Abstract
Coronavirus Disease-19 (COVID-19) outbreak has significantly burdened healthcare systems worldwide, leading to reorganization of healthcare services and reallocation of resources. The Italian Society for Study of Esophageal Diseases (SISME) conducted a national survey to evaluate changes in esophageal cancer management in a region severely struck by COVID-19 pandemic. A web-based questionnaire (26 items) was sent to 12 SISME units. Short-term outcomes of esophageal resections performed during the lockdown were compared with those achieved in the same period of 2019. Six (50%) centers had significant restrictions in their activity. However, overall number of resections did not decrease compared to 2019, while a higher rate of open esophageal resections was observed (40 vs. 21.7%; P = 0.034). Surgery was delayed in 24 (36.9%) patients in 6 (50%) centers, mostly due to shortage of anesthesiologists, and occupation of intensive care unit beds from intubated COVID-19 patients. Indications for neoadjuvant chemo (radio) therapy were extended in 14% of patients. Separate COVID-19 hospital pathways were active in 11 (91.7%) units. COVID-19 screening protocols included nasopharyngeal swab in 91.7%, chest computed tomography scan in 8.3% and selective use of lung ultrasound in 75% of units. Postoperative interstitial pneumonia occurred in 1 (1.5%) patient. Recovery from COVID-19 pandemic was characterized by screening of patients in all units, and follow-up outpatient visits in only 33% of units. This survey shows that clinical strategies differed considerably among the 12 SISME centers. Evidence-based guidelines are needed to support the surgical esophageal community and to standardize clinical practice in case of further pandemics.
Keywords: COVID-19, esophageal cancer, management, surgery, survey
INTRODUCTION
Coronavirus Disease-19 (COVID-19) outbreak has significantly burdened healthcare systems all around the world, leading to the reorganization of healthcare services and reallocation of resources to face the epidemic. COVID-19 rapidly spread in Italy, infecting almost 230,000 individuals and causing more than 34,000 deaths.1 Northern regions were the most highly affected, due to higher population and industrial density.2 Starting 8th March, lockdown was established in Northern Italy and extended to the whole Nation on March 11th.3 The vast majority of Italian hospitals were forced to change their structure, employing most intensive care units (ICU) facilities to treat COVID-19-infected patients.4
As a consequence, surgeries have been limited to urgent/emergent cases and cancer patients, to face the reduced number of ICU beds available for COVID-19-free patients.
Early reports suggest a higher risk of COVID-19 infection with associated severe morbidity and mortality in cancer patients.5,6 The complexity of the multidisciplinary approach to esophageal cancer patients and the high morbidity rates of esophageal surgery have challenged the treatment pathways of these patients. To help surgeons in their decision-making process and optimize the outcomes of these patients, several recommendations have been released and strategies advised. In particular, alternative treatment modalities and delaying esophageal resection have been proposed.7 However, these strategies were spontaneous and not supported by strong evidence, since the knowledge about the natural history of the disease is limited and tumor progression is highly variable among esophageal cancer patients.
The other main concern is the surgical approach. The International Society for Diseases of the Esophagus (ISDE)8 has transposed the recommendations of the Intercollegiate General Surgery Guidance (IGSG) in the United Kingdom9 and the Society of the American Gastrointestinal and Endoscopic Surgeons (SAGES).6 The first version of IGSG, published on 26 March suggested against the use of laparoscopy unless the benefits obviously outweigh the risks. This statement is based on the hypothesis that the risk of virus aerosolization is increased during minimally invasive surgery.10 However, clinical impact and real-life applicability of the above-reported recommendations have not been investigated. In the update released on 5 June, the Society changed its position, no longer coming out against laparoscopy, but stressing the need for risk mitigation strategies including use of technological protection and enhanced personal protective equipment (PPE).
The Italian Society for Study of Esophageal Diseases (SISME) has conducted a national survey among esophageal surgeons, in order to evaluate possible management strategies employed during the COVID-19 epidemic in Italy. We herein present the results of the SISME survey, focusing on the impact of COVID-19 pandemic on treatment strategies, use of minimally invasive approach and short-term outcomes.
