Abstract
In severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, viral load peaks early and declines quickly after symptom onset. Severe coronavirus disease 2019 (COVID-19) is marked by aberrant innate and adaptive immune responses with an abnormal cytokine profile and multiorgan system dysfunction that persists well after viral clearance. A purely antiviral treatment strategy may therefore be insufficient, and antiviral agents have not shown a benefit later in the illness course. A number of immunomodulatory strategies are being tested, including corticosteroids, cytokine and anticytokine therapies, small molecule inhibitors, and cellular therapeutics. To date, the only drug to show a mortality benefit for COVID-19 in a randomized, controlled trial is dexamethasone. However, there remains uncertainty about which patients may benefit most and about longer-term complications, including secondary infections. Here, we review the immune dysregulation of severe COVID-19 and the existing data behind various immunomodulatory strategies, and we consider future directions of study.
Keywords: COVID-19, SARS-CoV-2, immunomodulation, hyperinflammatory, cytokine storm
Evidence supports an acute viral followed by an immune dysregulation phase in severe COVID-19 that is associated with inadequate early type I interferon response and imbalanced innate and adaptive immunity. We review clinical data available for various immunomodulatory therapies.
(See the Editorial Commentary by Kaiser et al on pages e1144–5.)
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) results in a wide spectrum of disease. Among individuals with symptomatic coronavirus disease 2019 (COVID-19), approximately 15%–20% are estimated to develop severe presentations that require supplemental oxygen, including up to 5% who may develop critical illness [1]. Infection fatality rates are population- and age-dependent, with very low rates for children and young adults, but mortality rates >25% for individuals aged >90 years [2]. Areas with rapid surges of infections, associated with delayed access to care, may have higher fatality rates, as was found in Spain and New York City [3, 4]. The explanation for the profound differences in disease severity stratified by age are unknown and likely multifactorial. Current theories include possible increased likelihood of cross-protective cellular immune response from recent infection with common human coronaviruses and age-related changes in immunity, including decreased availability of naive T cells to respond to new viral antigens in older adults [5–7]. Autopsy studies demonstrate that the primary pulmonary pathology is diffuse alveolar damage, with micro- and macrovascular thrombosis [8].
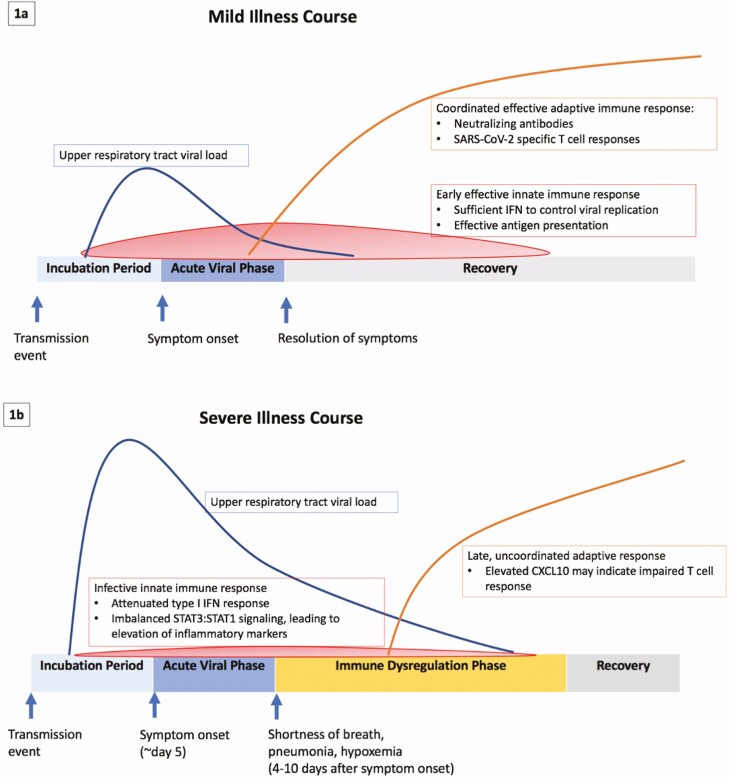
Symptomatic patients typically develop mild symptoms during an acute viral phase, although a subset of patients will progress to severe disease that can last weeks and often requires hospitalization [9–11]. This severe stage is typically marked by immune dysregulation and abnormal inflammatory markers (Figure 1). Higher upper respiratory tract viral loads are associated with more severe presentations [12, 13]. Numerous therapeutic agents are currently under investigation for treatment of COVID-19. During the early phase of the pandemic, many agents were given off-label or in the context of randomized, controlled trials (RCTs). While we will review randomized and nonrandomized series here, it is important to note that only data from high-quality RCTs should change practice in the next phase of the pandemic.
Figure 1.
