Abstract
Previously, an essential tremor-like phenotype has been noted in animals with a global knockout of the GABAAα1 subunit. Given the hypothesized role of the cerebellum in tremor, including essential tremor, we used transgenic mice to selectively knock out the GABAAα1 subunit from cerebellar Purkinje cells. We examined the resulting phenotype regarding impacts on inhibitory postsynaptic currents, survival rates, gross motor abilities, and expression of tremor. Purkinje cell specific knockout of the GABAAα1 subunit abolished all GABAA-mediated inhibition in Purkinje cells, while leaving GABAA-mediated inhibition to cerebellar molecular layer interneurons intact. Selective loss of GABAAα1 from Purkinje cells did not produce deficits on the accelerating rotarod, nor did it result in decreased survival rates. However, a tremor phenotype was apparent, regardless of sex or background strain. This tremor mimicked the tremor seen in animals with a global knockout of the GABAAα1 subunit, and, like essential tremor in patients, was responsive to ethanol. These findings indicate that reduced inhibition to Purkinje cells is sufficient to induce a tremor phenotype, highlighting the importance of the cerebellum, inhibition, and Purkinje cells in tremor.
NEW & NOTEWORTHY Animals with a global knockout of the GABAAα1 subunit show a tremor phenotype reminiscent of essential tremor. Here we show that selective knockout of GABAAα1 from Purkinje cells is sufficient to produce a tremor phenotype, although this tremor is less severe than seen in animals with a global knockout. These findings illustrate that the cerebellum can play a key role in the genesis of the observed tremor phenotype.
Keywords: alcohol, cerebellum, essential tremor, GABA, inhibition
INTRODUCTION
Essential tremor is one of the most common, yet least understood movement disorders, with prevalence rates surpassing Parkinson’s disease (Hopfner et al. 2016; Louis 2012; Louis and Ottman 2014; Louis and Vonsattel 2008; Shah 2017). A major limitation in the study of essential tremor is a lack of sufficient animal models available to study the underlying brain circuitry, the pharmacology behind antitremor drugs, and the development of novel treatments (Miwa 2007). Mice with a global knockout of the GABAAα1 subunit have been proposed as a model of essential tremor (Kralic et al. 2005). The tremor observed in global GABAAα1 knockout mice shows similar pharmacology to human essential tremor, including sensitivity to ethanol (Hopfner et al. 2015; Kralic et al. 2005). A role for dysfunction in GABAergic signaling in tremor is more broadly supported (Gironell 2014; Louis 2018; Málly and Baranyi 1994; Marin-Lahoz and Gironell 2016; Paris-Robidas et al. 2012). However, as GABAAα1 is expressed in a variety of cell types throughout the central nervous system, it is unclear which brain region(s) may be responsible for the tremor phenotype in global GABAAα1 knockout mice.
The cerebellum has been implicated in essential tremor (Benito-León and Labiano-Fontcuberta 2016; Handforth 2016; Llinás and Yarom 1986; Louis 2014; Marin-Lahoz and Gironell 2016) (but see also, e.g., Chahine and Ghosh 2009; Jankovic and Noebels 2005; Lenka et al. 2017; Pan et al. 2018; Pahapill et al. 1999; Pedrosa et al. 2017 regarding other important areas) and postmortem studies have repeatedly found significant pathology in the cerebellum of essential tremor patients (Erickson-Davis et al. 2010; Louis 2018; Louis and Vonsattel 2008; Louis et al. 2014b; Paris-Robidas et al. 2012; Shill et al. 2008). Similarly, tremor (rest, action, orthostatic, and intentional) phenotypes are noted in a range of disorders with significant cerebellar components, including spinocerebellar ataxias (SCAs) (Lenka and Louis 2019; Paulson et al. 2017). We hypothesized therefore that selective loss of GABAAα1 from cerebellar Purkinje cells could recapitulate the tremor phenotype observed in global GABAAα1 knockout animals. If a tremor phenotype is observable when GABAAα1 is removed exclusively from Purkinje cells, a key role of the cerebellum in tremor would be firmly established, and other nontremor phenotypes associated with the global knockout of GABAAα1 may be avoided.
As subunit composition contributes to channel kinetics (Bacci et al. 2003; Krook-Magnuson and Huntsman 2007; Lavoie et al. 1997), removal of the GABAAα1 subunit can alter the decay kinetics of GABAA-mediated inhibitory postsynaptic currents (IPSCs) (Kralic et al. 2006; Schneider Gasser et al. 2007). Therefore, in the global GABAAα1 knockout mice it is additionally unclear whether the tremor phenotype arises from a deficit of inhibition or an alteration in decay kinetics (Zhou et al. 2013). However, while most neurons express multiple isoforms of the α subunit, adult Purkinje cells appear to exclusively express the α1 isoform (Laurie et al. 1992; Patrizi et al. 2008). As all known functional GABAA receptors contain an α subunit (Chua and Chebib 2017) (with the notable exception of ρ-containing “GABAC” receptors), selective knockout of the GABAAα1 subunit can result in complete loss of GABAA-mediated inhibition in Purkinje cells (Fritschy and Panzanelli 2006; Fritschy et al. 2006; Patrizi et al. 2008). In this case, if a tremor phenotype is observed in animals with a selective knockout of the GABAAα1 subunit from Purkinje cells, it will be attributable specifically to a deficit of inhibition, rather than a change in the kinetics of remaining inhibition. This would provide further insight into the pathophysiology underlying tremor.
To examine these issues, we produced mice with a selective knockout of GABAAα1 from Purkinje cells. Previous studies have suggested that the phenotype displayed in the global knockout model may depend on the background strain of the mice (Arain et al. 2012). Therefore, we examined the resulting phenotype in Purkinje cell-specific GABAAα1 knockout mice on both mixed and predominately black6 genetic backgrounds. We find that a tremor phenotype is present in animals with a knockout of GABAAα1 selectively from Purkinje cells, regardless of the background of the animal. These findings firmly establish the relevance of the cerebellum (and inhibition to Purkinje cells in particular) to the observed tremor.
METHODS
All experimental protocols were approved by the University of Minnesota’s Institutional Animal Care and Use Committee.
Animals.
In brief, we generated mice with a global or a Purkinje cell specific knockout of GABAAα1, by crossing floxed-GABAAα1 mice with mice expressing Cre either globally or with Cre expression restricted to Purkinje cells, as detailed below. As the phenotype of the global GABAAα1 knockout mouse has been suggested to be dependent on the background of the animals (Arain et al. 2012; Kralic et al. 2005), we produced mice with a selective knockout (KO) of GABAAα1 from Purkinje cells (PC) on both a predominately black6 background (“PC-KO-Bl6” mice) and with a mixed background (“PC-KO-Mixed”).
For the black6 background, we crossed floxed-GABAAα1 mice on a black6 background (B6.129(FVB)-Gabra1tm1Geh/J; Jackson stock number: 004318) (Kralic et al. 2005; Vicini et al. 2001) with mice expressing Cre-recombinase in Purkinje cells, also on a black6 background (Pcp2-Cre mice; B6.Cg-Tg(Pcp2-cre)3555Jdhu/J; Jackson stock number: 010536) (Krook-Magnuson et al. 2014; Zhang et al. 2004). Note that Jackson Laboratories frequently moves donated lines to a black6 (or, more specifically, a C57BL/6J) background, as was done for floxed-GABAAα1 mice (donated to Jackson Laboratories by Dr. Gregg Homanics). Floxed-GABAAα1 mice were maintained in house by crossing floxed-GABAAα1 mice with floxed-GABAAα1 mice. Pcp-Cre mice were bred to C57BL/6N for at least 10 generations before arrival at Jackson Laboratory (original animals donated to Jackson by Dr. Jian-dong Huang) and were purchased from Jackson (/J) as hemizygotes. Pcp-Cre mice were maintained in house as hemizygotes, by crossing with black6 animals. Black6 animals used for breeding were either purchased directly from Jackson (C57BL/6J) or were bred in-house (with founders purchased from Jackson: C57BL/6J; stock number 004318). To generate experimental animals, Pcp-Cre-x-floxed-GABAAα1 mice were subsequently recrossed with floxed-GABAAα1 mice, producing 1) animals homozygous for wild-type (WT) GABAAα1 and/or Cre negative (“PC-WT-Bl6”), 2) animals that were Cre positive and hemizygous (HEMI) for floxed-GABAAα1 (“PC-HEMI-Bl6”), and 3) animals that were Cre positive and homozygous for floxed-GABAAα1 (“PC-KO-Bl6”).
As lines purchased from Jackson were on black6 backgrounds, to generate animals with a mixed background, additional initial breeding was necessary. Specifically, we crossed floxed-GABAAα1 breeders with both FVB/NJ (Jackson stock number 001800) and 129S1/SvlmJ (Jackson stock number 002448) mice. FVB/NJ-x-floxed-GABAAα1 mice were maintained by crossing with other FVB/NJ-x-floxed-GABAAα1 mice. 129S1/SvlmJ-x-floxed-GABAAα1 mice were similarly maintained by crossing with other 129S1/SvlmJ-x-floxed-GABAAα1 mice. Experimental animals were produced by further crossing with Pcp-Cre animals (on a black6 background, see above), ultimately producing 1) animals homozygous for WT GABAAα1 and/or Cre negative (“PC-WT-Mixed”), 2) animals that were Cre positive and hemizygous for floxed-GABAAα1 (“PC-HEMI-Mixed”), and 3) animals that were Cre positive and homozygous for floxed-GABAAα1 (“PC-KO-Mixed”). No attempt was made to make these mice genetically homogenous.
While Cre expression is also found in retinal bipolar neurons in Pcp2-Cre animals, the Pcp2-Cre line used [B6.Cg-Tg(Pcp2-cre)3555Jdhu/J] does not show widespread leaky expression (Krook-Magnuson et al. 2014; Witter et al. 2016). Work by others suggests that Purkinje cell expression of Cre starts within the first postnatal week (Sługocka et al. 2017), resulting in gradual loss of GABAAα1 immunoreactivity from Purkinje cells during the second and third postnatal weeks (Briatore et al. 2010), after the establishment of climbing fiber one-to-one innervation of Purkinje cells (Nakayama et al. 2012). While our previous work has examined the fidelity of the Pcp2-Cre line (Krook-Magnuson et al. 2014), to ensure not only selective expression of Cre in Purkinje cells, but also widespread expression of Cre in Purkinje cells across the cerebellum, we additionally crossed Pcp2-Cre animals with a tdTomato Cre-reporter mouse line [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, Jackson Laboratories Stock number 007905, also referred to as Ai9, donated to Jackson Laboratories by Dr. Hongkui Zeng]. This tissue was then cleared and imaged, as detailed more below, under Tissue clearing and imaging.
Animals with a global knockout of GABAAα1 (“Global-KO”) were also generated, allowing a direct comparison of tremor phenotypes in this study. To mimic previous work examining tremor in global knockout animals, we generated Global-KO animals on a mixed background. First, floxed-GABAAα1 mice were crossed with FVB/NJ and 129S1/SvlmJ mice as described above. FVB/NJ-x-floxed-GABAAα1 mice were then crossed with mice broadly expressing Cre recombinase [B6.C-Tg(CMV-cre)1Cgn/J; Jackson stock number: 006054; note that this includes germline expression, such that continued expression of Cre is not necessary]. Offspring were then crossed with 129S1/SvlmJ-x-floxed-GABAAα1 mice, offspring from this litter crossed, and the resulting litter then crossed with C57BL/6J animals (such that 129S1/SvlmJ, FVB/NJ, and C57BL/6J all contributed to the genetic “mixed” makeup and Cre expression was removed from the line). This resulted in a Global line, which was maintained by crossing with other Global animals, and which produces 1) homozygous, wild-type animals (“Global-WT”), 2) animals with one copy of the GABAAα1 gene (“Global-HEMI”), and 3) animals null for the GABAAα1 gene (Global-KO). No attempt was made to make these mice genetically homogenous.