METHODS
A web-based questionnaire was sent to 12 SISME esophageal surgery units, and all the fulfilled forms were collected and analyzed. All centers included in the survey are referral centers for esophageal surgery functioning as hubs. Almost all the population of Northern Italy and a significant percentage of Southern Italy are served by them. Center baseline data were asked in the first part of the questionnaire and are reported in the result section.
The restriction in surgical activity forced some centers to change their practice, both in diagnostic exams and neoadjuvant chemoradiotherapic schedule, as detailed in the result section.
The questionnaire included 26 items divided into three sections: (i) baseline characteristics of each surgical unit, including the number of esophageal resections performed per year and the preferred surgical approach; (ii) the impact of the COVID-19 pandemic on hospital reorganization, preoperative patients screening for COVID-19, alternative treatment modalities to surgery, surgical timing and perioperative patient management; (iii) implementation of strategies for recovery from COVID-19 outbreak.
Furthermore, centers were asked to complete a table summarizing the number and short-term outcomes of esophageal resections performed during the lockdown period (9 March–3 May 2020) and in the same period of time in 2019.
Statistical analyses
Continuous variables are reported as mean and standard deviation, while categorical variables as events number and percentages. Differences between 2019 and 2020 operative data were tested with the Fisher’s exact test for categorical variables and with the Mann–Whitney test for continuous ones.
RESULTS
All surgical units, members of SISME and located in five different regions of Northern Italy, answered the questionnaire.
Centres baseline characteristics
Eleven units (91.7%) were tertiary referral or academic centers, while 1 (8.3%) was a local district hospital. Six units (50%) were upper GI (UGI) dedicated units.
Three centers (25%) performed more than 50 procedures/year, 4 (33.3%) 20–50 procedures/year, while 5 (41.7%) less than 20 procedures/year.
Eight (66.6%) units adopted a minimally invasive approach: 5 (41.7%) totally minimally invasive and 3 (25%) hybrid. The robot assistance was used in 5 (41.7%) centers. Open esophageal resections were performed in 4 (33.3%) cases.
Impact of COVID-19 on hospital reorganization
Nearly, all centers (91.7%) had dedicated COVID-19-positive wards and ICUs, reflecting separate hospital pathways for COVID-19-positive and negative patients.
Patients screening for COVID-19 infection
Preoperatively, all patients were tested for COVID-19 infection with a nasopharyngeal swab in 11 centers (91.7%); in one center, the test was limited only to patients scheduled for postoperative ICU monitoring admission.
A chest computed tomography (CT) scan to rule out radiological features suspected for COVID-19 infection was routinely obtained before surgery in all patients in one center (8.3%), in patients with symptoms suggestive for COVID-19 infection (fever, cough, dyspnea) or at high risk for infection in 9 (75%) centers, and only in COVID-19-infected patients in 1 (8.3%) center. The chest CT scan was not part of the COVID-19 screening protocol in one unit (8.3%).
Preoperative lung ultrasound was available in 9 out of 12 centers (75%) and was used as an alternative (50%) or in addition (25%) to chest CT scan in high risk or COVID-19-infected patients (Fig. 1A–C).
Fig. 1.
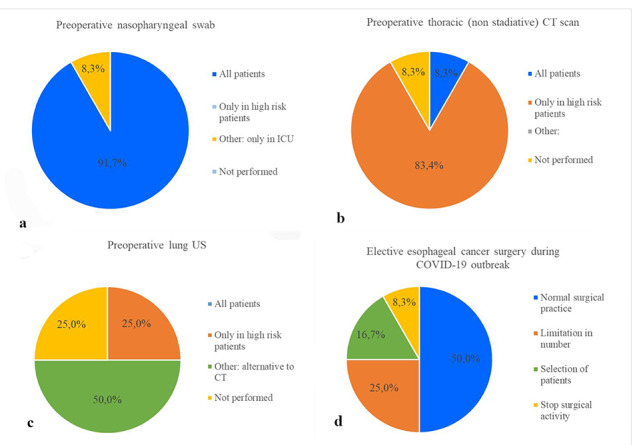
Preoperative screening measure among the Centers (A–C) and elective esophageal cancer surgery during COVID-19 (D).
