA3b (C), and CA3a (D). Error bars show SE. ***P < 0.001. n.s., Not significant. Dorsal: n = 6–15 neurons (5–8 mice) per group; ventral: n = 9–16 neurons (8 –11 mice) per group. E–G: AP frequency in response to a 1-s, +600-pA current injection plotted against normalized cell position from 2 single animals (E and F) and the population data (G). Dorsal: n = 27 neurons (11 mice); ventral: n = 48 neurons (14 mice). H: summary data for AP frequency in response to a +600-pA constant-current injection. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom = 73, Fdorsal-ventral(1, 73) = 58.28, Fabc(2, 73) = 74.77, ***P < 0.0003. Dorsal: n = 6–15 neurons (5–8 mice) per group; ventral: n = 11–19 neurons (8–13 mice) per group.
Topographic heterogeneity of CA3 pyramidal neurons in intrinsic membrane excitability.
To determine CA3 topographic heterogeneity of intrinsic excitability, we carried out whole cell current-clamp recordings to evoke AP output by injecting a series of constant currents with increasing amplitudes into the soma while holding the membrane potential at −72 mV. Consistent with our (Sun et al. 2017) previous findings from dorsal hippocampus, dCA3c neurons fired a greater number of APs in response to a set amount of current injections, whereas dCA3a was least excitable, followed by dCA3b (Fig. 2). A plot of AP frequency evoked by 600-pA current injection against normalized transverse position of recorded dCA3 neurons displayed a linear relationship (Fig. 2G). Surprisingly, however, we observed that vCA3 pyramidal neurons were significantly less excitable compared with dCA3 neurons along the proximodistal axis (Fig. 2). In addition, the differences of intrinsic excitability between dCA3 and vCA3 varied considerably along the proximodistal axis, such that proximal-to-mid vCA3 (vCA3c–b) neurons fired significantly fewer APs compared with dCA3c–b in response to a given current injection (Fig. 2, B, C, and H), whereas the firing rate of vCA3a neurons was only slightly, but not significantly, lower than dCA3a (Fig. 2, D and H). Furthermore, these dorsoventral and proximodistal differences were present at the levels of both single animals and population data (Fig. 2, E–H). As a result, although the proximodistal linear functions between AP frequency and normalized transverse position of recorded cells were observed in both dCA3 (r = −0.851, P < 0.001) and vCA3 (r = −0.764, P < 0.001), the slope was 188% steeper in dCA3 (slope = −26.3) than vCA3 (slope = −14.0; Fig. 2G). Thus these results reveal a marked topographic heterogeneity of CA3 intrinsic excitability along both proximodistal and dorsoventral axes.
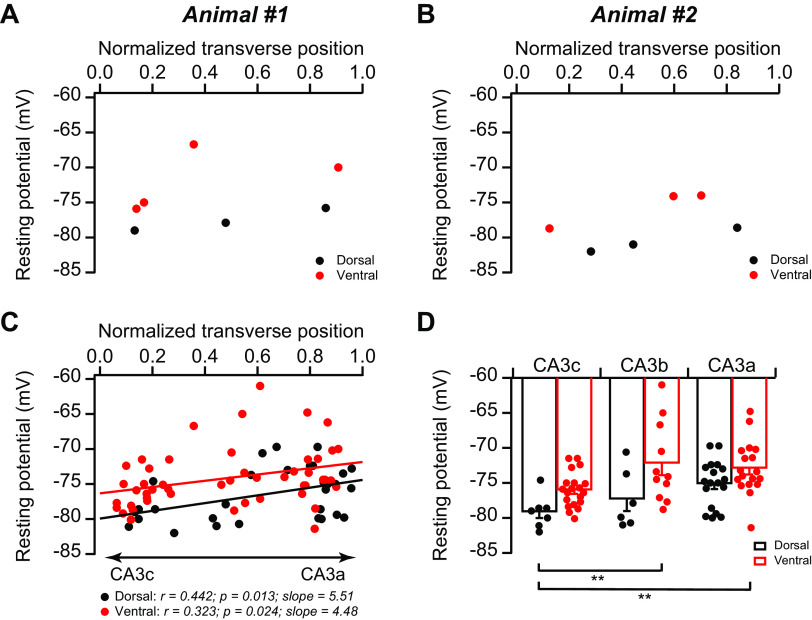
In addition, we also observed small but statistically significant continuous proximal-to-distal gradients in resting potential in both dCA3 (r = 0.442, P = 0.013) and vCA3 (r = 0.323, P = 0.024; Fig. 3), consistent with our (Sun et al. 2017) previous report. Moreover, the resting potential in vCA3 was slightly, but not significantly, more depolarized than that in dCA3 across the proximodistal axis (Fig. 3). As the membrane potential can influence the intrinsic excitability, we also examined the frequency-current curve at resting potential to provide a more physiological assessment. We found that the proximal-to-distal decreasing gradients of intrinsic excitability were present at resting potential in both dCA3 and vCA3 (Fig. 4), similar to that at −72 mV (Fig. 2). Thus, although the resting potential of vCA3b/c pyramidal neurons was slightly more depolarized than that of dCA3b/c, respectively, they fired significantly fewer APs in response to a set of current injections (Fig. 4). Moreover, dCA3c appears be the most intrinsically excitable CA3 subregion (Fig. 4G), despite its most negative resting potential (Fig. 3). Taken together, we conclude that variations of CA3 intrinsic excitability are present at both resting potential (Fig. 4) and at −72 mV (Fig. 2).
Fig. 3.
Topographic heterogeneity of area CA3 resting potential. A–C: the resting potential of CA3 pyramidal neurons plotted against normalized cell position from 2 single animals (A and B) and the population data (C). D: summary data for resting potential. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom = 79, Fdorsal-ventral(1, 79) = 14.42, Fabc(2, 79) = 6.34, **P < 0.005. Dorsal: n = 31 neurons (11 mice); ventral: n = 49 neurons (14 mice).
Fig. 4.