A, Schematic of the mild illness course for coronavirus disease 2019 (COVID-19) with an effective early innate response and an early, coordinated adaptive immune response. B, Schematic of the severe illness course for COVID-19 where an ineffective innate immune response including an attenuated type I interferon response and poor antigen presentation as well as a late uncoordinated adaptive immune response are associated with poor viral control (higher upper respiratory tract viral load) and proinflammatory, immune dysregulated profile. C-X-C motif chemokine ligand 10 (CXCL10) has been proposed as a possible biomarker for an ineffective specific T-cell response to SARS-CoV-2. Abbreviations: CXCL10, C-X-C motif chemokine ligand 10; IFN, interferon; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; STAT, signal transducer and activator of transcription protein.
The main therapeutic strategies are direct antiviral and immunomodulatory approaches. To date, remdesivir, a nucleoside analogue that targets the viral RNA-dependent RNA polymerase, has the most supportive data. It showed efficacy when given early to rhesus macaques as well as improvement in time to recovery in the large RCT Adaptive COVID-19 Treatment Trial (ACTT)-1, which compared remdesivir with placebo [14–16]. There was a suggestion of a mortality benefit in patients on supplemental oxygen but not in the intensive care unit (ICU), with no or minimal survival benefit seen for those with critical disease [16–18]. A challenge to antiviral therapy for COVID-19 is that patients with severe disease tend to present after 5–7 days of symptoms when viral loads are declining [19, 20].
The only agent yet to show a mortality benefit for COVID-19 is dexamethasone. In a large, open-label, UK-based RCT called Randomized Evaluation of Covid-19 Therapy (RECOVERY), more than 6000 patients were randomized in a 1:2 fashion to dexamethasone or standard of care, with a lower mortality recorded in the dexamethasone group (22.9% vs 25.7%, P < .001). The benefit was largest in those who were mechanically ventilated, and there was no benefit among those without hypoxemia. Additionally, a mortality benefit was seen for those who received dexamethasone after 7 days of symptoms but not if they received the agent before that time [21]. That remdesivir has not shown a significant benefit in critically ill patients and that the mortality benefit with dexamethasone is strongest in this group (and after 7 days of symptoms) further suggests a viral phase followed by an immune dysregulation phase.
That immunomodulation might impact COVID-19 outcomes is also suggested from some epidemiologic studies. While confounding and indication bias need to be considered, rheumatologists and inflammatory bowel specialists report early data suggestive of a lower incidence of severe COVID-19 for patients prescribed tumor necrosis factor (TNF)-α inhibitors compared with similar cohorts on chronic steroids [22, 23].
METHODS
We searched for English-language titles, abstracts, and relevant articles from LitCovid, an electronic literature hub for COVID-19–related articles indexed on PubMed from 1 January 2020 through 21 October 2020 using the following terms: corticosteroids, methylprednisolone, dexamethasone, tocilizumab, sarilumab, ruxolitinib, baricitinib, anakinra, and interferons. All RCTs were included, regardless of study size. Nonrandomized studies of at least 50 individuals treated with corticosteroids or tocilizumab were included. For other agents, reports were included if ≥20 individuals received the treatment. When no peer-reviewed data were available, press releases and preprints for major studies were included.
ACCUMULATING EVIDENCE FOR AN IMMUNE DYSREGULATION THAT CONTRIBUTE TO SEVERE COVID-19
Early reports of patients with severe COVID-19 describe marked derangements in inflammatory markers, including elevated levels of interleukin-6 (IL-6), ferritin, and C-reactive protein, each associated with severe outcomes [24, 25]. For example, high IL-6 levels are associated with progression to mechanical ventilation [26], elevated SARS-CoV-2 viremia, and longer viral RNA shedding [27–29]. Detailed cytokine profiling has noted significant differences between survivors and nonsurvivors in other markers of inflammation, including IL-2 receptor, IL-8, IL-10, and TNF-α [30]. The cytokine profile and clinical features of the second phase of severe COVID-19 illness have similarities to cytokine release syndrome (CRS) associated with chimeric antigen receptor (CAR) T-cell therapy. In both clinical scenarios, IL-6 that circulates at abnormally high levels may result in a signaling cascade, leading to vascular permeability and multisystem organ dysfunction [31]. In CRS, targeting the IL-6 axis was lifesaving [32] and led to early interest in IL-6 receptor inhibition as a possible treatment for severe COVID-19. More recently, several studies have reported that while certain cytokines are elevated in severe COVID-19, they are less elevated than for other conditions, including bacterial and non–COVID-19 acute respiratory distress syndrome (ARDS) [33, 34]. Therefore, it has become clear that severe or critical COVID-19 is not a true cytokine storm state.