Impact of COVID-19 on clinical activity
Six (50%) centers reported no changes in their oncologic surgical practice during the COVID-19 pandemic, while 6 units had significant restrictions in their activity: 3 (25%) declared a reduction in the number of esophageal resections, 2 (16.7%) limited esophageal resections only to selected patients without severe comorbidities, while 1 (8.3%) completely stopped its surgical activity (Fig. 1D).
In the event of a preoperative positive COVID-19 nasopharyngeal swab and/or imaging (chest CT scan, lung ultrasound), all centers agreed to postpone surgery, thus limiting surgery only to emergency cases.
Esophageal resection was delayed in 24 (36.9%) patients in 6 (50%) centers (Table 1), due to limited availability of ICU facilities (4 units) and complete lockdown (1 unit). At the time of completing the questionnaire, 7 (29.1%) patients were still on the waiting list; the delay of surgery in the remaining 17 patients who were operated ranged from 15 to 45 days.
Table 1.
Number and cause of delayed operation among centers
| Center | No. of patients | Reason for delay | Duration of delay (days) |
|---|---|---|---|
| 1 | 2 | Complete lockdown of the surgical activities | 15 |
| 2 | 1 | Preoperative positive swab | 20 (after two negative controls) |
| 3 | 2 | Limited ICU facility availability | 15 |
| 4 | 2 | Limited ICU facility availability | 15 |
| 5 | 10 | Limited ICU facility availability | 33–45 |
| 6 | 7 | Limited ICU facility availability | Not yet operated (3 months) |
Four surgical units that were forced to delay the scheduled operations for organizational issues opted to extend neoadjuvant chemotherapy in those patients who previously received neoadjuvant treatment or to start a neoadjuvant treatment in those patients who were scheduled for upfront surgery.
PPE
When asked regarding general protective measures adopted in the surgical theatre, the respondents reported the use of the following PPE: surgical mask (66.7%), filtering face piece mask (83.3%), eye protective glasses (58.3%), eye protective visor (50%), insulating suit (16.6%) and double gloves (8.3%) (Fig. 2A).
Fig. 2.
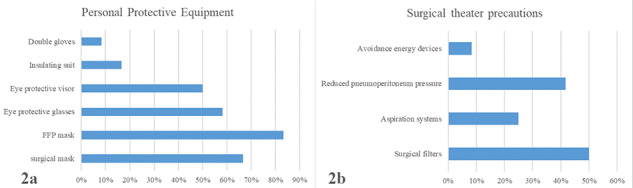
Protective measures against COVID-19 diffusion in the operating room; (A) PPE; (B) surgical theatre precautions.
During laparoscopic/thoracoscopic procedures, most centers adopted filters (50%) or aspiration systems (25%) for surgical smoke elimination. In addition, reduction of pneumoperitoneum pressure (41.7%) and avoidance of energy devices (8.3%) were reported (Fig. 2B). None of the surgical staff got infected by COVID-19.
Postoperative patient COVID-19 infection
In the postoperative period, 7 out of 65 patients (10.8%) from three centers developed symptoms suggestive for COVID-19 infection, but no SARS-Cov 2 RNA was detected in any patients both on nasopharyngeal swab and bronchoalveolar lavage. One of them developed bilateral interstitial pneumonia suggestive for COVID-19 infection diagnosed by a CT scan and died for pulmonary complications. The postoperative course was uneventful for the other six patients.
Implementation strategies for recovery from COVID-19 outbreak (phase 2)
With the advent of phase 2, all centers reported performing routine preoperative nasopharyngeal swab in all patients, with 2 (16.7%) centers requiring chest CT scan and/or lung ultrasound in patients with suspected symptoms or high risk factors for infection.