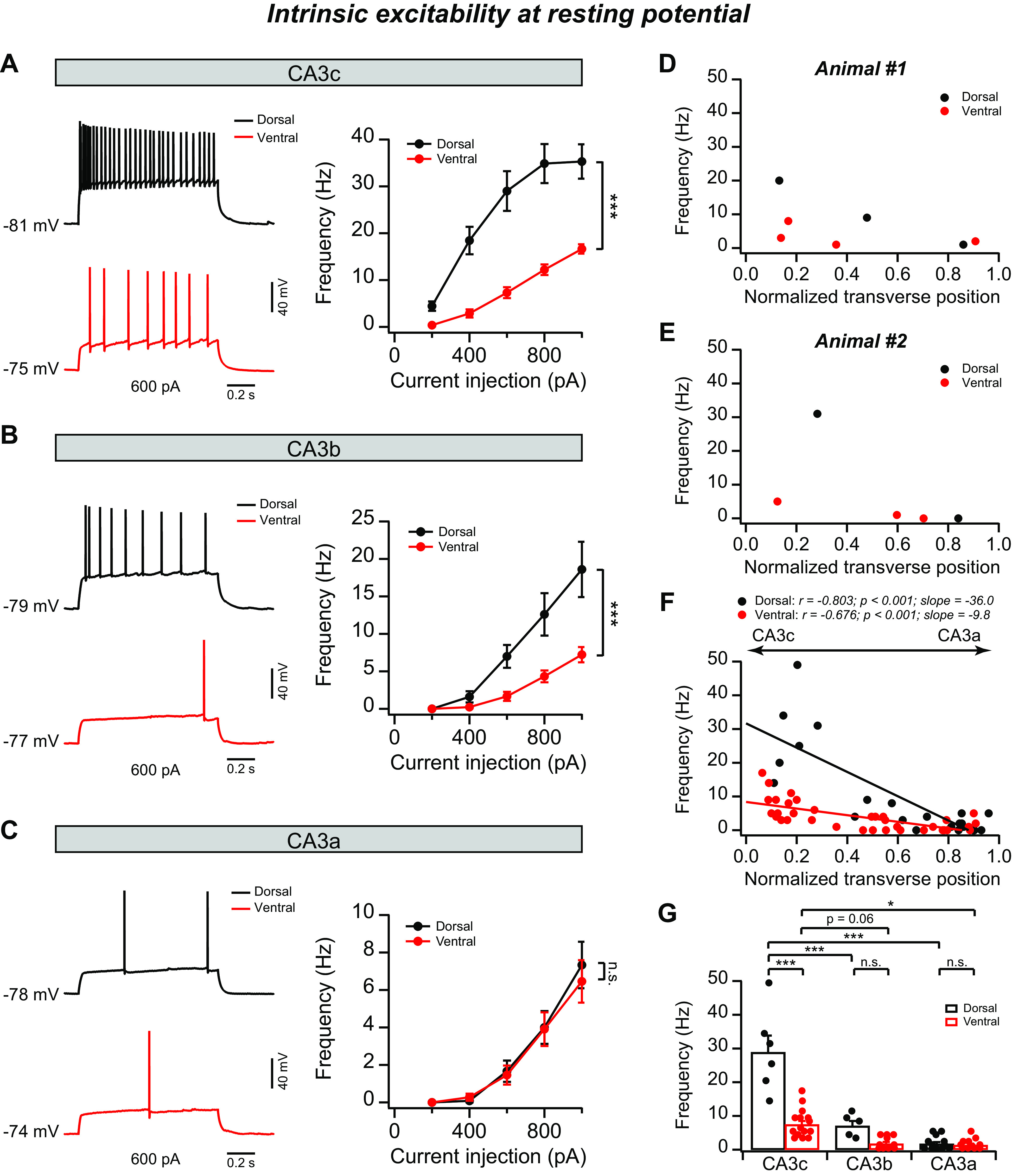
Topographic heterogeneity of area CA3 intrinsic excitability at resting potential. A–C, left: sample action potential (AP) traces of firing patterns in response to indicated constant-current injection from CA3c (A), CA3b (B), and CA3a (C). Right: mean AP frequency during the current step as function of current injection from CA3c (A), CA3b (B), and CA3a (C). Error bars show SE. ***P < 0.001. n.s., Not significant. Dorsal: n = 5–12 neurons (4–8 mice) per group; ventral: n = 9–14 neurons (7 –10 mice) per group. D–F: AP frequency in response to a 1-s, +600-pA current injection plotted against normalized cell position from 2 single animals (D and E) and the population data (F). Dorsal: n = 22 neurons (11 mice); ventral: n = 36 neurons (11 mice). G: summary data for AP frequency in response to a +600-pA constant-current injection. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom = 58, Fdorsal-ventral(1, 58) = 48.29, Fabc(2, 58) = 67.31, *P = 0.024, ***P < 4 × 10−9. Dorsal: n = 5–12 neurons (4–8 mice) per group; ventral: n = 10–15 neurons (7 –10 mice) per group.
Topographic heterogeneity of input resistance of CA3 pyramidal neurons.
What are the mechanism(s) underlying the heterogeneity of CA3 intrinsic excitability? We found there was no significant difference in AP voltage threshold along both proximodistal and dorsoventral axes (dCA3c: −36.9 ± 1.1 mV, n = 7 neurons; dCA3b: −39.4 ± 0.7 mV, n = 5 neurons; dCA3a: −41.0 ± 0.7 mV, n = 15 neurons; vCA3c: −38.0 ± 0.5 mV, n = 17 neurons; vCA3b: −41.3 ± 0.9 mV, n = 11 neurons; vCA3c: −40.3 ± 0.7 mV, n = 17 neurons; dorsal vs. ventral: P = 0.262, 2-way ANOVA). Next, we asked whether variations in input resistance contribute to the heterogeneity of intrinsic membrane excitability. In keeping with our (Sun et al. 2017) previous results, we found that dCA3 showed a decreasing proximal-to-distal gradient in input resistance (Fig. 5). In fact, input resistance in dCA3c was 3.8-fold greater than dCA3a (Fig. 5B). The magnitude of difference between dCA3a and dCA3c was bigger than that reported in our (Sun et al. 2017) previous study (2.6-fold). This is likely a result of the use of more stringent criteria to select dorsal slices here. The input resistance of vCA3 pyramidal neurons was lower than that of dCA3 across the proximodistal axis (Fig. 5). The dorsoventral difference was particularly prominent in proximal-to-mid CA3 (dCA3c: 464.6 ± 25.8 MΩ, vCA3c: 207.7 ± 18.9 MΩ, P = 3.4 × 10−13; dCA3b: 252.3 ± 32.7 MΩ, vCA3b: 115.3 ± 9.3 MΩ, P = 5.4 × 10−4, 2-way ANOVA; Fig. 5B), whereas in distal CA3 (CA3a), vCA3a (122.7 ± 13.6 MΩ) did not significantly differ from dCA3a (109.1 ± 7.8 MΩ; P = 1, 2-way ANOVA; Fig. 5B). Thus, although the plots of input resistance against the normalized cell transverse position revealed linear relationships in both dCA3 (Fig. 5E; r = −0.924, P < 0.001) and vCA3 (r = −0.638, P < 0.001), the slope in dCA3 was steeper than that in vCA3 (dCA3: slope = −497; vCA3: slope = −163). Overall, the topographic heterogeneity in input resistance mirrors almost perfectly the heterogeneity in intrinsic excitability (e.g., compare Fig. 2, G and H, with Fig. 5, B and E). Furthermore, AP frequency induced by somatic current injection displayed a linear function with the input resistance in both dCA3 and vCA3 of nearly identical slopes (Fig. 5, F–H), further supporting that the heterogeneity in input resistance is likely the dominant factor in determining variations of CA3 intrinsic excitability.
Fig. 5.