Animals were sexed at the time of weaning on the basis of external genitalia. Both male and female animals were used in experiments. Where found, sex differences are noted in the results section.
Slice electrophysiology.
Mice (23–392 days old) were anesthetized with isoflurane inhalation followed by intraperitoneal (i.p.) injection of 2,2,2-tribromoethanol or by 2,2,2-tribromoethanol injection alone and intracardially perfused with ice-cold cutting solution before decapitation. The brain was removed from the skull, and the cerebellum was dissected in ice-cold sucrose cutting solution containing (in mM) 85 NaCl, 75 sucrose, 2.5 KCl, 25 glucose, 1.25 NaH2PO4, 4 MgCl2, 0.5 CaCl2, and 24 NaHCO3. Sagittal slices of the cerebellum (300 μm) were cut and incubated for 30 min at 35°C in the same sucrose cutting solution and subsequently incubated at room temperature until recordings. Recordings were performed in artificial cerebrospinal fluid containing (in mM) 2.5 KCl, 10 glucose, 126 NaCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, and 26 NaHCO3, at ∼32°C. Pipettes (2–5 MΩ) were filled with an intracellular solution consisting of (in mM) 110 CsCl, 35 CsF, 10 HEPES, 10 EGTA.
Inhibitory postsynaptic currents (IPSCs) were recorded with a holding potential of −60 mV and in the presence of the AMPAR and NMDAR antagonists NBQX (5 µM; Tocris, no. 0373) and APV (10 µM; Tocris, no. 0106). In a subset of cells, zolpidem (1 µM; Sigma, no. Z103)—a GABAAα1 subunit-selective positive modulator—was bath applied to assay the subunit composition of GABAA receptors mediating IPSCs. To confirm that IPSCs were due to GABAA receptor activation, the GABAA receptor antagonist gabazine (5 µM; Sigma, no. S106) was bath applied at the end of the experiment. IPSCs were analyzed using Clampfit 10.7, including its event detection feature. A weighted τ was calculated per cell on an averaged IPSC according to the equation , where A is the amplitude of each component and τ is the associated time constant.
Tissue clearing and imaging.
To examine the extent of Cre expression in the cerebellum, Pcp2-Cre mice were crossed with tdTomato mice, as detailed above. A resulting Pcp2-tdTomato mouse was heavily anesthetized with isoflurane and perfused with ice-cold phosphate-buffered saline followed by a hydrogel hybridization solution (Chung et al. 2013; Epp et al. 2015; Yang et al. 2014). Following perfusion, the tissue was dissected out and prepared for tissue clearing: tissue was cross-linked and hybridized to hydrogel monomers (Chung et al. 2013; Epp et al. 2015; Yang et al. 2014). The tissue was then passively cleared in a 4% sodium dodecyl sulfate clearing solution at room temperature for 16 days (Yang et al. 2014). Once cleared, the tissue was stored in phosphate-buffered saline with 0.1% Triton and 20% sucrose to reduce tissue swelling. Twenty-four hours before imaging, the tissue was transferred to a refractive index-matched solution (RIMS, refractive index: 1.46; Yang et al. 2014) for equilibration. Tissue was then imaged in RIMS on a Caliber I.D. RS-G4 ribbon scanning confocal microscope equipped with a ×10 PlanApo glycerol immersion objective (0.5 NA, 5.5-mm working distance) with a correction collar. The whole cerebellum was imaged with assistance from the University of Minnesota’s Imaging Center. Supplemental Video S1 (available at https://doi.org/10.6084/m9.figshare.12733424), showing this tissue, was created using Imaris and ImageJ.
Kaplan–Meier survival curves.
To examine whether PC-KO animals displayed any early mortality, detailed record keeping was maintained, noting the date and cause of death for each animal. Animals euthanized solely for experimental needs (e.g., electrophysiology), or because the animal was no longer needed for experiments, were counted as censored observations. Euthanasia due to health concerns was considered a terminal time point (i.e., death, rather than a censored observation). Animals still alive at the time of calculation of survival curves had the date of calculation as their final observation point (censored time point).
Accelerating rotarod.
Accelerating rotarod testing and analysis was performed utilizing resources of the Mouse Behavior Core, University of Minnesota. The experimenter was blinded to the genotypes. The rotarod apparatus had a diameter of 3 cm (Ugo Basile, no. 47600) and was set to a minimum speed of 5 revolutions per minute (RPM) and a maximum speed of 50 RPM, with an acceleration rate of 9 RPM per minute. A trial was deemed complete when the animal fell onto the paddle below or made two consecutive rotations without recovery of stepping. Animals were run for 4 trials a day with 15–20 min of rest in their home cage between trials. During the “training period,” animals were run on the task for 4 consecutive days (4 trials a day). During the “testing period,” mice were tested once a week for 5 weeks (4 trials a day) to assess retention on the task.
Tail suspension.
Previous studies using a global knockout of GABAAα1 have examined tremor while the animal was suspended by the tail (Handforth et al. 2010; Kralic et al. 2005; Quesada et al. 2011). Tremor in this study was therefore also measured using the tail-suspension technique. Our custom-made tail-suspension apparatus included a miniature load cell (force transducer: Honeywell, Golden Valley, MN, model 31) attached to an in-line amplifier (Sensotec Inc., Columbus, OH, part number 008-0296-00). Signals were digitized (DAQ, National Instruments USB-6221) and recorded using custom MATLAB-based software. A rotating attachment (i.e., rotary joint or commutator) allowed the animals to be secured by the tail to the transducer while minimizing the risk of torque-induced damage to the transducer. Except when testing the pharmacological sensitivity of the tremor, trials lasted 10 min, with data averaged across trials per animal. When testing the pharmacological sensitivity of the tremor, an initial baseline trial of 5-min tail suspension was followed by an i.p. injection of either 1.25 g/kg ethanol (at 10 mL/kg) or saline (also 10 mL/kg). Tail-suspension trials (5 min duration each) were then completed 20 min, 40 min, and 60 min after i.p. injection to assess the impact of ethanol administration on the recorded tremor. Habituation, saline, and ethanol experiments were run on separate days, with at least 1 day between experiments.
For each file, body weight of the animal was calculated based on the average recorded signal; these values closely matched weights manually collected and recorded. In addition to a tremor phenotype, knockout animals often displayed other phenotypes (e.g., clasping) during tail suspension; these abnormalities were not quantified. To analyze tremor, recorded force transducer signals were converted into percent change in force (relative to the mean force, i.e., weight). Similar to previous studies which have examined the relative power of the signal in the tremor band (Martin et al. 2005; Paterson et al. 2009; Quesada et al. 2011; Wang and Fowler 2001), we examined the ratio of the power in the tremor range (20–30 Hz) to the power in the adjacent ranges (15–20 Hz and 30–35 Hz). Power in each band was calculated using the matlab function bandpower. Power spectral density (PSD) plots, and detailed analyses of bouts of tremor (described in the paragraph below), were done using the Chronux (http://chronux.org/) library (Mitra and Bokil 2007).
For a more detailed assessment of tremors, we additionally examined individual tremor bouts, using the following operational definition of a tremor bout: the maximum power in the tremor band (20–30 Hz) exceeds a threshold of 10−4.5 and the portion of power (from 0 to 50 Hz) present in the tremor band (i.e., 20–30 Hz) exceeds a threshold of 0.4 (40%); these thresholds were determined empirically using data sets with clear tremor envelopes. Initial detections were combined if the gap separating the detections was less than 0.1 s. The length of bouts was calculated with the start of the bout defined as the moment the enveloped signal (using matlab command envelope) first grew larger than a threshold of 9% change in force (relative to mean force), and the end as the moment it dropped below that threshold. Flagged “bouts” with a duration less than 0.15 s were removed and not further considered in analyses. Similar results were obtained if bouts were detected using a Z-score based approach (Supplemental Fig. S3; all Supplemental figures and legends are available at http://doi.org/10.6084/m9.figshare.12756206).
Statistical analysis software used.
Data sets were analyzed using Excel, Clampfit 10.7, OriginPro, and MATLAB, including the Chronux (http://chronux.org/) library (Mitra and Bokil 2007).
RESULTS
Selective knockout of the GABAAα1 subunit from Purkinje cells causes a loss of synaptic inhibition to Purkinje cells.
To confirm that our approach resulted in selective knockout of the GABAAα1 subunit from Purkinje cells, we performed whole-cell patch-clamp recordings of IPSCs from Purkinje cells in cerebellar slices from PC-KO, PC-WT, and PC-HEMI mice (Fig. 1).
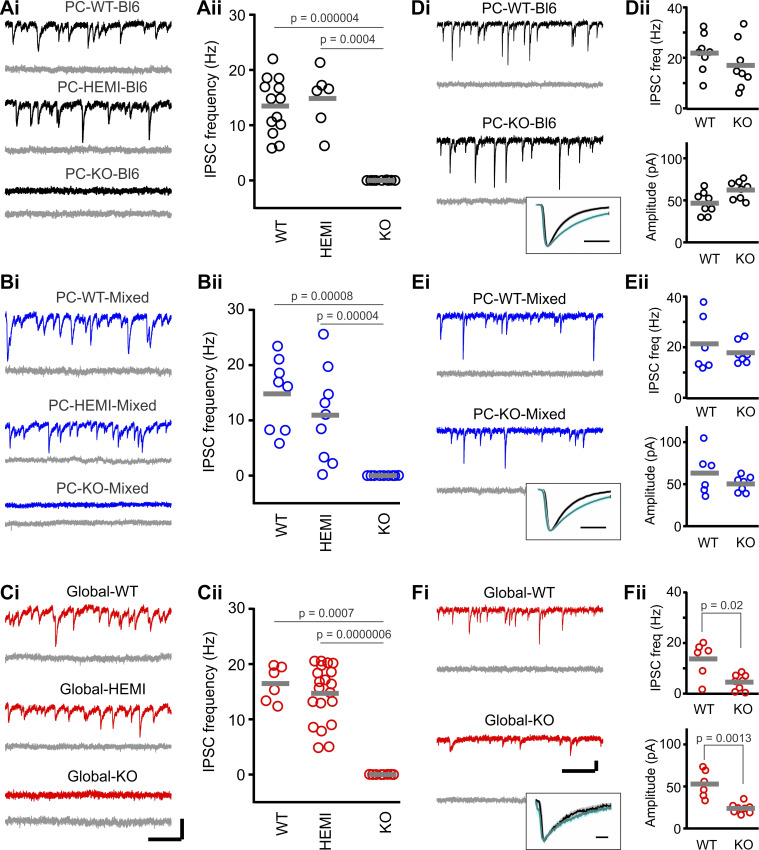
Fig. 1.
In PC-KO mice, GABAA-mediated inhibitory postsynaptic currents (IPSCs) are selectively lost in Purkinje cells (PCs). Spontaneous IPSCs recorded from Purkinje cells (subsequently blocked by gabazine, gray traces) in PC-Bl6 (A), PC-Mixed (B), and Global (C) wild-type (WT), hemizygous (HEMI), and knockout (KO) animals. D–F: IPSCs recorded from molecular layer interneurons. Insets: average molecular layer interneuron IPSCs (normalized amplitude) before (black) or in the presence of zolpidem (teal traces) for KO animals. Light shading indicates SE. Note that IPSCs are lost in Purkinje cells in KO animals, but are unaffected in PC-KO molecular layer interneurons. Gray bars in ii columns indicate means. Scale bars: A–C: 100 pA; 200 ms. D–F: 50 pA; 200 ms; 5 ms, insets. See also Table 1. Freq., frequency.