The four centers that treated patients with chemo (radiation) therapy instead of surgery due to lack of ICU facilities have planned to return to the standard of care.
At the time of completing the survey, three (25%) centers declared to still perform remote follow-up patient assessment (i.e. by phone), and five (41.7%) guaranteed outpatient follow-up visits only to symptomatic patients. Four (33.3%) units reported that visits in outpatient clinics were maintained for all patients.
Impact of COVID-19 on surgical outcomes
Data related to oncologic esophageal surgical procedures performed during the COVID-19 outbreak and the same period in 2019 are summarized in Table 2. The overall number of esophageal resections slightly increased (+8%). No significant differences were found in patients’ baseline characteristics. Regarding the surgical approach, a higher rate of open resections (40 vs. 21.7%; P = 0.034) and of conversions to open surgery (12.8 vs. 0%; P = 0.017) were performed during the COVID-19 period. The postoperative course was similar, with no significant differences in length of ICU stay, overall hospital stay and complications.
Table 2.
Esophageal resections: comparison between 2019 and 2020
| 2019 | 2020 | P-value | |||
|---|---|---|---|---|---|
| Operations (n; %) | 60 | 100% | 65 | 100% | |
| Age [years](mean; SD) | 66.4 (±3.5) | 67.7 (±6.8) | 0.596# | ||
| Tumor location | |||||
| Gastro-esophageal junction carcinoma | 34 | 56.7% | 43 | 66.2% | 0.358¶ |
| Esophageal carcinoma | 26 | 43.3% | 22 | 33.8% | |
| Surgical approach | |||||
| Open (n; %) | 13 | 21.7% | 26 | 40.0% | 0.034¶ |
| Hybrid (n; %) | 18 | 30.0% | 13 | 20.0% | 0.219¶ |
| Laparoscopic/thoracoscopic (n; %) | 17 | 28.3% | 18 | 27.7% | 1¶ |
| Robot assisted (n; %) | 12 | 20.0% | 8 | 12.3% | 0.329¶ |
| Conversion (n; %) | 0/47 | 0% | 5/39 | 12.8% | 0.017¶ |
| Length of ICU stay [years](mean; SD) | 2.3 (±1.6) | 2.8 (±2.4) | 0.628# | ||
| Length of Hospital stay [years] (mean; SD) | 12.0 (±4.4) | 11.5 (±3.0) | 0.767# | ||
| Complications (n; %) | 31 | 51.6% | 34 | 52.2% | 1¶ |
| Dindo 1–2 | 15 | 25.0% | 19 | 29.2% | 0.689¶ |
| Dindo 3 | 11 | 18.3% | 6 | 9.2% | 0.192¶ |
| Dindo 4 | 5 | 8.3% | 8 | 12.3% | 0.564¶ |
| Dindo 5 | 0 | 0% | 1 | 1.5% | 1¶ |
| Anastomotic leak (n; %) | 4 | 6.7% | 9 | 13.8% | 0.246¶ |
# t-test
¶Fisher’s exact test
DISCUSSION
The impact of COVID-19 outbreak on national health systems has been dramatic all around the world. The need for intensive treatments to manage COVID-19 patients rapidly increased over time, thus leading to a tremendous shortage of ICU resources and of anesthesiologist on duty for postoperative monitoring of patients undergoing surgery or to face possible post-surgical complications. As a result, the principal approach of most national governments quickly became rationing and prioritizing surgical procedures, thus allowing only urgent/emergent surgeries and cancer operations.
Surgery of esophageal cancer is burdened by postoperative major complications in up to 65% of patients11 and respiratory morbidity rates as high as 30%.12,13 As a consequence of these high postoperative complication rates, along with the technical aspects of the operation, most patients require postoperative ICU monitoring. The shortage of ICU facilities and the potential increased risk of mortality related to perioperative COVID-19 infection in cancer patients has raised several concerns about the most appropriate management of patients with esophageal cancer. Several scientific societies released recommendations aiming at supporting the decision-making process of the physicians worldwide; however, only a few studies14,15 specifically assessed the clinical impact of these guidelines on the management of patients with esophageal cancer.