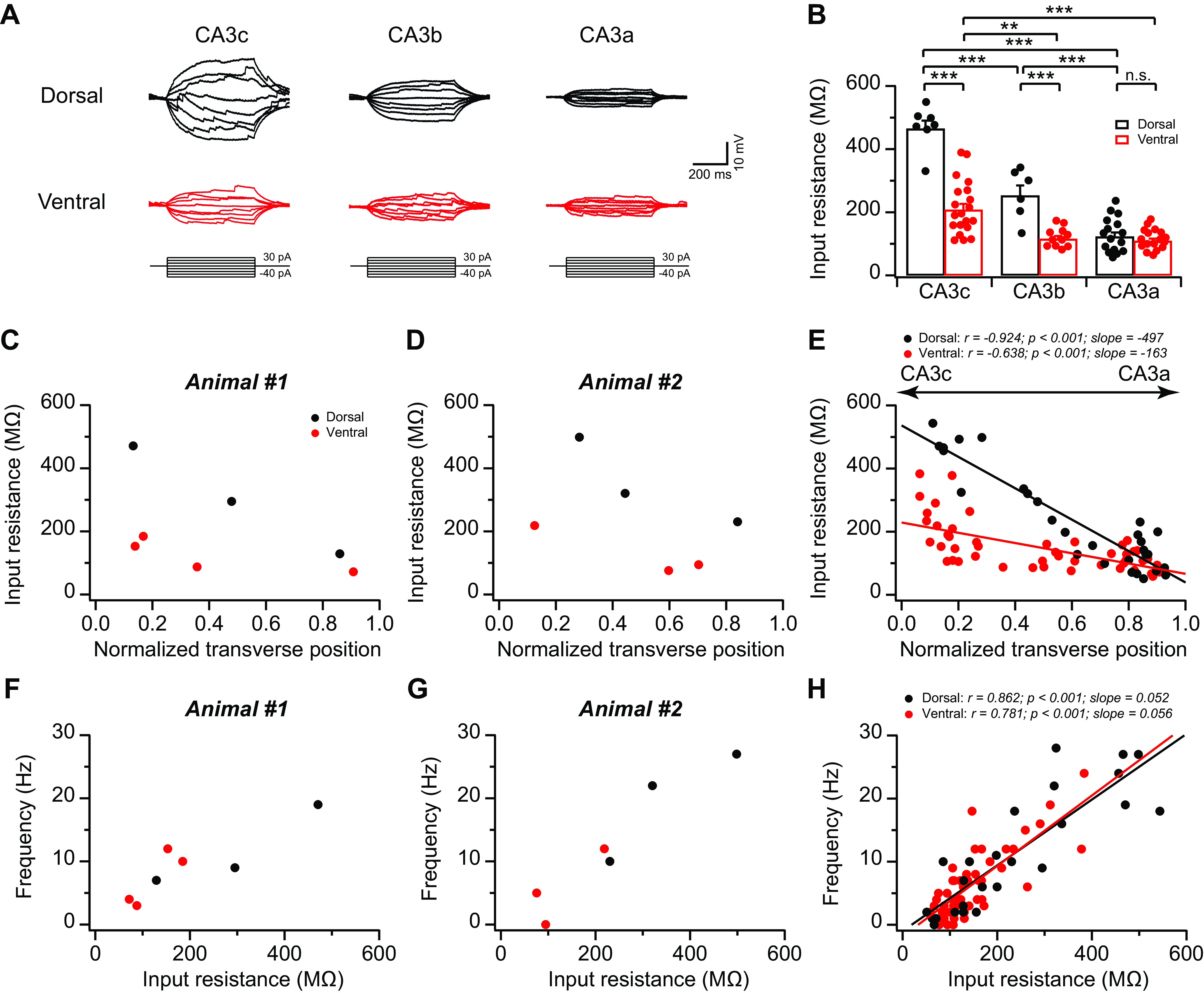
Topographic heterogeneity of input resistance in area CA3 pyramidal neurons. A: sample voltage traces in response to 500-ms constant increasing current injections with 10-pA step from pyramidal neurons in dorsal (top) vs. ventral (bottom) CA3c, -b, and -a, respectively. B: summary data for input resistance of dorsal CA3 (dCA3) and ventral CA3 (vCA3) neurons along the proximodistal axis. Dorsal: n = 6–16 neurons (5–9 mice) per group; ventral: n = 11–20 neurons (8–13 mice) per group. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom = 76, Fdorsal-ventral(1, 76) = 77.43, Fabc(2, 76) = 82.74, **P = 0.002, ***P < 0.0006. n.s., Not significant. C–E: input resistance plotted against normalized cell transverse position from 2 single animals (C and D) and the population data (E). Dorsal: n = 29 neurons (12 mice); ventral: n = 49 neurons (14 mice). F–H: action potential (AP) frequency plotted against input resistance from 2 single animals (F and G) and the population data (H). Dorsal: n = 25 neurons (11 mice); ventral: n = 48 neurons (14 mice).
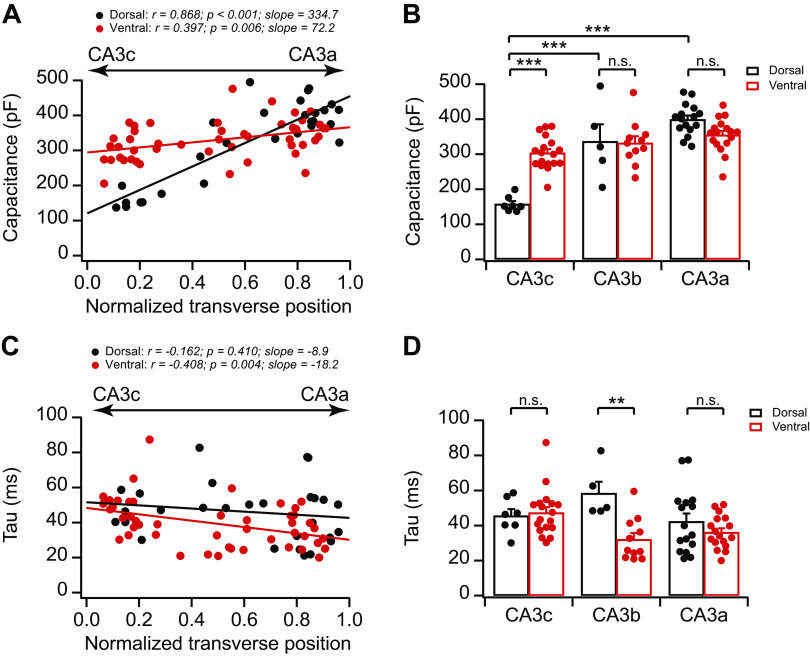
We next addressed the mechanism underlying variations in input resistance. Because the cell capacitance of individual neurons positively correlates with the size of the somatodendritic area, and thus has an inverse relationship with input resistance, we asked whether CA3 cell capacitance displays variations. Indeed, we found that CA3 cell capacitance nearly mirrored the topographic organization of input resistance (compare Fig. 6, A and B, with Fig. 5, B and E). Specifically, both dCA3 and vCA3 displayed increasing proximal-to-distal gradients in cell capacitance, with dCA3 exhibiting a steeper proximal-to-distal slope (Fig. 6, A and B). Moreover, consistent with the large dorsoventral difference of input resistance in proximal CA3 (Fig. 5B), dCA3c (158.1 ± 8.4 pF, n = 7 neurons) had significantly smaller cell capacitance than vCA3c (303.8 ± 10.9 pF, n = 18 neurons; P = 7.3 × 10−7, 2-way ANOVA; Fig. 6B). Interestingly, the membrane time constant displayed relatively more moderate variations along the proximodistal axis in dCA3 and vCA3 (Fig. 6, C and D). For example, the membrane time constant in dCA3c (45.6 ± 3.8 ms, n = 7 neurons) was not significantly different from that in vCA3c (47.4 ± 3.2 ms, n = 18 neurons; P = 1, 2-way ANOVA).
Fig. 6.
Topographic heterogeneity of area CA3 cell capacitance and membrane time constants. A: cell capacitance plotted against normalized cell transverse position. Dorsal: n = 28 neurons (12 mice); ventral: n = 48 neurons (14 mice). B: summary data for cell capacitance of dorsal CA3 (dCA3) and ventral CA3 (vCA3) neurons along the proximodistal axis. Dorsal: n = 5–16 neurons (5–9 mice) per group; ventral: n = 11–18 neurons (8–13 mice) per group. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom (df) = 73, Fdorsal-ventral(1, 73) = 5.41, Fabc(2, 73) = 47.26, ***P < 4 × 10−6. n.s., Not significant. C: membrane time constant (Tau) plotted against normalized cell transverse position. Dorsal: n = 28 neurons (12 mice); ventral: n = 48 neurons (14 mice). D: summary data for time constant of dCA3 and vCA3 neurons along the proximodistal axis. Dorsal: n = 5–16 neurons (5–9 mice) per group; ventral: n = 11–18 neurons (8–13 mice) per group. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 73, Fdorsal-ventral(1, 73) = 8.50, Fabc(2, 73) = 2.10, **P = 0.0084.