PC-HEMI mice, which still express one functional copy of the GABAAα1 gene, displayed a frequency of IPSCs that was not statistically different from WT animals (Table 1; Fig. 1, A and B). Similarly, amplitude and decay kinetics were not statistically different between PC-HEMI and PC-WT animals (Table 1), indicating that GABAA-mediated synaptic inhibition is maintained at wild-type levels in hemizygous animals.
Table 1.
Properties of Purkinje cell and molecular layer interneuron IPSCs
| Genotype | Frequency, Hz | Amplitude, pA | Decay Tau, ms | no. of cells | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC-BL6: Purkinje Cells | ||||||||||
| WT | 13.5 ± 1.4 | Kruskal–Wallis | P = 0.00012 | 80.4 ± 23.3 | MW WT vs. HEMI | 13.5 ± 1.5 | MW WT vs. HEMI | P = 0.18 | 13 | |
| HEMI | 14.8 ± 2.1 | MW WT vs. HEMI | P = 0.64 | 44.0 ± 6.1 | P = 0.15 | 10.5 ± 2.6 | 6 | |||
| KO | 0.01 ± 0.01 | MW KO vs. WT | P = 0.000004 | 9 | ||||||
| MW KO vs. HEMI | P = 0.0004 | |||||||||
| PC-BL6: Molecular Layer Interneurons | ||||||||||
| WT | 21.9 ± 2.6 | MW WT vs. KO | P = 0.19 | 46.6 ± 4.8 | MW WT vs. KO | 4.1 ± 0.4 | MW WT vs. KO | P = 0.4 | 8 | |
| KO | 17.0 ± 3.4 | 62.3 ± 3.8 | P = 0.05 | 3.6 ± 0.4 | 8 | |||||
| PC-Mixed: Purkinje Cells | ||||||||||
| WT | 14.8 ± 2.3 | Kruskal–Wallis | P = 0.00013 | 52.3 ± 11.8 | MW WT vs. HEMI | 12.5 ± 1.3 | MW WT vs. HEMI | P = 0.38 | 8 | |
| HEMI | 10.9 ± 2.8 | MW WT vs. HEMI | P = 0.37 | 45.9 ± 17.4 | P = 0.32 | 13.8 ± 1.4 | 9 | |||
| KO | 0.002 ± 0.002 | MW KO vs. WT | P = 0.00008 | 9 | ||||||
| MW KO vs. HEMI | P = 0.00004 | |||||||||
| PC-Mixed: Molecular Layer Interneurons | ||||||||||
| WT | 21.3 ± 4.5 | MW WT vs. KO | P = 0.84 | 63.1 ± 10.5 | MW WT vs. KO | 4.3 ± 0.4 | MW WT vs. KO | P = 0.14 | 6 | |
| KO | 17.8 ± 1.6 | 50.3 ± 3.5 | P = 0.53 | 4.0 ± 0.2 | 7 | |||||
| Global: Purkinje Cells | ||||||||||
| WT | 16.5 ± 1.3 | Kruskal–Wallis | P = 0.000124 | 106.7 ± 30.5 | MW WT vs. HEMI | 15.3 ± 1.6 | MW WT vs. HEMI | P = 0.92 | 6 | |
| HEMI | 14.7 ± 1.2 | MW WT vs. HEMI | P = 0.71 | 58.1 ± 5.7 | P = 0.32 | 15.3 ± 1.5 | 20 | |||
| KO | 0.01 ± 0.005 | MW KO vs. WT | P = 0.0007 | 8 | ||||||
| MW KO vs. HEMI | P = 0.0000006 | |||||||||
| Global: Molecular Layer Interneurons | ||||||||||
| WT | 13.7 ± 2.9 | MW WT vs. KO | P = 0.02 | 52.9 ± 6.6 | MW WT vs. KO | 4.3 ± 0.2 | MW WT vs. KO | P = 0.0007 | 6 | |
| KO | 4.5 ± 1.1 | 23.9 ± 2.1 | P = 0.0013 | 12.2 ± 1.1 | 8 | |||||
Average (±SE) values and statistical comparisons of inhibitory postsynaptic currents (IPSCs) by cell type and mouse line. Note that no values are given for amplitude or decay for Purkinje cells from KO animals, and one cell was excluded from PC-Mixed-HEMI decay analysis, due to too few IPSCs. HEMI, hemizygous; KO, knockout; MW, Mann–Whitney tests; PC, Purkinje cell; WT, wild-type.
In contrast, Purkinje cells from PC-KO animals displayed a loss of IPSCs (Table 1; Fig. 1, A and B). This was true of all Purkinje cells recorded from either strain (Fig. 1, A and B). These findings support previous work indicating that the α1 subunit is the only α subunit expressed in adult Purkinje cells, and loss of the α1 subunit results in a loss of all GABAA-mediated synaptic inhibition in Purkinje cells, without a compensatory increase in the expression of a different GABAAα subunit (Fritschy and Panzanelli 2006; Fritschy et al. 2006; Patrizi et al. 2008). For comparison, we also examined IPSCs in animals with a global, rather than Purkinje cell-specific, knockout of GABAAα1 (“Global-KO”). As expected, similar to PC-KO animals, global knockout of the GABAAα1 subunit resulted in a loss of IPSCs in Purkinje cells (Table 1; Fig. 1C). These data show our approach is able to knockout the α1 subunit from Purkinje cells, which results in a loss of synaptic GABAergic IPSCs in Purkinje cells.
To further confirm the widespread expression of Cre in Purkinje cells across the cerebellum, we crossed Pcp2-Cre mice with a tdTomato reporter, euthanized the animal at P23, performed tissue clearing, and imaged the entire cerebellum. Selective expression was visible in Purkinje cells across the entire cerebellum (Supplemental Video S1; available at https://doi.org/10.6084/m9.figshare.12733424).
Selective knockout of GABAAα1 from Purkinje cells leaves inhibition to other cells intact.
To check the specificity of our Purkinje cell-specific GABAAα1 knockout, we recorded IPSCs from cerebellar molecular layer interneurons which should not be directly impacted in Purkinje cell selective knockout animals. As anticipated, IPSCs recorded from molecular layer interneurons displayed similar amplitude, decay kinetics, and frequency in PC-WT and PC-KO animals (Table 1, Fig. 1, D and E). In a subset of molecular layer interneurons, we applied zolpidem, a positive allosteric modulator of GABAA receptors containing the α1 subunit (Olsen and Sieghart 2008), to further confirm the presence of the GABAAα1 subunit. As expected, we found a consistent increase in the decay tau after the application of 1 µM zolpidem (weighted τ pre-zolpidem: 4.2 ± 0.2 ms, vs. post-zolpidem: 8.6 ± 1.0 ms, n = 11 molecular layer interneurons, P = 0.001, Wilcoxon, Fig. 1, D and E, insets), further confirming the expression of the GABAAα1 subunit in molecular layer interneurons in PC-KO animals.
We also examined IPSCs in molecular layer interneurons in Global-KO animals, as done previously by Vicini and colleagues (Vicini et al. 2001), and confirmed that the frequency and amplitude of IPSCs were significantly reduced (Table 1, Fig. 1F). Moreover, due to lack of α1 expression, the decay kinetics of IPSCs in molecular layer interneurons were altered in Global-KO animals (Table 1) and were insensitive to zolpidem (n = 6 cells, P = 0.16 Wilcoxon, Fig. 1F insets). These results are in contrast to IPSCs in molecular layer interneurons in PC-KO animals and further affirms the sensitivity of our methods.
Therefore, in Global-KO animals, observed phenotypes may be due to effects on Purkinje cells and/or other cell types, and due to deficits of inhibition (in the case of Purkinje cells) and/or changes in kinetics of inhibition (e.g., in molecular layer interneurons). In contrast, phenotypes observed in PC-KO animals can be attributed, specifically, to loss of inhibition to Purkinje cells.
Selective knockout of GABAAα1 from Purkinje cells does not reduce survival rates.
Having confirmed the specificity of our selective knockout approach, we next sought to examine resulting phenotypes. Mice with a global knockout of the GABAAα1 subunit can display early death (Arain et al. 2012; Sur et al. 2001). We also observed this early mortality in our hands (P < 0.0001, Log Rank, comparing survival rates between genotypes in the global knockout line). Early mortality in Global-KO animals was concentrated around the time of weaning (Fig. 2A), with higher rates of mortality in males (Fig. 2B). There was also, as previously reported (Arain et al. 2012; Kralic et al. 2002), a decrease in body weight among Global-KO animals [WT vs. HEMI: P = 0.88, KO vs. WT: P < 0.0001, KO vs. HEMI: P < 0.0001, F test].
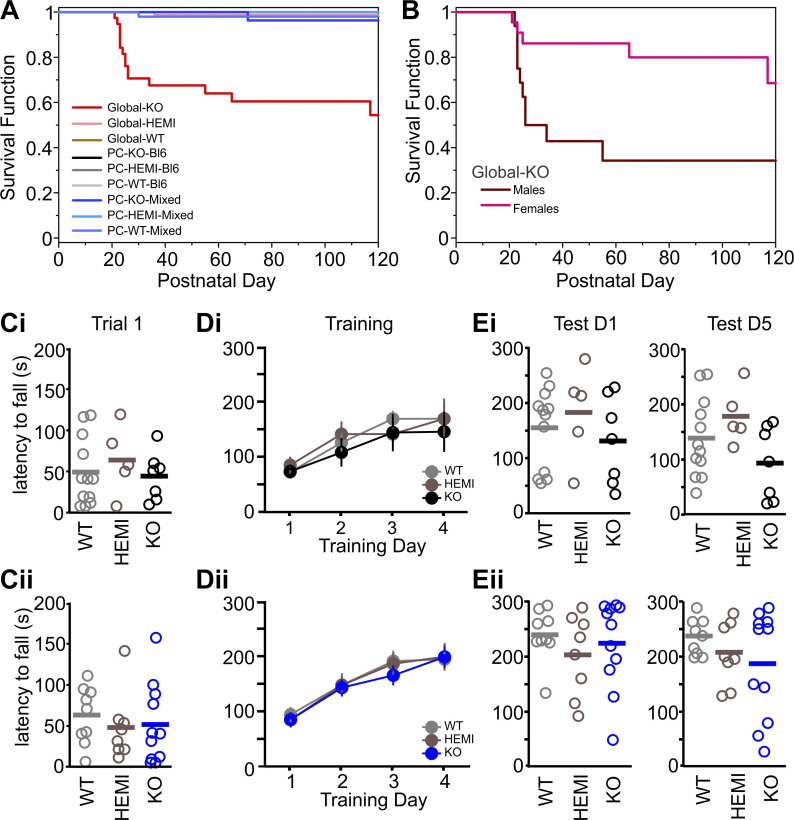
Fig. 2.
PC-KOs do not show reduced survival or impaired rotarod performance. A: Kaplan–Meier survival curves show decreased survival rates in Global-KO animals (red trace), especially around the time of weaning (P < 0.0001 across genotypes and mouse lines, Log Rank). Note that PC-KO animals show no increased mortality, and therefore lines overlap. B: decreased survival in Global-KO animals is especially pronounced in male animals (maroon line: male; pink line: female; male versus female P = 0.01, Log Rank). C–E: PC-KOs show no impairment on the accelerating rotarod task: mean latency to fall from the rotarod for the first trial of the first training day (C), across the 4-day training period (D), and on the first testing day (1 wk posttraining, Test D1, E left) and last testing day (5 wk posttraining, Test D5, E right) by genotype in PC-WT, PC-HEMI, and PC-KO animals on a Bl6 background (i) or Mixed background (ii). Bars: mean. HEMI, hemizygous; KO, knockout; PC, Purkinje cell; WT, wild-type.