The purpose of this survey was to provide a snapshot of esophageal cancer surgery in an area strongly hit by the pandemic. This survey across 12 Italian SISME institutions shows that the overall number of surgeries during the COVID-19 pandemic did not decrease when compared to the same period of 2019. This might reflect the fact that most centers were tertiary referral or academic institutions, with well-defined pathways for COVID-19-positive and COVID-19-negative patients and, during the pandemic, they functioned as hubs within the oncologic hub-and-spoke program developed in Northern Italy. However, there was great variability among the centers in terms of surgical activity during COVID-19 pandemic. Despite only 50% of them maintained the routine caseload, the remaining tertiary referral centers able to operate without significant restrictions increased the number of esophageal resections, in order to guarantee a global number of cases comparable to the same period of the year before.
Since the beginning of the pandemic, it became clear that preoperative screening of patients was mandatory, in order to avoid potentially severe postoperative complications in surgical patients. Several diagnostic tools have been implemented. A study from Wuhan on 1,014 patients showed a sensitivity of 97% of chest CT in suggesting COVID-19 infection, even in patients with a negative swab16. COVID-19 pneumonia also shows typical patterns at lung ultrasound17, but studies on diagnostic accuracy are still lacking.
The routine preoperative testing for COVID-19 was heterogeneous among the units. While the swab test was used in most centers (91.7%), chest CT scan was routinely used only in one unit, and reserved to patients with symptoms in 75% of centers. Main indications for lung ultrasound were patients with high risk of infection, as an alternative or in addition to chest CT scan. The results obtained in this survey are consistent with those recently published by the Oesophagogastric Anastomosis Audit Group14. In this international survey, they found that routine testing was not performed in only 14.7% units, while CT scan was routinely used in only 6% of units; in 32% of centers, a combination of symptoms, swab test and chest CT scan was employed for patients screening.
In 11 out of 12 centers, pathways for COVID-19-positive and COVID-19-negative patients were strictly separated. This turned into a very low rate of postoperative COVID-19 infection (1.5%) in this very high-risk and frail patient population. This finding shows how preoperative screening measures and separate pathways inside the hospital represented essential measures to guarantee patients’ safety.
The preoperative screening for COVID-19 deeply influenced surgical planning in all Italian centers: in patients with a positive swab or suggestive imaging, the operation was delayed unless in case of emergency surgery.
Postponing esophagectomy in favor of alternative treatment modalities to surgery has been recently proposed for esophageal cancer patients.18 A delay in esophageal cancer resections, ranging between 15 and 45 days, occurred in 37% of patients in 50% of the centers. Notably, at the time of completing this survey, one-third of these patients were still awaiting surgery. This was due to limited ICU facility availability in four centers and a complete lockdown of the surgical activity in one.
Indications and timing of neoadjuvant treatment were extended in all patients who had their operation postponed. A similar shift towards increased use of chemo (radiation) therapy was also observed in the international survey by Kamarajah et al.14 with changes in the treatment in 60% of patients. In particular, definitive chemoradiation therapy was offered to 30% of patients, and neoadjuvant chemoradiation therapy was planned in 21% of T2 N0 cancers. In addition, there was a 5-fold increase in centers planning surgery 10 to 12 weeks after the end of neoadjuvant chemoradiation therapy.
Despite the restrictions related to the epidemic, none of the centers included in the survey opted for a therapeutic shift to definitive chemo (radiation) therapy in patients scheduled for surgery. A recently published population study comparing survival outcomes in patients who underwent definitive chemoradiotherapy versus neoadjuvant therapy followed by esophagectomy clearly showed a significant survival benefit in the surgical group. Authors concluded that surgery remains an integral component of the management of patients with esophageal cancer.19 To date, the oncologic implications of these changes in treatment timing and modalities are unclear, with some studies showing that longer the interval between neoadjuvant treatment and surgery, the higher the recurrence rates20, while others suggest the safety of postponing surgery up to 28 or 30 weeks21. Further studies are needed to elucidate the real impact of delaying surgery on survival with esophageal cancer.