Topographic heterogeneity of CA3 somatodendritic morphology can, in part, account for the heterogeneity of input resistance.
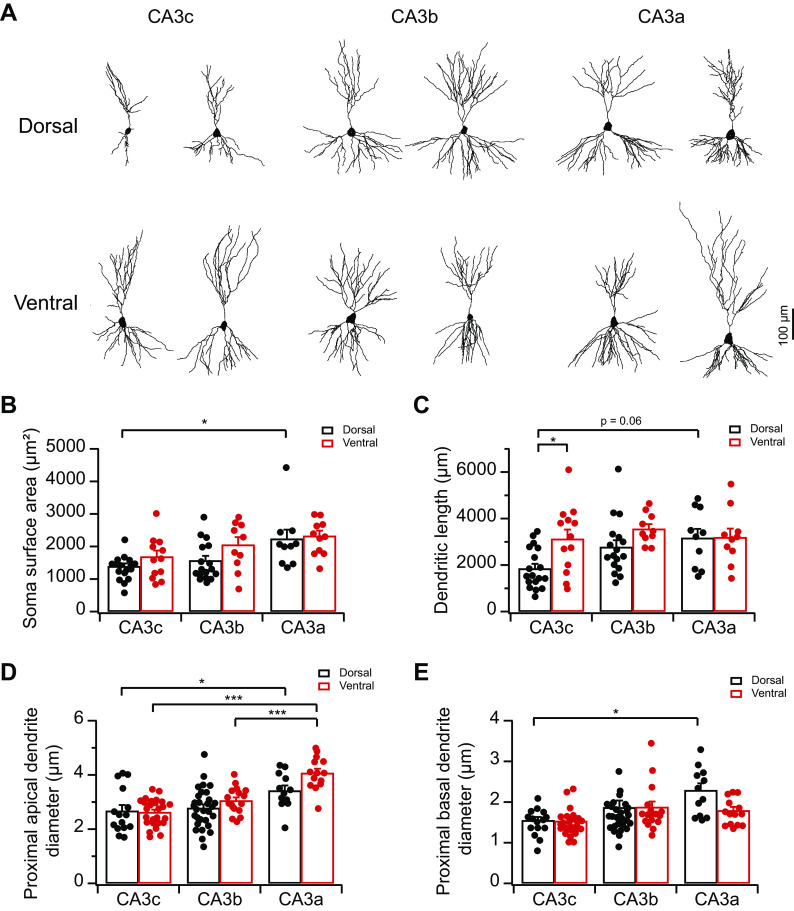
Cell capacitance results above suggest strongly that vCA3, in particular proximal vCA3, likely exhibits more complex somatodendritic structures than dCA3. As there have been no prior studies on the dorsoventral heterogeneity of CA3 neuron morphology, we next performed Golgi staining to examine the somatodendritic morphology of CA3 pyramidal neurons along different anatomic axes (Fig. 7). Consistent with previous studies on dorsal hippocampus (Ishizuka et al. 1995; Sun et al. 2017), we found dCA3 soma surface area, dendritic length, and proximal dendritic diameter displayed proximal-to-distal increasing gradients, with dCA3c showing the most compact somatodendritic structures (Fig. 7). By contrast, somatodendritic morphology in vCA3 were relatively homogeneous along the proximodistal axis. In addition, in keeping with a larger cell capacitance in vCA3c than dCA3c (Fig. 6B), the dendritic length of vCA3c was significantly greater than that of dCA3c (Fig. 7C; P = 0.04, 2-way ANOVA). Overall, topographic heterogeneity of CA3 somatodendritic morphology matches with variations of cell capacitance and likely contributes to the heterogeneity of CA3 intrinsic excitability.
Fig. 7.
Topographic heterogeneity of area CA3 somatodendritic morphology. A: representative CA3 pyramidal neuron morphology along the proximodistal and dorsoventral axes reconstructed from Golgi staining. B: summary data for soma surface area (in square micrometers) of CA3 pyramidal neurons. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom (df) = 76, Fdorsal-ventral(1, 76) = 4.08, Fabc(2, 76) = 8.78, *P = 0.014. n = 10–18 neurons (3–4 mice) per group. C: summary data for total dendritic length (in micrometers) of CA3 pyramidal neurons. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 77, Fdorsal-ventral(1, 77) = 6.92, Fabc(2, 77) = 3.36, *P = 0.04. n = 10–18 neurons (3–4 mice) per group. D: summary data for the diameter of CA3 proximal apical dendrites (in micrometers). Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 113, Fdorsal-ventral(1, 113) = 5.34, Fabc(2, 113) = 23.65, ***P < 4 × 10−4, *P = 0.047. n = 12–30 neurons (3–4 mice) per group. E: summary data for the diameter of CA3 proximal basal dendrites (in micrometers). Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 113, Fdorsal-ventral(1, 113) = 1.96, Fabc(2, 113) = 5.91, *P = 0.03. n = 12–30 neurons (3–4 mice) per group.
Topographic heterogeneity of Ih in CA3 pyramidal neurons.
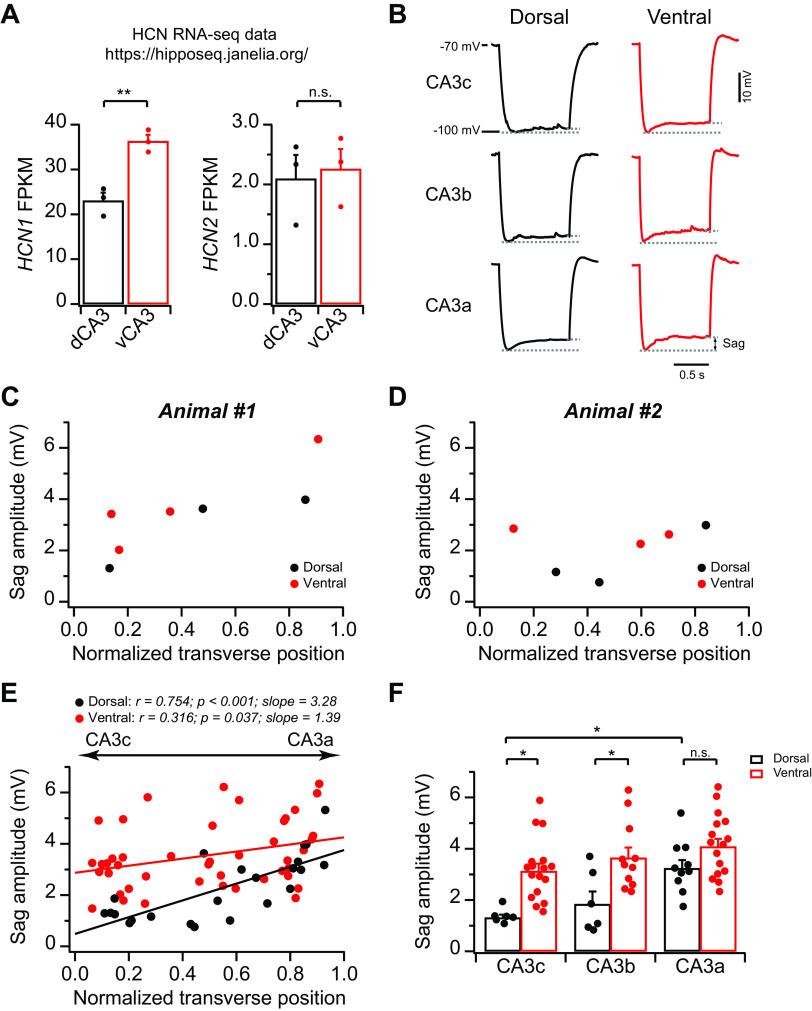
In addition to somatodendritic morphology, the variations in ion conductance(s) can also contribute to the topographic heterogeneity in input resistance and intrinsic excitability. In fact, we (Sun et al. 2017) have previously discovered that in dCA3 an increasing proximal-to-distal gradient of Ih expression contributes to a decreasing gradient in intrinsic membrane excitability. To explore whether Ih differs along the dorsoventral axis, we first searched the hippocampus RNA-sequencing (RNA-Seq) atlas generated by Cembrowski et al. (2016). This searchable atlas provides a comprehensive RNA-Seq database for different hippocampal cell types from different anatomic positions, including dorsoventral variations of RNA-Seq results from the CA3 region (Cembrowski et al. 2016). The RNA-Seq results revealed the differential expression of hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (HCN1), but not HCN2, between dCA3 and vCA3: HCN1 RNA expression in vCA3 was significantly greater than dCA3, indicating a greater Ih in vCA3 than dCA3 (Fig. 8A). To control for the potential dorsoventral variations of general RNA expression, we searched the expression level of two neuronal housekeeper genes: β-actin (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). We found no difference of ACTB RNA level between dCA3 [896.1 ± 96.6 fragments per kilobase million (FPKM), n = 3 replicates] and vCA3 (826.4 ± 105.6 FPKM, n = 3 replicates; P = 0.65). GAPDH RNA level in dCA3 (1,603.6 ± 96.8 FPKM, n = 3 replicates) was greater than vCA3 (1,182.5 ± 21.3 FPKM, n = 3 replicates; P = 0.013), an opposite trend compared with the dorsoventral difference of HCN1. In fact, if HCN1 is scaled to the GAPDH level, the dorsoventral difference becomes even more robust (vCA3: ∼214% of dCA3). Overall, we conclude that the higher HCN1 RNA expression in vCA3, compared with dCA3, unlikely resulted from the greater RNA levels in vCA3 in general.