In contrast, PC-KO mice were generated at the expected Mendelian ratios, and there were no differences in survival rates between PC-KO, PC-HEMI, and PC-WT animals, for either background (Fig. 2A). These data indicate that the decreased survival in Global-KO animals is not due to the loss of GABAAα1 from Purkinje cells.
Selective knockout of GABAAα1 from Purkinje cells does not produce deficits on the accelerating rotarod test.
As an initial assessment of gross motor abilities in the PC-KO animals, we utilized the accelerating rotarod test (Fig. 2, C–E). This allowed us to examine both baseline performance (by examining the first trial of the first testing day) as well as motor learning (by examining performance across training days) and retention (by examining performance across testing days). We found no significant differences between PC-KO, PC-HEMI, and PC-WT mice at baseline performance, regardless of background strain [training day 1, trial 1, latency to fall: PC-WT-Bl6: 47 ± 11.8 s, PC-HEMI-Bl6: 62 ± 18.4 s, PC-KO-Bl6: 42 ± 11.0 s, P = 0.71 Kruskal–Wallis test (KW); PC-WT-Mixed: 62 ± 11.6 s, PC-HEMI-Mixed: 47 ± 14.4 s, PC-KO-Mixed: 51 ± 14.6 s, P = 0.55 KW; Fig. 2C], indicating similar initial motor performance. We also found no difference between PC-KO, PC-HEMI, and PC-WT mice, regardless of background strain, during training (training day * genotype: Black6 background P = 0.83, Mixed background P = 0.72; two-way mixed-effects ANOVAs; Fig. 2D), indicating similar levels of motor learning across training trials. Finally, no difference was observed in performance on testing days (first test day: Black6 background P = 0.62 KW, Mixed background P = 0.59 KW; last test day: Black6 background P = 0.14 KW, Mixed background P = 0.51 KW; Fig. 2E), suggesting similar retention of motor learning.
Selective knockout of GABAAα1 from Purkinje cells results in a tremor phenotype.
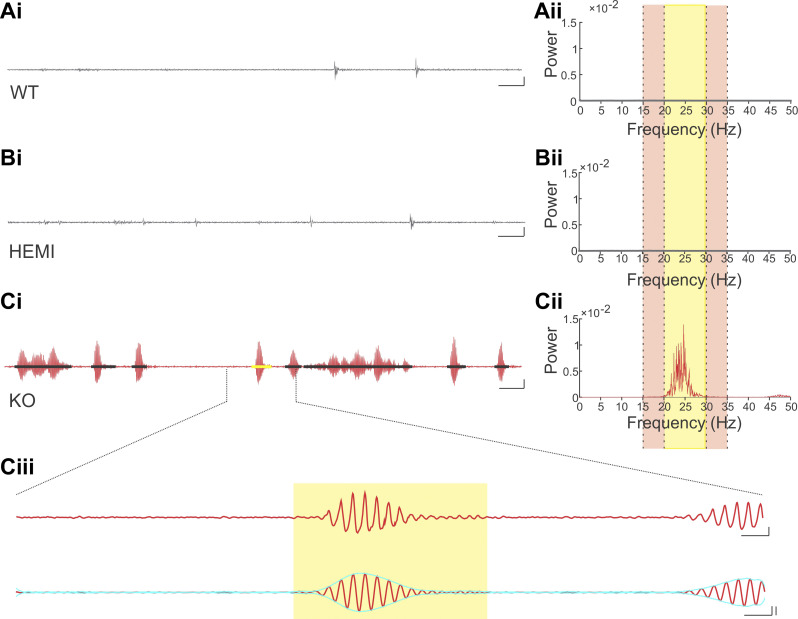
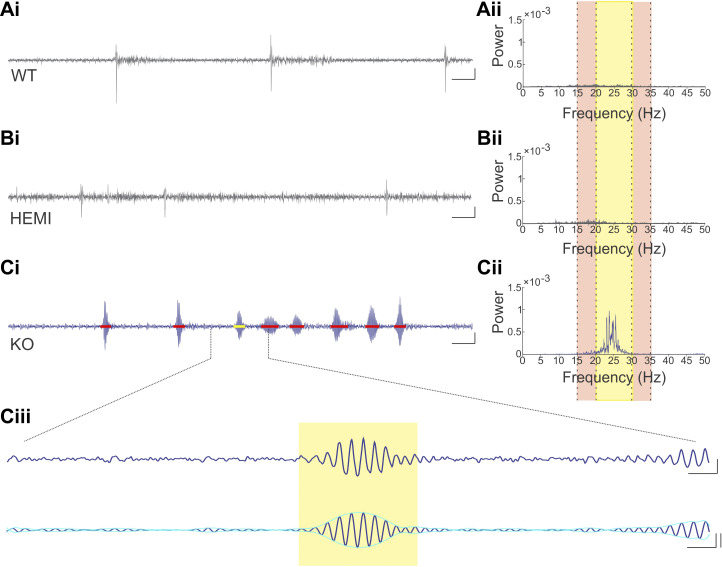
In global GABAAα1 subunit knockout mice a tremor phenotype has been observed when animals are suspended by their tail (Kralic et al. 2005; Handforth et al. 2010; Quesada et al. 2011). We therefore tail-suspended Global-KO animals while using a force transducer to measure tremor, to confirm our ability to reliably detect tremor and provide a point of comparison for observations in PC-KO animals. Matching previous literature, we observed a tremor phenotype in our Global-KO animals when tail-suspended (Fig. 3, Supplemental Video S2; available at https://doi.org/10.6084/m9.figshare.12733526). This tremor was evident between 20 Hz and 30 Hz (Fig. 3Cii), as previously described for Global-KO animals (Handforth et al. 2010; Quesada et al. 2011). Global- KO, but not Global-HEMI, animals displayed a tremor phenotype relative to Global-WT animals [tremor band ratio: “TBR” Global-KO: 4.12 ± 0.34, n = 36 animals; TBR Global-HEMI: 0.97 ± 0.02, n = 58 animals; TBR Global-WT: 0.92 ± 0.04, n = 27 animals; P < 0.0001 KW; WT vs. HEMI P = 0.25, WT vs. KO P < 0.0001, HEMI vs. KO P < 0.0001, post hoc Mann–Whitney tests (MWs)]. We observed that the tremor was not always present, but rather occurred in bouts (Fig. 3, Ci and Ciii), as has been noted in other models of tremor (Wang and Fowler 2001; Paterson et al. 2009).
Fig. 3.
Tremor is present in Global-KO animals. Example raw traces (i) from wild-type (WT; A), hemizygous (HEMI; B), and knockout (KO; C) animals, and corresponding power spectral density (PSD) plots (ii). Yellow highlighted region in PSD plots represents the tremor band (20–30 Hz); adjacent reference bands are highlighted in the light tan color (15–20 Hz and 30–35 Hz). The tremor band ratio (TBR) is the ratio of the power in the tremor band to the power in these adjacent bands. In the KO animal, there is a clear increase in power in the tremor band relative to the adjacent bands (Cii), and relative to WT (Aii) and HEMI (Bii) animals. Note that the PSD plot is present for both WT and HEMI animals, but it is difficult to see as it hovers near zero in the plots. In WT and HEMI animals, periods of movement are visible, but these are not associated with a strong oscillatory signal. In contrast, in KO animals, bouts of oscillatory movement (i.e., tremor) were present (Ci, detected tremor bouts are indicated by black bars, yellow bar highlights the tremor bout expanded in Ciii). Ciii: an expanded view of a tremor bout, showing both the wideband trace (top) and the signal filtered to the tremor band (20–30 Hz; bottom trace). The yellow box indicates the detected tremor bout, with onset and offset determined by the envelope of the filtered signal (aqua overlay). Note that the beginning of the next tremor bout is visible to the far right and is not marked. Scale bars: Ai, Bi, Ci: 1 s; 50% change in force. Ciii, top: 100 ms; 50% change in force. Ciii, bottom: 100 ms; 50% change in force, left vertical; 3 SD, right vertical.
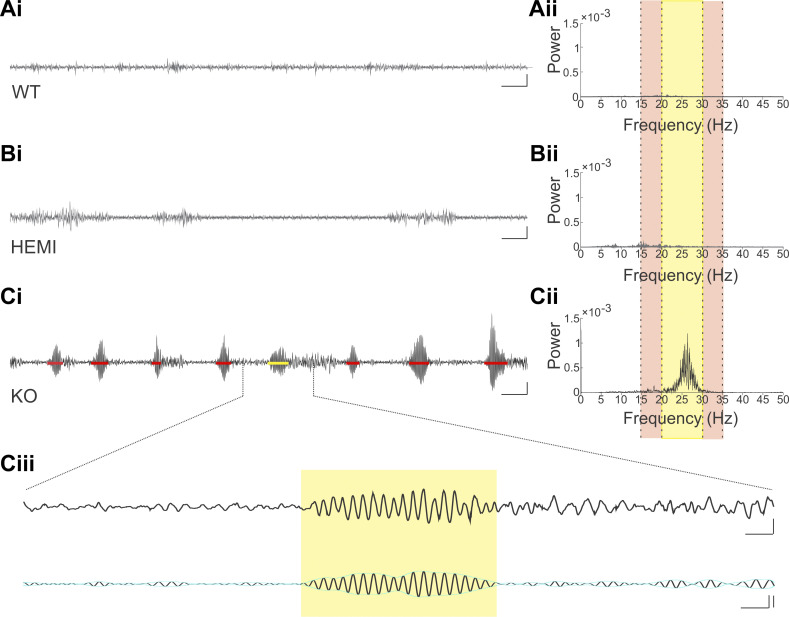
To test whether selective knockout of the GABAAα1 subunit from Purkinje cells would be sufficient to induce a tremor phenotype, we performed similar tail-suspension recordings from PC-KO-Bl6 (Fig. 4) and PC-KO-Mixed (Fig. 5) animals. As with Global-KO animals, a tremor phenotype was observed in PC-KO-Bl6 animals as compared with PC-WT-Bl6 and PC-HEMI-Bl6 animals, with a corresponding increase in power between 20 Hz and 30 Hz (PC-KO-Bl6 TBR: 1.05 ± 0.07, n = 30 animals; PC-WT-Bl6 TBR: 0.69 ± 0.03, n = 42 animals; PC-HEMI-Bl6 TBR: 0.64 ± 0.04, n = 14 animals; black6 background TBR across genotypes: P < 0.0001, KW; Fig. 4, Supplemental Video S2). A similar tremor phenotype was observed in PC-KO-Mixed animals (PC-KO-Mixed TBR: 1.2 ± 0.10, n = 38 animals; PC-WT-Mixed TBR: 0.74 ± 0.03, n = 28 animals; PC-HEMI-Mixed TBR: 0.76 ± 0.03, n = 30 animals; mixed background TBR across genotypes: P < 0.0001, KW; Fig. 5, Supplemental Video S2). A tremor phenotype was observed in PC-KO animals regardless of the background strain of the animal (PC-KO-Bl6 TBR vs. PC-KO-Mixed TBR: P = 0.45, MW; Fig. 6A), indicating that background strain does not play a pivotal role in the expression of the tremor phenotype. There was also no significant difference in tremor severity between males and females (TBR PC-KO-Bl6 males: 1.1 ± 0.09, n = 18 animals, females: 0.9 ± 0.1, n = 12 animals, P = 0.10, MW; TBR PC-KO-Mixed males: 1.2 ± 0.12, n = 22 animals, females: 1.1 ± 0.17, n = 16 animals, P = 0.14, MW, Fig. 6A). We also detected no difference in tremor severity between male and female Global-KO animals (TBR male: 4.47 ± 0.73, female: 3.92 ± 0.35, P = 0.74 MW, Fig. 6A). We did find a slight increase in tremor with age in KO animals (Supplemental Figs. S1 and S2).