Regarding the surgical approach used during the COVID-19 pandemic, the rate of open resection and conversions to open surgery significantly increased during the lockdown. The increased rate of open resections was probably due to the influence of the statements released by several international societies recommending to avoid minimally invasive approach during the epidemic. This statement is based on the hypothesis of increased risk of virus aerosolization when electrosurgical devices are used.10 Even though there is no evidence of COVID-19 emission and transmission during laparoscopic surgery, this suggestion has been quickly shared by surgeons worldwide via the social networks and the use of laparoscopy/thoracoscopy banned in several areas, thus potentially depriving patients from the major advantages of the minimally invasive approach. De Leeuw et al. 22 recently published a review of the current literature and local expertise on COVID-19 and laparoscopic surgery, concluding that there is no reason to abandon laparoscopy in favor of open surgery. None of the surgical staff of the units involved in the survey got infected by COVID-19. The use of adequate measures of prevention, both in the operative theatre and in the wards, was effective in preventing surgical staff contamination. The minimally invasive approach did not influence COVID-19 diffusion among operators, showing how the use of safe smoke evacuation systems was able to avoid virus transmission.
With the end of the lockdown, the Northern Italian units planned to progressively return to their usual routine activities. However, the fear of a further peak of COVID-19 infection has led all centers to continue to test all patients with a nasopharyngeal swab preoperatively. In addition, at the time of the survey, only 33.3% of units reported that visits in outpatient clinics were maintained for all patients. The other centers still conduct remote follow-up assessment or guarantee outpatient follow-up visits only to symptomatic patients. These data show how the return to routine is slow, underlining a high level of caution towards the epidemic.
No significant differences were recorded in the short-term surgical outcomes between 2019 and 2020. Similar rates and grades of severity of postoperative complications were observed. In particular, infectious pulmonary complications occurred only in one (1.5%) patient, who eventually died of respiratory failure.
One of the major concern dealing with the period of lockdown is the number of delayed or missed cancer diagnoses. Outpatient visits were completely stopped for 2 months and only partially restored from June 2020. These data are not available at the moment, but it is likely that a higher number of locally cases will be diagnosed in the next months.
In conclusion, COVID-19 outbreak has radically changed surgical practice in most esophageal cancer surgical units in Northern Italy. The heterogeneity of clinical strategies adopted was a consequence of the absence of guidelines based on strong evidence. Collecting and sharing data within national and international study groups will help to better define the long-term impact of COVID-19 epidemic on patients with esophageal cancer. Furthermore, we believe that the surgical community should carefully analyze the impact of COVID-19 pandemic on surgical and oncologic results, in order to be prepared in case of a new widespread of COVID-19 or other viral pandemics.
AUTHOR CONTRIBUTIONS
Study concept and design: Rebecchi, Arolfo and Ugliono. Analysis and interpretation of data: Rebecchi, Arolfo and Ugliono. Drafting of the manuscript: Arolfo and Ugliono. Critical revision of the manuscript for important intellectual content: Morino and Rebecchi. Statistical analysis: Arolfo and Ugliono.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGEMENTS
None.
Specific author contributions: SISME (The Italian Society for Study of Esophageal Diseases) COVID-19 group. The individuals included in the SISME COVID-19 group are collaborators of the study.
Contributor Information
Fabrizio Rebecchi, Department of Surgical Sciences, University of Turin, Turin, Italy.
Simone Arolfo, Department of Surgical Sciences, University of Turin, Turin, Italy.
Elettra Ugliono, Department of Surgical Sciences, University of Turin, Turin, Italy.
Mario Morino, Department of Surgical Sciences, University of Turin, Turin, Italy.