Fig. 8.
Topographic heterogeneity of hyperpolarization‐activated current in area CA3 pyramidal neurons. A: summary data for hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (HCN1; left) and HCN2 (right) fragments per kilobase million (FPKM) from dorsal CA3 (dCA3) and ventral CA3 (vCA3). Two-tailed Student’s t test, **P = 0.0045. n.s., Not significant. The results were obtained from the Hipposeq database (Cembrowski et al. 2016). RNA-seq, RNA-sequencing. B: sample traces of hyperpolarizing voltage response (from −70 to −100 mV) in response to negative constant-current injections from dCA3 (left) and vCA3 (right). C–E: sag amplitude plotted against normalized transverse cell position from 2 single animals (C and D) and population data (E). Dorsal: n = 26 neurons (10 mice); ventral: n = 47 neurons (15 mice). F: summary data for sag amplitude. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom = 65, Fdorsal-ventral(1, 65) = 23.15, Fabc(2, 65) = 8.42, *P < 0.05. dCA3: n = 6–10 neurons (5–7 mice) per group; vCA3: n = 11–17 neurons (8–12 mice) per group.
Next, we performed whole cell current-clamp recordings to measure voltage sag amplitude, a measurement of Ih, by injecting negative currents to hyperpolarize cell membrane potential from −70 to −100 mV. Consistent with the higher HCN1 RNA expression in vCA3 than in dCA3 (Fig. 8A), we observed that the sag amplitude in vCA3b and -c was significantly greater than dCA3b and -c, respectively, whereas in CA3a it did not reach statistical difference (Fig. 8, B–F). As a result, although the sag amplitude had a linear proximal-to-distal increasing function with normalized transverse cell position in both dCA3 and vCA3, the slope in vCA3 (1.39) was smaller than dCA3 (3.28; Fig. 8E). Because a larger Ih can lead to a smaller input resistance and reduced intrinsic excitability (Robinson and Siegelbaum 2003), we conclude that a combination of differential Ih expression and variations of somatodendritic area (as indicated by cell capacitance and somatodendritic morphology) likely contributes to the differences in CA3 intrinsic excitability and input resistance along the proximodistal and dorsoventral axes.
Topographic heterogeneity of action potential properties in CA3 pyramidal neurons.
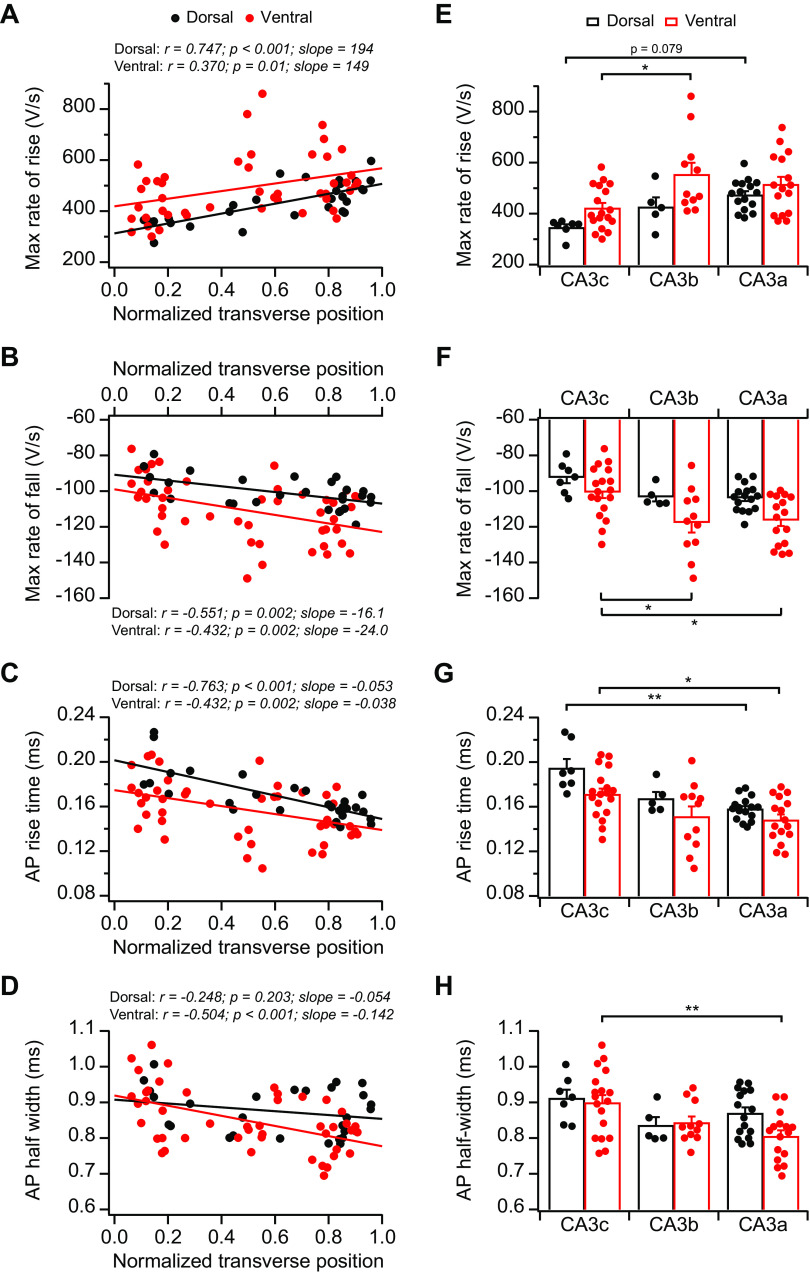
We (Sun et al. 2017) have previously observed no significant differences in AP threshold and AP amplitude along the proximodistal axis in dCA3. Interestingly, recent studies have identified a proximodistal gradient of voltage-dependent Kv2.2 potassium channel in CA3 (Palacio et al. 2017; Raus Balind et al. 2019). These findings prompted us to perform additional analyses to explore possible topographic variations of active membrane properties. Thus we analyzed several parameters of AP properties, including the maximum rate of rise, the maximum rate of fall, AP rise time, and AP half-width (Fig. 9). These measurements also revealed orderly proximal-to-distal gradients in both dCA3 and vCA3: absolute values of both maximum rates of rise and fall displayed increasing proximal-to-distal gradients, whereas AP rise time and half-width exhibited decreasing proximal-to-distal gradients (Fig. 9). Interestingly, unlike dorsoventral heterogeneity in passive membrane properties described above, active properties did not show significant dorsoventral differences, with vCA3 displaying similar proximal-to-distal gradients as dCA3 (Fig. 9).