Fig. 4.
Tremor is visible in PC-KO-Bl6 but not PC-WT-Bl6 or PC-HEMI-Bl6 animals. Example raw traces (i) from wild-type (WT; A), hemizygous (HEMI; B), and knockout (KO; C) animals, and corresponding power spectral density (PSD) plots (ii). Yellow highlighted region in PSD plots represents the tremor band (20–30 Hz); adjacent reference bands are highlighted in the light tan color (15–20 Hz and 30–35 Hz). The tremor band ratio (TBR) is the ratio of the power in the tremor band to the power in these adjacent bands. In the KO animal, there is a clear increase in power in the tremor band relative to the adjacent bands (Cii), and relative to WT (Aii) and HEMI (Bii) animals. Note that the PSD plot is present for both WT and HEMI animals, but it is difficult to see as it hovers near zero in the plots. In KO animals, bouts of tremor were present (Ci, detected tremor bouts are indicated by red bars, yellow bar highlights the tremor bout expanded in Ciii). Ciii: an expanded view of a tremor bout, showing both the wideband trace (top) and the signal filtered to the tremor band (20–30 Hz; bottom trace). The yellow box indicates the detected tremor bout, with onset and offset determined by the envelope of the filtered signal (aqua overlay). Note that while this example tremor bout is followed by nontremor movement, the nontremor movement is not included in the tremor bout, as it does not show a high signal specifically in the tremor band (compare top and bottom traces in Ciii). Note that periods of (nontremor) movement are visible in Ai and Bi, but these did not have a strong oscillatory component, and were not detected as tremor bouts. Scale bars: Ai, Bi, Ci: 1 s; 20% change in force. Ciii, top: 100 ms; 20% change in force. Ciii, bottom: 100 ms; 20% change in force, left vertical; 3 SD, right vertical. PC, Purkinje cell.
Fig. 5.
Tremor is visible in PC-KO-Mixed animals. Example raw traces (i) from wild-type (WT; A), hemizygous (HEMI; B), and knockout (KO; C) animals, and corresponding power spectral density (PSD) plots (ii). Yellow highlighted region (ii) represents the tremor band (20–30 Hz); adjacent reference bands are highlighted in the light tan color (15–20 Hz and 30–35 Hz). The tremor band ratio (TBR) is the ratio of the power in the tremor band to the power in these adjacent bands. In the KO animal, there is a clear increase in power in the tremor band relative to the adjacent bands (Cii), and relative to WT (Aii) and HEMI (Bii) animals. Note that the PSD plot is present for both WT and HEMI animals, but it is difficult to see as it hovers near zero in the plots. In KO animals, bouts of tremor were present (Ci; detected tremor bouts are indicated by red bars; yellow bar highlights the tremor bout expanded in Ciii). While periods of (nontremor) movements are visible in Ai and Bi, these were not associated with strong oscillatory signals and were not detected as tremor bouts. Ciii: an expanded view of a tremor bout, showing both the wideband trace (top) and the signal filtered to the tremor band (20–30 Hz; bottom trace). The yellow box indicates the detected tremor bout, with onset and offset determined by the envelope of the filtered signal (aqua overlay). Note that the beginning of the next tremor bout is visible to the far right and is not marked. Scale bars: Ai, Bi, Ci: 1 s; 20% change in force. Ciii, top: 100 ms; 20% change in force. Ciii, bottom: 100 ms; 20% change in force, left vertical; 3 SD, right vertical. HEMI, hemizygous; KO, knockout; PC, Purkinje cell; WT, wild-type.
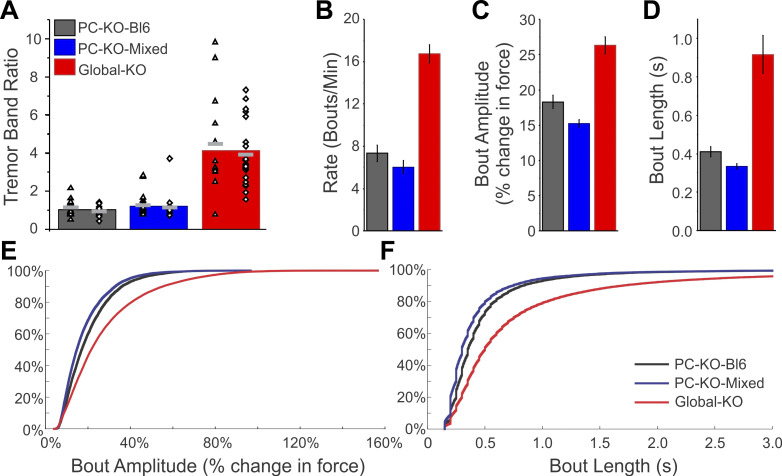
Fig. 6.
The tremor phenotype is stronger in Global-KO than in PC-KO animals. A: summary tremor band ratio (TBR) data for PC-KO-Bl6 animals (gray column), PC-KO-Mixed animals (blue), and Global-KO animals (red). While PC-KO-Bl6 and PC-KO-Mixed animals showed similar TBR values, Global-KO animals showed greater levels of tremor (P < 0.0001), as measured by the TBR. Individual animal data points are also presented by sex: triangles represent male animals; diamonds represent female animals. Note that many data points are overlapping. Light gray bars represent averages by sex within each group. B–F: Global-KO animals also showed a more severe tremor phenotype (P < 0.0001) as assessed by average tremor bout rate (B), bout amplitude (C), and bout length (D). Cumulative histograms of individual bout amplitudes (E) and length (F) by strain. HEMI, hemizygous; KO, knockout; PC, Purkinje cell; WT, wild-type.
Together, these data indicate that selective knockout of the GABAAα1 subunit from Purkinje cells is sufficient to induce a tremor, regardless of the background strain or sex of the animal. Additionally, the frequency band of the tremor (20–30 Hz) in PC-KO animals (Fig. 4Cii, Fig. 5Cii) is similar to the tremor observed in global GABAAα1 knockout animals (Fig. 3Cii) (Handforth et al. 2010; Quesada et al. 2011), suggesting similar underlying mechanisms. However, the tremor was more prominent in the Global-KO mice (TBR in KOs between lines: P < 0.0001, KW; Global-KO vs. PC-KO-Bl6 P < 0.0001, MW; Global-KO vs. PC-KO-Mixed P < 0.0001, MW; Fig. 6A), suggesting that additional circuit elements (Zhang and Santaniello 2019) or the timing of the knockout contribute to the overall phenotype in animals with a global knockout.
Visually, it appeared bouts occurred at a lower rate in PC-KO animals than in Global-KO animals, which may contribute to a lower overall TBR value. To further explore the tremor phenotype, we therefore quantified the rate of bouts, the length of bouts, and the amplitude of bouts (percent change in force) per animal and compared across mouse lines (Fig. 6, B–F). Global-KO mice displayed a higher rate of bouts (Global-KO: 16.7 ± 0.8 bouts/min, across mouse lines P < 0.0001, KW; Fig. 6B), a greater amplitude of tremor per bout (Global-KO: 26 ± 1% change in force; comparing across lines P < 0.0001, KW; Fig. 6C), and longer bouts (Global-KO: 0.92 ± 0.10 s; comparing across lines P < 0.0001, KW; Fig. 6D) than PC-KO-Bl6 (rate, length, amplitude, P < 0.001 each, MWs) and PC-KO-Mixed animals (P < 0.001 each, MWs). This was apparent both in the mean values across animals (Fig. 6, B–D) and in the cumulative distributions of all recorded bouts (Fig. 6, E and F). There were no differences, however, between Purkinje cell specific knockout strains in the rate of tremor bouts (PC-KO-Bl6: 7.3 ± 0.8 bouts/min; PC-KO-Mixed: 6.0 ± 0.6 bouts/min, P = 0.21, MW), and only subtle differences in length (PC-KO-Bl6: 0.41 ± 0.03 s; PC-KO-Mixed: 0.33 ± 0.02 s, P = 0.03, MW) and amplitude (PC-KO-Bl6: 18 ± 1% change in force; PC-KO-Mixed: 15 ± 0.5% change in force, P = 0.007, MW; Fig. 6, B–D), again suggesting that the background of the animal is not critical for expression of the tremor phenotype. As seen with the TBR, examination of bout measurements (rate, length, and amplitude) generally also suggested an overall slight increase in tremor severity in KO mice with age (Supplemental Fig. S2). Our examination of bouts was not strongly dependent on the exact measures used to analyze bouts; similar results were obtained using a detection of bouts based on Z-score, rather than percent change in force (Supplemental Fig. S3).
Tremor in PC-KO animals is sensitive to ethanol.
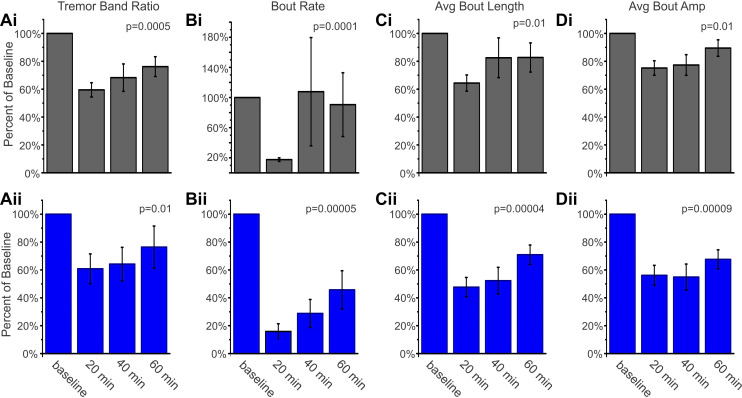
Having determined that a selective knockout of the GABAAα1 subunit from Purkinje cells is sufficient to induce a tremor phenotype, we next examined the pharmacology of this tremor—specifically, whether this tremor would be sensitive to ethanol (Fig. 7). The tremor in Global-KO mice is sensitive to pharmacological agents including ethanol (Kralic et al. 2005), similar to essential tremor in patients. We found that ethanol (1.25 g/kg ip) significantly reduced tremor in both PC-KO-Bl6 and PC-KO-Mixed animals (Bl6: P = 0.005, Mixed: P = 0.01, Friedman’s test; Fig. 7A), and the reduction in tremor largely persisted over the course of the hour examined, regardless of background (PC-KO-Bl6: TBR reduced to 60 ± 5% 20 min postinjection, 68 ± 10% at 40 min, and 76 ± 7% of baseline at 60 min; PC-KO-Mixed: TBR reduced to 61 ± 11% of baseline at 20 min postinjection, 64 ± 12% at 40 min, 76 ± 15% at 60 min). In contrast, no reduction was seen following saline injection (PC-KO-Bl6: P = 0.36 Friedman, TBR 130 ± 20%, 135 ± 23%, and 151 ± 24% of baseline at 20, 40, and 60 min postinjection, respectively; PC-KO-Mixed: P = 0.50 Friedman, TBR 118 ± 29%, 105 ± 19%, and 147 ± 29% of baseline at 20, 40, and 60 min postinjection, respectively).