Emanuele Asti, Department of General and Foregut Surgery, University of Milan, IRCCS, Policlinico San Donato, Milan, Italy.
Luigi Bonavina, Department of General and Foregut Surgery, University of Milan, IRCCS, Policlinico San Donato, Milan, Italy.
Felice Borghi, General and Oncologic Surgery Unit, Santa Croce e Carle Hospital, Cuneo, Italy.
Andrea Coratti, Division of Oncological and Robotic Surgery, Careggi University Hospital of Florence, Florence, Italy.
Andrea Cossu, Gastrointestinal Surgery Unit, San Raffaele Hospital, Milan, Italy.
Giovanni De Manzoni, General, Esophageal and Gastric Surgery Unit, University Hospital of Verona, Verona, Italy.
Stefano De Pascale, Digestive Surgery, European Institute of Oncology -IRCCS, Milan, Italy.
Giovanni Carlo Ferrari, Mini-Invasive Oncological Surgical Department, Niguarda Hospital, Milan, Italy.
Uberto Fumagalli Romario, Digestive Surgery, European Institute of Oncology -IRCCS, Milan, Italy.
Simone Giacopuzzi, General, Esophageal and Gastric Surgery Unit, University Hospital of Verona, Verona, Italy.
Monica Gualtierotti, Mini-Invasive Oncological Surgical Department, Niguarda Hospital, Milan, Italy.
Massimo Guglielmetti, Thoracic Surgery, S. Anna Hospital, Como, Italy.
Stefano Merigliano, Center for Esophageal Disease, Department of Surgery, Oncology and Gastroenterology, University Hospital of Padova, Padua, Italy.
Giovanni Pallabazzer, Esophageal Surgery Unit, University Hospital of Pisa, Pisa, Italy.
Paolo Parise, Gastrointestinal Surgery Unit, San Raffaele Hospital, Milan, Italy.
Andrea Peri, Department of Surgery, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Pavia, Italy.
Andrea Pietrabissa, Department of Surgery, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Pavia, Italy.
Riccardo Rosati, Gastrointestinal Surgery Unit, San Raffaele Hospital, Milan, Italy.
Stefano Santi, Esophageal Surgery Unit, University Hospital of Pisa, Pisa, Italy.
Angela Tribuzi, Division of Oncological and Robotic Surgery, Careggi University Hospital of Florence, Florence, Italy.
Michele Valmasoni, Center for Esophageal Disease, Department of Surgery, Oncology and Gastroenterology, University Hospital of Padova, Padua, Italy.
Jacopo Viganò, Department of Surgery, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Pavia, Italy.
Jacopo Weindelmayer, General, Esophageal and Gastric Surgery Unit, University Hospital of Verona, Verona, Italy.
References
- 1. Ministero della salute italiano (2020) COVID-19 Italian situation Available from: http://www.protezionecivile.gov.it/ [accessed 15 May 2020].
- 2. Distante C, Piscitelli P, Miani A. Covid-19 outbreak progression in Italian regions: approaching the peak by the end of march in northern Italy and first week of April in southern Italy. Int J Environ Res Public Health 2020; 27(17): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Decreto del Presidente del Consiglio dei Ministri 8 marzo 2020. Gazzetta Ufficiale della Repubblica Italiana Available from: https://www.gazzettaufficiale.it/eli/id/2020/03/08/20A01522/sg.