Fig. 9.
Topographic heterogeneity of action potential (AP) properties in area CA3 pyramidal neurons. A–D: maximum (max) rate of rise (A), maximum rate of fall (B), AP rise time (C), and AP half-width (D) plotted against normalized transverse cell position from dorsal CA3 (dCA3) and ventral CA3 (vCA3). Dorsal: n = 28 neurons (12 mice); ventral: n = 47 neurons (14 mice). E: summary data for maximum rate of rise. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, degrees of freedom (df) = 72, Fdorsal-ventral(1, 72) = 10.33, Fabc(2, 72) = 8.70, *P = 0.011. F: summary data for maximum rate of fall. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 72, Fdorsal-ventral(1, 72) = 12.14, Fabc(2, 72) = 7.95, *P < 0.02. G: summary data for AP rise time. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 72, Fdorsal-ventral(1, 72) = 9.57, Fabc(2, 72) = 13.73, **P = 0.0026, *P = 0.023. H: summary data for AP half-width. Error bars show SE. Two-way ANOVA with Bonferroni test for multiple comparison, df = 72, Fdorsal-ventral(1, 72) = 1.85, Fabc(2, 72) = 6.02, **P = 0.004. dCA3: n = 5–16 neurons (5–9 mice) per group; vCA3: n = 11–18 neurons (8–12 mice) per group.
DISCUSSION
This study, to the best of our knowledge, represents the first demonstration of the dorsoventral heterogeneity of CA3 pyramidal neuron somatodendritic structures and cellular electrical properties. Our combined morphology and electrophysiology findings extend previous reports on topographic heterogeneity in CA3 axonal projections (Besnard et al. 2020; Ishizuka et al. 1990) and molecular profiles (Cembrowski et al. 2016; Thompson et al. 2008), uncovering a complex, yet orderly, pattern of topographic organization of intrinsic membrane properties in CA3 pyramidal neurons along both proximodistal and dorsoventral axes. Although, compared with CA1 and DG, the in vivo functional and behavioral studies on CA3 heterogeneity remain surprisingly scarce, we predict this orderly, topographic organization likely has significant impact on how the information processes through CA3 to contribute to various behavioral tasks (Kesner 2007).
The mechanisms underlying topographic heterogeneity of intrinsic excitability.
Our results show that AP voltage threshold of CA3 pyramidal neurons does not exhibit significant difference along the proximodistal and dorsoventral axes and thus cannot explain the heterogeneity of intrinsic excitability. Rather, our data indicate that the variations of intrinsic excitability are largely determined by the heterogeneity of input resistance along different anatomic axes. Furthermore, our combined electrophysiological and morphological results provide the evidence that heterogeneity of somatodendritic morphology and Ih can, in large part, account for topographic variations of input resistance and intrinsic excitability. We do not rule out the possibility that the differential distributions of additional ion conductances may also play a role. In addition, the basal spontaneous synaptic conductances can influence the intrinsic properties. As we did not block synaptic transmission here, it remains to be determined whether the variations of spontaneous synaptic activity in CA3 contribute to the heterogeneity of intrinsic properties.
In addition to the passive membrane properties, we also observed the proximodistal, but not dorsoventral, gradients in AP properties. The underlying mechanisms are unclear, but we speculate that the heterogeneous distributions of certain voltage-gated ion channels may play a role. Consistent with this idea, recent studies reported the proximodistal heterogeneity of Kv2.2 potassium channel subunit in CA3 (Palacio et al. 2017; Raus Balind et al. 2019), raising the possibility of the presence of the topographic heterogeneity of additional active ion conductances in CA3.
Contrasting dorsoventral variations of intrinsic excitability between CA1 and CA3.
Of particular interest is that dorsoventral differences in CA3 intrinsic excitability and dendritic morphology appear to be the opposite to what have been reported in CA1 dorsoventral heterogeneity. Previous studies show that dCA1 pyramidal neurons are less intrinsically excitable than vCA1, in part because dCA1 pyramidal neurons exhibit lower input resistance and more complex dendritic arborizations than vCA1 (Dougherty et al. 2012, 2013; Malik et al. 2016; Marcelin et al. 2012a). This is in stark contrast with dorsoventral heterogeneity of CA3 intrinsic excitability and neuronal morphology, as we found dCA3 pyramidal neurons are significantly more excitable and have higher input resistance and less complex somatodendritic structures than vCA3. In addition, dCA1 has been found to express a higher level of HCN channels compared with vCA1 (Marcelin et al. 2012a). This is also in contrast with CA3, as we observed a significantly lower level of Ih in dCA3 than vCA3. This finding was supported by both our current-clamp recordings measuring Ih and single-cell RNA-Seq results from an independent study measuring HCN RNA expression (Cembrowski et al. 2016). Whether the functional heterogeneity of Ih in CA3 can be solely explained by differential expression level of HCN1 RNA remains an open question, as the levels of RNA are not necessarily correlated with protein expression and the variations of signaling pathway that regulates Ih can also play a role, as reported in CA1 (Dougherty et al. 2013; Marcelin et al. 2012a, 2012b). Another notable difference between CA1 and CA3 is that both dCA3 and vCA3 display considerable proximodistal gradients in intrinsic excitability and input resistance, whereas in CA1 the intrinsic excitability is relatively uniform along the proximodistal axis (Jarsky et al. 2008; Masurkar et al. 2020).
Based on the contrasting dorsoventral differences in intrinsic excitability of CA1 (Dougherty et al. 2012; Malik et al. 2016; Marcelin et al. 2012a) versus CA3 revealed here, we conclude that there are no general principles that can be applied to the topographic organization in cellular properties across different cell types in the hippocampus, including CA1, CA2, and CA3 pyramidal neurons and DG granule cells. Of particular note is that the majority of prior studies have focused on understanding the heterogeneity of CA1 cellular properties, with a wide range of important findings on topographic heterogeneity in dendritic morphology, ion channel distribution, dendritic integration and excitability, and synaptic properties and plasticity (Dougherty et al. 2012, 2013; Malik et al. 2016; Malik and Johnston 2017; Masurkar et al. 2020; Papatheodoropoulos 2018; Papatheodoropoulos et al. 2002; Papatheodoropoulos and Kostopoulos 2000). Apparently, these results deriving from CA1 cannot be readily applied to CA3. Instead, the opposing dorsoventral difference between CA1 and CA3 highlights the importance of further scrutiny of the heterogeneity of CA3 cellular and dendritic properties (Guzman et al. 2016; Kim et al. 2012; Makara and Magee 2013; Mishra et al. 2016), an area that remains relatively underexplored compared with CA1.