Fig. 7.
Ethanol inhibits tremor in PC-KO mice. Tremor metric (A), rate of bouts (B), length of bouts (C), and amplitude of bouts (D) are all significantly reduced after intraperitoneal injection of 1.25 g/kg ethanol in PC-KO-Bl6 (i, black) and PC-KO-Mixed (ii, blue) animals. Percent of baseline (mean ± SE) at 20, 40, and 60 min post-drug administration is shown for n = 11 animals for each mouse line; P values from Friedman tests. Amp, amplitude; Avg., average; KO, knockout; PC, Purkinje cell.
To determine whether this decrease in the TBR was due to a reduced rate of tremor bouts, shorter tremor bouts, and/or a reduced amplitude per bout, we performed a more detailed analysis of the tremor bouts observed. Ethanol strongly reduced the rate of detected bouts (P < 0.001, Friedman’s test), especially at the earliest time point examined (Fig. 7B). The length of bouts was also reduced, although this reduction was less dramatic than the initial reduction in the rate of bouts (Bl6: P = 0.01; Mixed: P < 0.01, Friedman’s test; Fig. 7C). The amplitude of individual bouts was also reduced (Bl6: P = 0.01; Mixed: P < 0.01, Friedman’s test; Fig. 7D), indicating a broad reduction in the rate, length, and strength of remaining bouts by ethanol. These data illustrate that tremor is strongly attenuated by ethanol administration, reminiscent of the global GABAAα1 knockout model (Kralic et al. 2005) and human essential tremor (Hopfner et al. 2015).
DISCUSSION
To further investigate the tremor phenotype seen in mice with a global knockout of the GABAAα1 subunit, we produced mice with a more restricted cerebellar Purkinje cell specific knockout of GABAAα1. This resulted in an abolishment of all GABAA-mediated synaptic inhibition to Purkinje cells but left GABAA-mediated inhibition to cerebellar molecular layer interneurons intact. As a result, any phenotype observed can be attributed, specifically, to a loss of inhibition to Purkinje cells. Purkinje cell specific knockout of GABAAα1 did not result in reduced survival in animals, nor gross motor deficits as assessed by the accelerating rotarod test but did result in a tremor phenotype. This tremor phenotype was less severe than tremor observed in Global-KO animals but had similar properties (i.e., the tremor was focused in the 20- to 30-Hz range). Hemizygous animals displayed GABAA IPSCs in Purkinje cells with similar frequency, amplitude, and decay kinetics as wild-type animals, and, correspondingly, did not display a tremor phenotype. Finally, the tremor phenotype in PC-KO animals, similar to the tremor phenotype in global knockout animals (Kralic et al. 2005), is sensitive to pharmacological inhibition through systemic administration of ethanol. In this regard, the tremor in PC-KO animals is reminiscent of the tremor seen in human essential tremor (Hopfner et al. 2015). These findings highlight a potentially key role in tremor for the cerebellum, Purkinje cells, and inhibition (and specifically, inhibition to Purkinje cells).
We were interested in testing the hypothesis that Purkinje cells play a key role in the observed tremor phenotype in global GABAAα1 knockout animals, given the hypothesized role of the cerebellum in tremor phenotypes (Benito-León and Labiano-Fontcuberta 2016; Handforth 2016; Lenka and Louis 2019; Louis 2014; Marin-Lahoz and Gironell 2016). The cerebellum has routinely been implicated in human essential tremor, with noted postmortem pathologies including Purkinje cell loss (Louis and Faust 2014), dendritic (Louis et al. 2014a; Yu et al. 2012), and axonal (Babij et al. 2013) abnormalities, dense and tangled “hairy” baskets (Erickson-Davis et al. 2010), and altered climbing fiber innervation (Kuo et al. 2017; Lin et al. 2014). While human tissue provides critical insight into the human condition, in isolation, it is difficult to discern causative, compensatory, and incidental changes from one another. Recent animal work has shown that neurotransmission from Purkinje cells can be necessary for the expression of tremor in the harmaline model (Brown et al. 2020), and that rhythmic, coordinated stimulation of Purkinje cells can produce tremors (Brown et al. 2020; Pan et al. 2020). Further cementing the importance of the cerebellum in tremor, we show here that a genetic deficit specifically in Purkinje cells, in the absence of rhythmic external inputs via a chemical or optogenetic approach, is sufficient to generate a tremor phenotype. While our study highlights the importance of Purkinje cells, our findings in no way discount the importance of other circuit elements. For example, tremors may be driven by altered activity in the inferior olives in harmaline-induced tremors (though Purkinje cells are necessary for expression) (Brown et al. 2020), by altered innervation of Purkinje cells by climbing fibers in GluRδ2-insufficient animals (Pan et al. 2020), or by alterations downstream of Purkinje cells in the cerebellar nuclei (Zhang and Santaniello 2019). Speculatively, decreased numbers of Purkinje cells could result in simplified, more coherent input to nuclear neurons, resulting in abnormal synchrony at this downstream node and a tremor phenotype. Diversity in tremor, including mechanisms supporting tremor, seems clear (Kuo et al. 2019; Pan et al. 2018). Indeed, determining the genetic underpinnings of essential tremor has likely been delayed by the heterogeneity of the disorder (Odgerel et al. 2019) and by the existence of multiple potential pathways to a tremor phenotype.
Specific knockout of the GABAAα1 subunit from Purkinje cells resulted in a loss of GABAA-mediated inhibition to Purkinje cells, without compensatory expression of other α subunits. This indicates that a loss of inhibition, rather than altered kinetics of inhibition, to Purkinje cells is responsible for the tremor phenotype observed in PC-KO animals. Therefore, other manipulations to the circuitry that could similarly reduce inhibition to Purkinje cells (e.g., loss of molecular layer interneurons, or reduced drive to molecular layer interneurons) may also result in a tremor phenotype. It will be of interest to see the functional consequences of hairy baskets (Erickson-Davis et al. 2010) and determine whether this pathology observed in human tissue may functionally contribute to (or, conversely, dampen) the tremor phenotype. As noted above, a key element to tremor pathology may be the abnormal firing patterns of Purkinje cells, either their burstiness or synchrony across cells (Brown et al. 2020; Pan et al. 2020). While our findings provide some important insights, questions remain, including whether the loss of inhibition to Purkinje cells simply alters their net excitability or regularity of firing (Brown et al. 2019), or whether the loss of inhibition (also) produces an increase in synchronization of Purkinje cells, and this specifically is what drives a tremor phenotype. Similarly, future work could explore why tremor in these animals occurs specifically in the 20–30 Hz range and why tremor frequencies vary across different animal models.
Abnormal synchrony of downstream circuit elements may also feed into a tremor phenotype. Features of the tremor (including strong oscillatory power in the 20–30 Hz band) observed in the PC-KO animals mirror the tremor observed in global knockout animals sufficiently to suggest similar underlying mechanisms. However, the magnitude of the tremor was reduced in PC-KO animals. This further supports the importance of conceptualizing tremors within a broader network context. In PC-KO animals, intact inhibition to other circuit elements may help dampen the expression of tremor, such that tremor is amplified, and more frequent, in Global-KO animals.
Tremor, both in essential tremor patients and across animal models, is not constantly present, but appears to be induced by certain situations, including but not limited to, movement (Pan et al. 2020; Reich 2019; Shanker 2019; Wang and Fowler 2001). Here, we used a tail-suspension apparatus to measure tremor, which, while often associated with studies examining depressive phenotypes (Cryan et al. 2005), has a long history of use in measurement of tremor in global GABAAα1 knockout animals (Handforth et al. 2010; Kralic et al. 2005; Quesada et al. 2011). Using tail suspension to measure tremor in our animals allowed us to most directly compare our study to these previous studies. However, future work using additional methods may provide important additional insight into the observed tremor phenotype, including why tremor occurs in bouts, and what may switch the system into a pathological “tremor state.” Of interest, a recent study using optogenetic stimulation of Purkinje terminals found that stronger light stimulation (which could result in a larger degree of synchronous activity in the cerebellar nucleus) caused more robust tremor phenotypes (Brown et al. 2020). Lower light intensities produced more mild and intermittent tremor.
Our work builds on previous work using global GABAAα1 knockout animals. Previous studies using global GABAAα1 knockout animals reported different phenotypes, with some studies reporting a tremor phenotype (Handforth et al. 2010; Kralic et al. 2005; Quesada et al. 2011) but others finding instead increased mortality and an absence epilepsy phenotype (Arain et al. 2012). It was suggested that the background strain of the animals may underlie differences in the expressed phenotype (Arain et al. 2012). We therefore examined the impact of Purkinje cell specific knockout of GABAAα1 on two different backgrounds. However, we found a similar tremor phenotype on both backgrounds, suggesting that the tremor phenotype is robust and not heavily dependent on the background strain. We have not yet examined PC-KO animals for an epilepsy phenotype. Of interest, in addition to reports of epilepsy in mice with a loss of GABAAα1, a recent study reported generalized epilepsy in zebrafish lacking GABAAα1 (Samarut et al. 2018). While it may seem unlikely that an epilepsy phenotype would be present in PC-KO animals, the engagement of the cerebellum during seizures has been repeatedly noted (Caveness et al. 1977; Gartside 1979; Julien and Laxer 1974; Kandel and Buzsáki 1993; Krook-Magnuson et al. 2014; Kros et al. 2015; Miller 1992; Streng and Krook-Magnuson 2020). Additionally, mice with a loss of P/Q channels in the cerebellum display absence epilepsy (Maejima et al. 2013; Mark et al. 2011). In the future, it would therefore be interesting to examine whether a cerebellar specific loss of the GABAAα1 subunit can also produce an epilepsy phenotype, and, more generally, when abnormal cerebellar activity results in an epilepsy versus a tremor phenotype.
While no decreased mortality was seen on either background in the PC-KO animals, we did find increased mortality in our Global-KO animals. We took care to have our Global-KO animals on a mixed background (matching previous studies which reported a tremor phenotype, but not increased mortality or epilepsy). Therefore, our findings suggest that background strain is not a likely explanation for differences in reported mortality. Early mortality does, however, appear to be a result of the global nature and/or timing of the knockout, as PC-KO animals do not display early mortality. The early mortality in Global-KO animals peaks around the time of weaning, is not reminiscent of human essential tremor, and provides experimental challenges. Early mortality is therefore not a desirable phenotype in a model of essential tremor, and PC-KO animals avoid this confound. Perhaps the greatest drawback to the global knockout approach, however, is a lack of circuit insights, which is also overcome by more selective approaches.
Future work could also examine the potential for other non-tremor-related phenotypes in PC-KO animals. Given the strength of the impact on inhibition to Purkinje cells (i.e., there was an abolishment of IPSCs in Purkinje cells), it was notable that no effects were detected on the accelerating rotarod test. This suggests a minimal role of inhibition to Purkinje cells (or a compensatory mechanism) in learning and performance on the accelerating rotarod task. Results on the accelerating rotarod in previous studies in animals with a global knockout of GABAAα1 have been mixed (Kralic et al. 2003; Sur et al. 2001). It would be of interest to see what phenotypes can, and cannot, be observed in a variety of cerebellar dependent tasks in PC-KO animals in future studies, as this could provide increased insight into the role of GABAergic inhibition to Purkinje cells (both from molecular layer interneurons and from other Purkinje cells) in cerebellar function (Barmack and Yakhnitsa 2008; Brown et al. 2019; Chu et al. 2011, 2012; de Solages et al. 2008; Lee et al. 2015; Oldfield et al. 2010; Orduz and Llano 2007; Witter et al. 2016; Wulff et al. 2009). Similarly, the cerebellum is important for functions beyond motor control (Ivry and Baldo 1992; Rochefort et al. 2011; Schmahmann et al. 2007; Stoodley and Schmahmann 2010; Wagner et al. 2017; Yu and Krook-Magnuson 2015; Zeidler et al. 2020), and it will therefore also be of interest to examine potential nonmotor phenotypes in PC-KO animals. That is, PC-KO animals provide a useful tool to study not only tremor, but also the role of the cerebellum, and in particular inhibition to Purkinje cells, in a range of conditions.