- 4. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet 2020; 11(395(10231)): 1225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu P W Y, Hassan C, Yip H C, Antonelli G, Sharma P. Management of Upper-GI endoscopy and surgery in COVID-19 outbreak. Dis Esophagus 2020; Esophagus website Available from: https://isde.net/covid19-guidance accessed 30 Mar 2020; 00: 1–4. doi: 10.1093/dote/doaa029. [DOI] [Google Scholar]
- 6. Francis N, Dort J, Cho E, Feldman L, Keller D, Lim R et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc 2020; 34(6): 2327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuech J-J, Gangloff A, Di Fiore F, Michel P, Brigand C, Slim K et al. Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Visc Surg 2020; 157(3S1): S7–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yip HC, Chiu P, Hassan C, Antonelli G, Sharma P. ISDE guidance statement: management of upper gastrointestinal endoscopy and surgery in COVID-19 outbreak. Dis Esophagus 2020; 33(5). 10.1093/dote/doaa029. [DOI] [Google Scholar]
- 9. Royal College of Surgeons of Edinburgh (2020) , Intercollegiate general surgery guidance on COVID-19 update. Available from: https://www.rcseng.ac.uk/coronavirus/joint-guidance-for-surgeons-v2/ [accessed 15 May 2020].
- 10. Barbieri L, Talavera Urquijo E, Parise P, Nilsson M, Reynolds J V, Rosati R. Esophageal oncologic surgery in SARS-CoV-2 (COVID-19) emergency. Dis Esophagus 2020; 33(5): doaa028. doi: 10.1093/dote/doaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mariette C, Markar S, Dabakuyo-Yonli T S, Meunier B, Pezet D, Collet D et al. Health-related quality of life following hybrid minimally invasive versus open Esophagectomy for patients with Esophageal cancer, analysis of a Multicenter, open-label, randomized phase III controlled trial: the MIRO trial. Ann Surg 2020; 271(6): 1023–9. [DOI] [PubMed] [Google Scholar]
- 12. Bartlett E K, Roses R E, Kelz R R, Drebin J A, Fraker D L, Karakousis G C. Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of Surgeons National Surgical Quality Improvement Program database. Surgery 2014; 156(2): 298–304. [DOI] [PubMed] [Google Scholar]
- 13. Wang W-J, Li R, Guo C-A, Li H-T, Yu J-P, Wang J et al. Systematic assessment of complications after robotic-assisted total versus distal gastrectomy for advanced gastric cancer: a retrospective propensity score-matched study using Clavien-Dindo classification. Int J Surg 2019; 71: 140–8. [DOI] [PubMed] [Google Scholar]
- 14. Kamarajah S K, Markar S R, Singh P, Griffiths E A. Oesophagogastric Anastomosis Audit Group. The influence of the SARS-CoV-2 pandemic on esophagogastric cancer services: an international survey of esophagogastric surgeons. Dis Esophagus 2020; 33(7): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torzilli G, Vigano L, Galvanin J, Castoro C, Quagliuolo V, Spinelli A et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg 2020; 272(2): e112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296(2): E32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Convissar D, Gibson L E, Berra L, Bittner E A, Chang M G. Application of lung ultrasound during the COVID-19 pandemic: a narrative review. Anesth Analg 2020; 131(2): 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Fiore F, Bouché O, Lepage C, Sefrioui D, Gangloff A, Schwarz L et al. COVID-19 epidemic: proposed alternatives in the management of digestive cancers: a French intergroup clinical point of view (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis 2020; 52(6): 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamarajah S K, Phillips A W, Hanna G B, Low D, Markar S R. Definitive Chemoradiotherapy Compared to Neoadjuvant Chemoradiotherapy With Esophagectomy for Locoregional Esophageal Cancer: National Population-Based Cohort Study. Ann Surg 2020. doi: 10.1097/SLA.0000000000003941 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20. Qin Q, Xu H, Liu J, Zhang C, Xu L, Di X et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg 2018; 59: 11–8. [DOI] [PubMed] [Google Scholar]
- 21. Turaga K K, Girotra S. Are we harming cancer patients by delaying their cancer surgery during the COVID-19 pandemic? Ann Surg 2020; 2: 10.1097/SLA.0000000000003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leeuw R A, Burger N B, Ceccaroni M, Zhang J, Tuynman J, Mabrouk M et al. COVID-19 and laparoscopic surgery, a scoping review of current literature and local expertise. JMIR Public Health Surveill 2020; 6(2): e18928. [DOI] [PMC free article] [PubMed] [Google Scholar]