In this study, our dorsal and ventral slices were well separated longitudinally (Fig. 1). Although this approach enables us to detect dorsal versus ventral differences more robustly, it is unclear whether the dorsoventral differences in intrinsic properties are graded or segmented. The large-scale studies are required to address these questions in the future (Malik et al. 2016; Pastoll et al. 2020).
Implication for data interpretation of CA3 studies.
This study, together with our (Sun et al. 2017) previous report focusing on dCA3 heterogeneity, offers one of likely explanations for a wide range of values of CA3 intrinsic membrane properties obtained from many studies (Hemond et al. 2008; Raus Balind et al. 2019). For instance, coronal and horizontal cuttings are two commonly used orientations to cut hippocampal slices for electrophysiological experiments. Our findings indicate that CA3 electrical properties and dendritic morphology from horizontal slices (intermediate to ventral) can be dramatically different from coronal slices (dorsal). For certain parameters, such as input resistance and Ih, the difference between dCA3 and vCA3 can be substantial even within the same transverse position (e.g., >2-fold in CA3b-c). Similarly, intrinsic properties and dendritic morphology from proximal CA3 (CA3c) can markedly differ from distal CA3 (CA3a) from both dorsal and ventral hippocampus (e.g., 3.8-fold difference in dCA3 for input resistance). Given the significant variations of intrinsic properties and dendritic morphology of CA3 pyramidal neurons along the proximodistal and dorsoventral axes, we believe that it is crucial to provide specific anatomic positions where CA3 neurons are obtained in any studies (e.g., dorsal vs. ventral or proximal vs. distal).
Functional implication of CA3 intrinsic excitability heterogeneity.
In vivo CA3 place cell recordings have demonstrated variations of place cell properties from proximal to distal area in dCA3 (Lee et al. 2015; Lu et al. 2015; Oliva et al. 2016) and from dorsal to ventral pole of CA3 (Kjelstrup et al. 2008). For instance, the scale of place field in CA3 increases almost linearly from the dorsal to ventral pole by nearly 10 times (Kjelstrup et al. 2008). It is unclear whether the heterogeneity of CA3 intrinsic excitability contributes to such proximodistal and dorsoventral variations in place cell formation. In addition, as intrinsic properties are known to play an important role in determining subthreshold resonance of hippocampal pyramidal neurons (Borel et al. 2013; Zemankovics et al. 2010), whether topographic variations of CA3 intrinsic properties can result in differential response of CA3 neurons to inputs at different frequencies remains to be tested. Genetic or pharmacological manipulations that can alter intrinsic excitability from selective geometric population of CA3 neurons or computational models that incorporate intrinsic excitability variations have the potential to help address these questions.
GRANTS
This work was supported by Grant K01MH117444 from the National Institute of Mental Health (to Q. Sun) and institutional support from Case Western Reserve University (to Q. Sun).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.S. conceived and designed research; Q.S., Y.-Q.J., and M.L. performed experiments; Q.S. and Y.-Q.J. analyzed data; Q.S. interpreted results of experiments; Q.S. and Y.-Q.J. prepared figures; Q.S. drafted manuscript; Q.S. edited and revised manuscript; Q.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank D. M. Katz and S. U. Lad for helping us with the Neurolucida software, Z. Dong for helping us with Golgi staining, and K. Jiao for assistance with Zeiss LSM 800 confocal microscope imaging.
REFERENCES
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci 9: 357–369, 2008. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- Besnard A, Miller SM, Sahay A. Distinct dorsal and ventral hippocampal CA3 outputs govern contextual fear discrimination. Cell Reports 30: 2360–2373.e5, 2020. doi: 10.1016/j.celrep.2020.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkey DK, Schwartzkroin PA. Variation in electrophysiology and morphology of hippocampal CA3 pyramidal cells. Brain Res 514: 77–83, 1990. doi: 10.1016/0006-8993(90)90437-G. [DOI] [PubMed] [Google Scholar]
- Borel M, Guadagna S, Jang HJ, Kwag J, Paulsen O. Frequency dependence of CA3 spike phase response arising from h-current properties. Front Cell Neurosci 7: 263, 2013. doi: 10.3389/fncel.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Maggi L, Di Castro MA, Catalano M, Migliore R, Migliore M, Scattoni ML, Calamandrei G, Cherubini E. Premature changes in neuronal excitability account for hippocampal network impairment and autistic-like behavior in neonatal BTBR T+tf/J mice. Sci Rep 6: 31696, 2016. [Erratum in Sci Rep 7: 39726, 2017.] doi: 10.1038/srep31696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, Spruston N. Heterogeneity within classical cell types is the rule: lessons from hippocampal pyramidal neurons. Nat Rev Neurosci 20: 193–204, 2019. doi: 10.1038/s41583-019-0125-5. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife 5: e14997, 2016. doi: 10.7554/eLife.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Dougherty KA, Islam T, Johnston D. Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J Physiol 590: 5707–5722, 2012. doi: 10.1113/jphysiol.2012.242693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KA, Nicholson DA, Diaz L, Buss EW, Neuman KM, Chetkovich DM, Johnston D. Differential expression of HCN subunits alters voltage-dependent gating of h-channels in CA1 pyramidal neurons from dorsal and ventral hippocampus. J Neurophysiol 109: 1940–1953, 2013. doi: 10.1152/jn.00010.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19, 2010. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman SJ, Schlögl A, Frotscher M, Jonas P. Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science 353: 1117–1123, 2016. doi: 10.1126/science.aaf1836. [DOI] [PubMed] [Google Scholar]
- Hemond P, Epstein D, Boley A, Migliore M, Ascoli GA, Jaffe DB. Distinct classes of pyramidal cells exhibit mutually exclusive firing patterns in hippocampal area CA3b. Hippocampus 18: 411–424, 2008. doi: 10.1002/hipo.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus 18: 1064–1073, 2008. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Hunt DL, Linaro D, Si B, Romani S, Spruston N. A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nat Neurosci 21: 985–995, 2018. doi: 10.1038/s41593-018-0172-7. [DOI] [PubMed] [Google Scholar]
- Hyun JH, Eom K, Lee KH, Ho WK, Lee SH. Activity-dependent downregulation of D-type K+ channel subunit Kv1.2 in rat hippocampal CA3 pyramidal neurons. J Physiol 591: 5525–5540, 2013. doi: 10.1113/jphysiol.2013.259002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol 362: 17–45, 1995. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol 295: 580–623, 1990. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jarsky T, Mady R, Kennedy B, Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J Comp Neurol 506: 535–547, 2008. doi: 10.1002/cne.21564. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem 14: 771–781, 2007. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kim S, Guzman SJ, Hu H, Jonas P. Active dendrites support efficient initiation of dendritic spikes in hippocampal CA3 pyramidal neurons. Nat Neurosci 15: 600–606, 2012. doi: 10.1038/nn.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science 321: 140–143, 2008. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kowalski J, Gan J, Jonas P, Pernía-Andrade AJ. Intrinsic membrane properties determine hippocampal differential firing pattern in vivo in anesthetized rats. Hippocampus 26: 668–682, 2016. doi: 10.1002/hipo.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Wang C, Deshmukh SS, Knierim JJ. Neural population evidence of functional heterogeneity along the CA3 transverse axis: pattern completion versus pattern separation. Neuron 87: 1093–1105, 2015. doi: 10.1016/j.neuron.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente De Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol (Leipzig) 46: 113–117, 1934. [Google Scholar]