The pathophysiology of essential tremor largely remains a mystery. This underscores the need for models to study the disorder but also makes finding great animal models especially challenging. The global GABAAα1 knockout model provides a potential tool to study essential tremor, complementing other genetic and chemical models of tremor. The relevance of the global GABAAα1 knockout tremor phenotype to essential tremor is not firmly established, but, importantly, the observed tremor phenotype is sensitive to both ethanol and propranolol (Kralic et al. 2005). Minimally, therefore, the global GABAAα1 knockout model provides a tool to examine this pharmacology. In this regard, it is important to note that the tremor observed in PC-KO animals is similarly sensitive to ethanol, while also benefiting from a more restricted loss GABAAα1 (which further assists with interpretation of findings). The loss of GABAergic IPSCs in Purkinje cells in knockout animals, combined with the sensitivity of tremor in PC-KO animals to ethanol, indicates that the tremor-reducing effects of ethanol are unlikely to be mediated by an increase in GABAergic inhibition to Purkinje cells (e.g., through increased GABA release from molecular layer interneurons) (Hirono et al. 2009; Kaplan et al. 2013; Lin et al. 1991; Valenzuela and Jotty 2015). Either ethanol is working through a different mechanism to inhibit Purkinje cells or it is working upstream (e.g., to reduce excitatory drive to Purkinje cells) or downstream (e.g., in the cerebellar nuclei, or further downstream).
Our findings of a tremor phenotype in PC-KO animals highlights the relevance of Purkinje cells in tremor, illustrates that a deficit in inhibition can produce a tremor phenotype, and paves the way for future investigations into tremor and the role of inhibition to Purkinje cells in cerebellar functions.
GRANTS
This work was funded in part through a McKnight Land Grant Professorship award, the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative, NIH NINDS R01 NS112518, and an International Essential Tremor Foundation (IETF) grant (to EKM).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.N., J.K., Z.C., and E.K. conceived and designed research; A.N., H.G., J.K., C.S., I.H., and Z.C. performed experiments; A.N., C.K., H.G., J.K., Z.C., and E.K. analyzed data; A.N., J.K., Z.C., and E.K. interpreted results of experiments; A.N., J.K., I.H., Z.C., and E.K. prepared figures; A.N., C.K., J.K., C.S., Z.C., and E.K. drafted manuscript; A.N., C.K., J.K., I.H., Z.C., and E.K. edited and revised manuscript; A.N., C.K., H.G., J.K., I.H., Z.C., and E.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michael Benneyworth and the University of Minnesota’s Mouse Behavior Core for assistance with the accelerating rotarod testing, the other members of the Krook-Magnuson laboratory, and Stephen Fowler for assistance with establishing tremor measurements in our laboratory. We thank Alexander Skorput, Jennifer Cook, Lucy Vulchanova, Alexandra Scott, Mark Sanders, Guillermo Marques, and the University of Minnesota’s University Imaging Center for assistance with tissue processing and imaging.
REFERENCES
- Arain FM, Boyd KL, Gallagher MJ. Decreased viability and absence-like epilepsy in mice lacking or deficient in the GABAA receptor α1 subunit. Epilepsia 53: e161–e165, 2012. doi: 10.1111/j.1528-1167.2012.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 136: 3051–3061, 2013. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci 23: 9664–9674, 2003. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci 28: 1140–1152, 2008. doi: 10.1523/JNEUROSCI.3942-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-León J, Labiano-Fontcuberta A. Linking essential tremor to the cerebellum: clinical evidence. Cerebellum 15: 253–262, 2016. doi: 10.1007/s12311-015-0741-1. [DOI] [PubMed] [Google Scholar]
- Briatore F, Patrizi A, Viltono L, Sassoè-Pognetto M, Wulff P. Quantitative organization of GABAergic synapses in the molecular layer of the mouse cerebellar cortex. PLoS One 5: e12119, 2010. doi: 10.1371/journal.pone.0012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Arancillo M, Lin T, Catt DR, Zhou J, Lackey EP, Stay TL, Zuo Z, White JJ, Sillitoe RV. Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells. Sci Rep 9: 1742, 2019. doi: 10.1038/s41598-018-38264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, White JJ, van der Heijden ME, Zhou J, Lin T, Sillitoe RV. Purkinje cell misfiring generates high-amplitude action tremors that are corrected by cerebellar deep brain stimulation. eLife 9: e51928, 2020. doi: 10.7554/eLife.51928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caveness WF, Kosaka K, Hosokawa S, O’Neill RR. Cerebral-cerebellar paroxysmal activity in experimental focal seizures. Ann Neurol 1: 287–289, 1977. doi: 10.1002/ana.410010317. [DOI] [PubMed] [Google Scholar]
- Chahine LM, Ghosh D. Essential tremor after ipsilateral cerebellar hemispherectomy: support for the thalamus as the central oscillator. J Child Neurol 24: 861–864, 2009. doi: 10.1177/0883073808329528. [DOI] [PubMed] [Google Scholar]
- Chu CP, Bing YH, Liu H, Qiu DL. Roles of molecular layer interneurons in sensory information processing in mouse cerebellar cortex Crus II in vivo. PLoS One 7: e37031, 2012. doi: 10.1371/journal.pone.0037031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CP, Bing YH, Qiu DL. Sensory stimulus evokes inhibition rather than excitation in cerebellar Purkinje cells in vivo in mice. Neurosci Lett 487: 182–186, 2011. doi: 10.1016/j.neulet.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Chua HC, Chebib M. GABAA receptors and the diversity in their structure and pharmacology. Adv Pharmacol 79: 1–34, 2017. doi: 10.1016/bs.apha.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature 497: 332–337, 2013. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29: 571–625, 2005. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, Rousseau C, Barbour B, Léna C. High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron 58: 775–788, 2008. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Epp JR, Niibori Y, Hsiang HL, Mercaldo V, Deisseroth K, Josselyn SA, Frankland PW. Optimization of CLARITY for clearing whole-brain and other intact organs. eNeuro 2: ENEURO.0022-15.2015, 2015. doi: 10.1523/ENEURO.0022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol 69: 262–271, 2010. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P. Molecular and synaptic organization of GABAA receptors in the cerebellum: effects of targeted subunit gene deletions. Cerebellum 5: 275–285, 2006. doi: 10.1080/14734220600962805. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoè-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J Neurosci 26: 3245–3255, 2006. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside IB. The activity of cerebellar neurones during epileptiform activity induced by penicillin in the cerebral cortex of the rat. Electroencephalogr Clin Neurophysiol 46: 189–196, 1979. doi: 10.1016/0013-4694(79)90068-3. [DOI] [PubMed] [Google Scholar]
- Gironell A. The GABA hypothesis in essential tremor: lights and shadows. Tremor Other Hyperkinet Mov (N Y) 4: 254, 2014. doi: 10.5334/tohm.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handforth A. Linking essential tremor to the cerebellum-animal model evidence. Cerebellum 15: 285–298, 2016. doi: 10.1007/s12311-015-0750-0. [DOI] [PubMed] [Google Scholar]
- Handforth A, Homanics GE, Covey DF, Krishnan K, Lee JY, Sakimura K, Martin FC, Quesada A. T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology 59: 380–387, 2010. doi: 10.1016/j.neuropharm.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Yamada M, Obata K. Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells. Neuropharmacology 57: 109–120, 2009. doi: 10.1016/j.neuropharm.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Hopfner F, Erhart T, Knudsen K, Lorenz D, Schneider SA, Zeuner KE, Deuschl G, Kuhlenbäumer G. Testing for alcohol sensitivity of tremor amplitude in a large cohort with essential tremor. Parkinsonism Relat Disord 21: 848–851, 2015. doi: 10.1016/j.parkreldis.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Hopfner F, Haubenberger D, Galpern WR, Gwinn K, Van’t Veer A, White S, Bhatia K, Adler CH, Eidelberg D, Ondo W, Stebbins GT, Tanner CM, Helmich RC, Lenz FA, Sillitoe RV, Vaillancourt D, Vitek JL, Louis ED, Shill HA, Frosch MP, Foroud T, Kuhlenbäumer G, Singleton A, Testa CM, Hallett M, Elble R, Deuschl G. Knowledge gaps and research recommendations for essential tremor. Parkinsonism Relat Disord 33: 27–35, 2016. doi: 10.1016/j.parkreldis.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Baldo JV. Is the cerebellum involved in learning and cognition? Curr Opin Neurobiol 2: 212–216, 1992. doi: 10.1016/0959-4388(92)90015-D. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Noebels JL. Genetic mouse models of essential tremor: are they essential? J Clin Invest 115: 584–586, 2005. doi: 10.1172/JCI24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien RM, Laxer KD. Cerebellar responses to penicillin-induced cerebral cortical epileptiform discharge. Electroencephalogr Clin Neurophysiol 37: 123–132, 1974. doi: 10.1016/0013-4694(74)90002-9. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsáki G. Cerebellar neuronal activity correlates with spike and wave EEG patterns in the rat. Epilepsy Res 16: 1–9, 1993. doi: 10.1016/0920-1211(93)90033-4. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Mohr C, Rossi DJ. Opposite actions of alcohol on tonic GABAA receptor currents mediated by nNOS and PKC activity. Nat Neurosci 16: 1783–1793, 2013. doi: 10.1038/nn.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Criswell HE, Osterman JL, O’Buckley TK, Wilkie ME, Matthews DB, Hamre K, Breese GR, Homanics GE, Morrow AL. Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice. J Clin Invest 115: 774–779, 2005. doi: 10.1172/JCI200523625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABAA receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther 302: 1037–1045, 2002. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol 495: 408–421, 2006. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Wheeler M, Renzi K, Ferguson C, O’Buckley TK, Grobin AC, Morrow AL, Homanics GE. Deletion of GABAA receptor alpha 1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther 305: 600–607, 2003. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Huntsman MM. The transience of interneuron circuit diversity just “sped” up. Proc Natl Acad Sci USA 104: 16723–16724, 2007. doi: 10.1073/pnas.0708149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1: ENEURO.0005-14.2014, 2014. doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros L, Eelkman Rooda OH, Spanke JK, Alva P, van Dongen MN, Karapatis A, Tolner EA, Strydis C, Davey N, Winkelman BH, Negrello M, Serdijn WA, Steuber V, van den Maagdenberg AM, De Zeeuw CI, Hoebeek FE. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol 77: 1027–1049, 2015. doi: 10.1002/ana.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Lin CY, Wang J, Sims PA, Pan MK, Liou JY, Lee D, Tate WJ, Kelly GC, Louis ED, Faust PL. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol 133: 121–138, 2017. doi: 10.1007/s00401-016-1626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Louis ED, Faust PL, Handforth A, Chang SY, Avlar B, Lang EJ, Pan MK, Miterko LN, Brown AM, Sillitoe RV, Anderson CJ, Pulst SM, Gallagher MJ, Lyman KA, Chetkovich DM, Clark LN, Tio M, Tan EK, Elble RJ. Current opinions and consensus for studying tremor in animal models. Cerebellum 18: 1036–1063, 2019. doi: 10.1007/s12311-019-01037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci 12: 1063–1076, 1992. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on alpha-subunit isoform. Biophys J 73: 2518–2526, 1997. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Mathews PJ, Reeves AM, Choe KY, Jami SA, Serrano RE, Otis TS. Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron 86: 529–540, 2015. doi: 10.1016/j.neuron.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, Bharath RD, Yadav R, Pal PK. Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology 59: 157–168, 2017. doi: 10.1007/s00234-016-1771-1. [DOI] [PubMed] [Google Scholar]
- Lenka A, Louis ED. Revisiting the clinical phenomenology of “cerebellar tremor”: beyond the intention tremor. Cerebellum 18: 565–574, 2019. doi: 10.1007/s12311-018-0994-6. [DOI] [PubMed] [Google Scholar]
- Lin AM, Freund RK, Palmer MR. Ethanol potentiation of GABA-induced electrophysiological responses in cerebellum: requirement for catecholamine modulation. Neurosci Lett 122: 154–158, 1991. doi: 10.1016/0304-3940(91)90846-L. [DOI] [PubMed] [Google Scholar]
- Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 137: 3149–3159, 2014. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol 376: 163–182, 1986. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. Treatment of essential tremor: are there issues we are overlooking? Front Neurol 2: 91, 2012. doi: 10.3389/fneur.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. Essential tremor: from bedside to bench and back to bedside. Curr Opin Neurol 27: 461–467, 2014. doi: 10.1097/WCO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. Essential tremor and the cerebellum. Handb Clin Neurol 155: 245–258, 2018. doi: 10.1016/B978-0-444-64189-2.00016-0. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL. Purkinje cell loss in essential tremor. Mov Disord 29: 1329–1330, 2014. doi: 10.1002/mds.25965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Lee M, Babij R, Ma K, Cortés E, Vonsattel JP, Faust PL. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 137: 3142–3148, 2014a. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (N Y) 4: 259, 2014. doi: 10.5334/tohm.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord 23: 174–182, 2008. doi: 10.1002/mds.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis RJ, Lee M, Kuo SH, Vonsattel JP, Louis ED, Faust PL. Cellular density in the cerebellar molecular layer in essential tremor, spinocerebellar ataxia, and controls. Parkinsonism Relat Disord 20: 1270–1273, 2014b. doi: 10.1016/j.parkreldis.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Wollenweber P, Teusner LU, Noebels JL, Herlitze S, Mark MD. Postnatal loss of P/Q-type channels confined to rhombic-lip-derived neurons alters synaptic transmission at the parallel fiber to purkinje cell synapse and replicates genomic Cacna1a mutation phenotype of ataxia and seizures in mice. J Neurosci 33: 5162–5174, 2013. doi: 10.1523/JNEUROSCI.5442-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málly J, Baranyi M. Change in the concentrations of amino acids in cisternal CSF of patients with essential tremor. J Neurol Neurosurg Psychiatry 57: 1012–1013, 1994. doi: 10.1136/jnnp.57.8.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Lahoz J, Gironell A. Linking essential tremor to the cerebellum: neurochemical evidence. Cerebellum 15: 243–252, 2016. doi: 10.1007/s12311-015-0735-z. [DOI] [PubMed] [Google Scholar]
- Mark MD, Maejima T, Kuckelsberg D, Yoo JW, Hyde RA, Shah V, Gutierrez D, Moreno RL, Kruse W, Noebels JL, Herlitze S. Delayed postnatal loss of P/Q-type calcium channels recapitulates the absence epilepsy, dyskinesia, and ataxia phenotypes of genomic Cacna1a mutations. J Neurosci 31: 4311–4326, 2011. doi: 10.1523/JNEUROSCI.5342-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord 20: 298–305, 2005. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- Miller JW. The role of mesencephalic and thalamic arousal systems in experimental seizures. Prog Neurobiol 39: 155–178, 1992. doi: 10.1016/0301-0082(92)90009-4. [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. Oxford, UK: Oxford University Press, 2007. [Google Scholar]
- Miwa H. Rodent models of tremor. Cerebellum 6: 66–72, 2007. doi: 10.1080/14734220601016080. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Miyazaki T, Kitamura K, Hashimoto K, Yanagawa Y, Obata K, Sakimura K, Watanabe M, Kano M. GABAergic inhibition regulates developmental synapse elimination in the cerebellum. Neuron 74: 384–396, 2012. doi: 10.1016/j.neuron.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Odgerel Z, Sonti S, Hernandez N, Park J, Ottman R, Louis ED, Clark LN. Whole genome sequencing and rare variant analysis in essential tremor families. PLoS One 14: e0220512, 2019. doi: 10.1371/journal.pone.0220512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield CS, Marty A, Stell BM. Interneurons of the cerebellar cortex toggle Purkinje cells between up and down states. Proc Natl Acad Sci USA 107: 13153–13158, 2010. doi: 10.1073/pnas.1002082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60: 243–260, 2008. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Llano I. Recurrent axon collaterals underlie facilitating synapses between cerebellar Purkinje cells. Proc Natl Acad Sci USA 104: 17831–17836, 2007. doi: 10.1073/pnas.0707489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahapill PA, Levy R, Dostrovsky JO, Davis KD, Rezai AR, Tasker RR, Lozano AM. Tremor arrest with thalamic microinjections of muscimol in patients with essential tremor. Ann Neurol 46: 249–252, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Pan MK, Li YS, Wong SB, Ni CL, Wang YM, Liu WC, Lu LY, Lee JC, Cortes EP, Vonsattel JG, Sun Q, Louis ED, Faust PL, Kuo SH. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med 12: eaay1769, 2020. doi: 10.1126/scitranslmed.aay1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MK, Ni CL, Wu YC, Li YS, Kuo SH. Animal models of tremor: relevance to human tremor disorders. Tremor Other Hyperkinet Mov (N Y) 8: 587, 2018. doi: 10.5334/tohm.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, Pilote M, Tremblay C, Di Paolo T, Rajput AH, Rajput A, Calon F. Defective dentate nucleus GABA receptors in essential tremor. Brain 135: 105–116, 2012. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol 616: 73–80, 2009. doi: 10.1016/j.ejphar.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, Strata P, Varoqueaux F, Brose N, Fritschy JM, Sassoè-Pognetto M. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc Natl Acad Sci USA 105: 13151–13156, 2008. doi: 10.1073/pnas.0802390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson HL, Shakkottai VG, Clark HB, Orr HT. Polyglutamine spinocerebellar ataxias - from genes to potential treatments. Nat Rev Neurosci 18: 613–626, 2017. doi: 10.1038/nrn.2017.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa DJ, Nelles C, Brown P, Volz LJ, Pelzer EA, Tittgemeyer M, Brittain JS, Timmermann L. The differentiated networks related to essential tremor onset and its amplitude modulation after alcohol intake. Exp Neurol 297: 50–61, 2017. doi: 10.1016/j.expneurol.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Bui PH, Homanics GE, Hankinson O, Handforth A. Comparison of mibefradil and derivative NNC 55-0396 effects on behavior, cytochrome P450 activity, and tremor in mouse models of essential tremor. Eur J Pharmacol 659: 30–36, 2011. doi: 10.1016/j.ejphar.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich SG. Essential Tremor. Med Clin North Am 103: 351–356, 2019. doi: 10.1016/j.mcna.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, André M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science 334: 385–389, 2011. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- Samarut É, Swaminathan A, Riché R, Liao M, Hassan-Abdi R, Renault S, Allard M, Dufour L, Cossette P, Soussi-Yanicostas N, Drapeau P. γ-Aminobutyric acid receptor alpha 1 subunit loss of function causes genetic generalized epilepsy by impairing inhibitory network neurodevelopment. Epilepsia 59: 2061–2074, 2018. doi: 10.1111/epi.14576. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum— insights from the clinic. Cerebellum 6: 254–267, 2007. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schneider Gasser EM, Duveau V, Prenosil GA, Fritschy JM. Reorganization of GABAergic circuits maintains GABAA receptor-mediated transmission onto CA1 interneurons in alpha1-subunit-null mice. Eur J Neurosci 25: 3287–3304, 2007. doi: 10.1111/j.1460-9568.2007.05558.x. [DOI] [PubMed] [Google Scholar]
- Shah B. Essential tremor: a comprehensive overview. J Neurol Disord 5: 2, 2017. doi: 10.4172/2329-6895.1000343. [DOI] [Google Scholar]
- Shanker V. Essential tremor: diagnosis and management. BMJ 366: l4485, 2019. doi: 10.1136/bmj.l4485. [DOI] [PubMed] [Google Scholar]
- Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, Beach TG. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology 70: 1452–1455, 2008. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- Sługocka A, Wiaderkiewicz J, Barski JJ. Genetic targeting in cerebellar purkinje cells: an update. Cerebellum 16: 191–202, 2017. doi: 10.1007/s12311-016-0770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46: 831–844, 2010. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng ML, Krook-Magnuson E. The cerebellum and epilepsy. Epilepsy Behav: 106909, 2020. doi: 10.1016/j.yebeh.2020.106909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O’Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J Neurosci 21: 3409–3418, 2001. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Jotty K. Mini-Review: Effects of ethanol on GABAA receptor-mediated neurotransmission in the cerebellar cortex—recent advances. Cerebellum 14: 438–446, 2015. doi: 10.1007/s12311-014-0639-3. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 21: 3009–3016, 2001. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature 544: 96–100, 2017. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Fowler SC. Concurrent quantification of tremor and depression of locomotor activity induced in rats by harmaline and physostigmine. Psychopharmacology (Berl) 158: 273–280, 2001. doi: 10.1007/s002130100882. [DOI] [PubMed] [Google Scholar]
- Witter L, Rudolph S, Pressler RT, Lahlaf SI, Regehr WG. Purkinje cell collaterals enable output signals from the cerebellar cortex to feed back to Purkinje cells and interneurons. Neuron 91: 312–319, 2016. doi: 10.1016/j.neuron.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Schonewille M, Renzi M, Viltono L, Sassoè-Pognetto M, Badura A, Gao Z, Hoebeek FE, van Dorp S, Wisden W, Farrant M, De Zeeuw CI. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci 12: 1042–1049, 2009. doi: 10.1038/nn.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E, Shah S, Cai L, Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158: 945–958, 2014. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ma K, Faust PL, Honig LS, Cortés E, Vonsattel JP, Louis ED. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol 19: 625–630, 2012. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Krook-Magnuson E. Cognitive collaborations: bidirectional functional connectivity between the cerebellum and the hippocampus. Front Syst Neurosci 9: 177, 2015. doi: 10.3389/fnsys.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler Z, Hoffmann K, Krook-Magnuson E. HippoBellum: acute cerebellar modulation alters hippocampal dynamics and function. J Neurosci 40: 6910–6926, 2020. doi: 10.1523/JNEUROSCI.0763-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Santaniello S. Role of cerebellar GABAergic dysfunctions in the origins of essential tremor. Proc Natl Acad Sci USA 116: 13592–13601, 2019. doi: 10.1073/pnas.1817689116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ng AH, Tanner JA, Wu WT, Copeland NG, Jenkins NA, Huang JD. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis 40: 45–51, 2004. doi: 10.1002/gene.20062. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Z, Ding L, Deel ME, Arain FM, Murray CR, Patel RS, Flanagan CD, Gallagher MJ. Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J Biol Chem 288: 21458–21472, 2013. doi: 10.1074/jbc.M112.444372. [DOI] [PMC free article] [PubMed] [Google Scholar]