- Lu L, Igarashi KM, Witter MP, Moser EI, Moser MB. Topography of place maps along the CA3-to-CA2 axis of the hippocampus. Neuron 87: 1078–1092, 2015. doi: 10.1016/j.neuron.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Makara JK, Magee JC. Variable dendritic integration in hippocampal CA3 pyramidal neurons. Neuron 80: 1438–1450, 2013. doi: 10.1016/j.neuron.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Dougherty KA, Parikh K, Byrne C, Johnston D. Mapping the electrophysiological and morphological properties of CA1 pyramidal neurons along the longitudinal hippocampal axis. Hippocampus 26: 341–361, 2016. doi: 10.1002/hipo.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Johnston D. Dendritic GIRK channels gate the integration window, plateau potentials, and induction of synaptic plasticity in dorsal but not ventral CA1 neurons. J Neurosci 37: 3940–3955, 2017. doi: 10.1523/JNEUROSCI.2784-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin B, Liu Z, Chen Y, Lewis AS, Becker A, McClelland S, Chetkovich DM, Migliore M, Baram TZ, Esclapez M, Bernard C. Dorsoventral differences in intrinsic properties in developing CA1 pyramidal cells. J Neurosci 32: 3736–3747, 2012a. doi: 10.1523/JNEUROSCI.5870-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin B, Lugo JN, Brewster AL, Liu Z, Lewis AS, McClelland S, Chetkovich DM, Baram TZ, Anderson AE, Becker A, Esclapez M, Bernard C. Differential dorso-ventral distributions of Kv4.2 and HCN proteins confer distinct integrative properties to hippocampal CA1 pyramidal cell distal dendrites. J Biol Chem 287: 17656–17661, 2012b. doi: 10.1074/jbc.C112.367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa LM, Benardo LS, Prince DA. Variations in electrophysiological properties of hippocampal neurons in different subfields. Brain Res 242: 341–344, 1982. doi: 10.1016/0006-8993(82)90320-1. [DOI] [PubMed] [Google Scholar]
- Masurkar AV, Tian C, Warren R, Reyes I, Lowes DC, Brann DH, Siegelbaum SA. Postsynaptic integrative properties of dorsal CA1 pyramidal neuron subpopulations. J Neurophysiol 123: 980–992, 2020. doi: 10.1152/jn.00397.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milshtein-Parush H, Frere S, Regev L, Lahav C, Benbenishty A, Ben-Eliyahu S, Goshen I, Slutsky I. Sensory deprivation triggers synaptic and intrinsic plasticity in the hippocampus. Cereb Cortex 27: 3457–3470, 2017. doi: 10.1093/cercor/bhx084. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Kim S, Guzman SJ, Jonas P. Symmetric spike timing-dependent plasticity at CA3-CA3 synapses optimizes storage and recall in autoassociative networks. Nat Commun 7: 11552, 2016. doi: 10.1038/ncomms11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13: 3916–3925, 1993. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura NH, Flasbeck V, Maingret N, Kitsukawa T, Sauvage MM. Proximodistal segregation of nonspatial information in CA3: preferential recruitment of a proximal CA3-distal CA1 network in nonspatial recognition memory. J Neurosci 33: 11506–11514, 2013. doi: 10.1523/JNEUROSCI.4480-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319: 1260–1264, 2008. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297: 211–218, 2002. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A. Spatial coding and physiological properties of hippocampal neurons in the Cornu Ammonis subregions. Hippocampus 26: 1593–1607, 2016. doi: 10.1002/hipo.22659. [DOI] [PubMed] [Google Scholar]
- Palacio S, Chevaleyre V, Brann DH, Murray KD, Piskorowski RA, Trimmer JS. Heterogeneity in Kv2 channel expression shapes action potential characteristics and firing patterns in CA1 versus CA2 hippocampal pyramidal neurons. eNeuro 4: ENEURO.0267-17.2017, 2017. doi: 10.1523/ENEURO.0267-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoropoulos C. Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Front Biosci 23: 109–145, 2018. doi: 10.2741/4584. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Asprodini E, Nikita I, Koutsona C, Kostopoulos G. Weaker synaptic inhibition in CA1 region of ventral compared to dorsal rat hippocampal slices. Brain Res 948: 117–121, 2002. doi: 10.1016/S0006-8993(02)02958-X. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. Dorsal-ventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neurosci Lett 286: 57–60, 2000. doi: 10.1016/S0304-3940(00)01084-3. [DOI] [PubMed] [Google Scholar]
- Pastoll H, Garden DL, Papastathopoulos I, Sürmeli G, Nolan MF. Inter- and intra-animal variation in the integrative properties of stellate cells in the medial entorhinal cortex. eLife 9: e52258, 2020. doi: 10.7554/eLife.52258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raus Balind S, Magó Á, Ahmadi M, Kis N, Varga-Németh Z, Lőrincz A, Makara JK. Diverse synaptic and dendritic mechanisms of complex spike burst generation in hippocampal CA3 pyramidal cells. Nat Commun 10: 1859, 2019. doi: 10.1038/s41467-019-09767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Carta M, Mulle C. Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding. Nat Rev Neurosci 18: 208–220, 2017. doi: 10.1038/nrn.2017.10. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A quantitative theory of the functions of the hippocampal CA3 network in memory. Front Cell Neurosci 7: 98, 2013a. doi: 10.3389/fncel.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci 7: 74, 2013b. doi: 10.3389/fnsys.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin D, Hattori S, Ybarra N, Musial TF, Buss EW, Richter H, Oh MM, Nicholson DA, Disterhoft JF. Aging-related hyperexcitability in CA3 pyramidal neurons is mediated by enhanced A-type K+ channel function and expression. J Neurosci 35: 13206–13218, 2015. doi: 10.1523/JNEUROSCI.0193-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldado-Magraner S, Brandalise F, Honnuraiah S, Pfeiffer M, Moulinier M, Gerber U, Douglas R. Conditioning by subthreshold synaptic input changes the intrinsic firing pattern of CA3 hippocampal neurons. J Neurophysiol 123: 90–106, 2020. doi: 10.1152/jn.00506.2019. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15: 655–669, 2014. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, Siegelbaum SA. Proximodistal heterogeneity of hippocampal CA3 pyramidal neuron intrinsic properties, connectivity, and reactivation during memory recall. Neuron 95: 656–672.e3, 2017. doi: 10.1016/j.neuron.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Srinivas KV, Sotayo A, Siegelbaum SA. Dendritic Na+ spikes enable cortical input to drive action potential output from hippocampal CA2 pyramidal neurons. eLife 3: e04551, 2014. doi: 10.7554/eLife.04551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir I, Daninos M, Yaari Y. Plasticity of intrinsic firing response gain in principal hippocampal neurons following pilocarpine-induced status epilepticus. Neuroscience 357: 325–337, 2017. doi: 10.1016/j.neuroscience.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, Cusick A, Riley ZL, Sunkin SM, Bernard A, Puchalski RB, Gage FH, Jones AR, Bajic VB, Hawrylycz MJ, Lein ES. Genomic anatomy of the hippocampus. Neuron 60: 1010–1021, 2008. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Turner DA, Li XG, Pyapali GK, Ylinen A, Buzsaki G. Morphometric and electrical properties of reconstructed hippocampal CA3 neurons recorded in vivo. J Comp Neurol 356: 580–594, 1995. doi: 10.1002/cne.903560408. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci 34: 89–103, 2011. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Witter MP. Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn Mem 14: 705–713, 2007. doi: 10.1101/lm.725207. [DOI] [PubMed] [Google Scholar]
- Zemankovics R, Káli S, Paulsen O, Freund TF, Hájos N. Differences in subthreshold resonance of hippocampal pyramidal cells and interneurons: the role of h-current and passive membrane characteristics. J Physiol 588: 2109–2132, 2010. doi: 10.1113/jphysiol.2009.185975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885–900, 2003. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]