The picture that is emerging is far more complex, with dysfunction of both the innate and adaptive immune system contributing to severe COVID-19. Transcriptional changes in host cells after SARS-CoV-2 infection include upregulation of cytokines such as IL-6 and IL-1 receptor antagonist as well as reduced interferon expression. There is also induction of other cytokines and chemokines, including chemokine ligand 2 (CCL2) and chemokine ligand 8 (CCL8) (which recruit macrophages) and CXCL2 and CXCL8 (which recruit neutrophils) [35]. This “imbalanced host response” is a hallmark of COVID-19 as the proinflammatory state starts within days after infection and persists long after viral clearance [35]. This cytokine milieu recruits and activates neutrophils, macrophages, and T lymphocytes [36]. In another study of critically ill patients with COVID-19, circulating CD8+ T lymphocytes showed significant reductions in cytokines, and natural killer cells had decreased intracellular expression of antiviral cytotoxic mediators granzyme A and perforin, consistent with an “exhausted phenotype” [37].
The type I interferon signaling pathway has emerged as likely playing a central role in COVID-19 pathogenesis. Inborn errors of the type I interferon pathway and autoantibodies against type I interferons are present (and overrepresented) in some patients with severe COVID-19 [38, 39]. Additionally, the SARS-CoV-2 genome encodes structural and nonstructural proteins that antagonize type I interferons [40]. At the same time, interferon-ß inhibits SARS-CoV-2 replication [41]. Attenuation of the type I interferon response is associated with inhibition of signal transducer and activator of transcription protein (STAT1) and activation of STAT3 signaling, which has myriad downstream effects, including induction of various inflammatory cytokines and dampening an effective T-cell response [42].
The adaptive immune response to SARS-CoV-2 infection is also under intense study. A coordinated early adaptive immune response with generation of SARS-CoV-2–specific CD4+ and CD8+ T cells and neutralizing antibodies is associated with less severe outcomes [7]. CXCL10 has been found to have a strong negative correlation with SARS-CoV-2–specific T-cell responses and has been proposed as a potential biomarker for poor T-cell responses in severe COVID-19 [7].
A now well-described inflammatory syndrome related to COVID-19, multisystem inflammatory syndrome in children, may have clinical features of Kawasaki disease, with some patients also meeting criteria for macrophage activation syndrome [43–46]. The syndrome is now also well described in adults [47]. These cases are often diagnosed weeks after SARS-CoV-2 infection, and treatment includes intravenous immunoglobulin and other immunomodulating agents such as steroids and anakinra. There is some overlap between the immune changes associated with this syndrome and those seen in severe COVID-19, including lymphopenia, elevation of various cytokines, and impaired antigen presentation [48].
CLINICAL DATA FOR IMMUNOMODULATION FOR COVID-19
Corticosteroids are widely used immunomodulatory agents for a variety of conditions. There was initial hesitation in using them for COVID-19 given an association with prolonged viral shedding when used for other SARS or Middle East respiratory syndrome (MERS) in nonrandomized settings [49]. Steroid use in non-COVID-19–related ARDS has had mixed results, with some studies suggesting a possible mortality benefit; however, steroid use for influenza pneumonia is associated with increased mortality [50, 51]. We identified 531 references on “steroids,” “dexamethasone,” or “methylprednisolone” and included 14 studies (Table 1). While studies have reported mixed efficacy for steroids for COVID-19, they have become standard of care for people with severe or critical COVID-19 based on the RECOVERY trial. Importantly, there is a dearth of data regarding infectious complications of corticosteroids for COVID-19. Additionally, multiple retrospective studies suggest steroid use in mild COVID-19 may be associated with prolonged viral RNA shedding [52–55].
Table 1.
Review of Major Coronavirus Disease 2019 Series That Used Corticosteroids as Therapy
| Agent [Ref] | Country | Study Design | Target Population (n)a | Endpoint Measured | Outcome and Multivariable Analysis | Infectious Complications | Conclusion or Recommendation | Strength of Evidenceb |
|---|---|---|---|---|---|---|---|---|
| Dexamethasone [21] | United Kingdom | Open-label, RCT | Hospitalized patients (2104) | Mortality at 28 days | 22.9% vs 25.7% favoring dexamethasone, age-adjusted rate ratio 0.83 (95% CI, .75 to .93) | Not reported | Mortality benefit favoring dexamethasone, strongest effect on those receiving mechanical ventilation | A |
| Hydrocortisone [56] | France | RCT, double-blind | Critically ill patients (76) | Death or persistent mechanical ventilation or high-flow nasal cannula at day 21 | 42.1% vs 50.7% favoring hydrocortisone, difference of proportions –8.6% (95% CI, –24.9% to 7.7%; P = .29) | 37.3% for hydrocortisone and 41.1% for placebo (HR, 0.81; 95% CI, .49 to 1.35; P = .42) | No significant difference in primary outcome; study stopped early (underpowered) | A |
| Methylprednisolone [57] | Brazil | RCT, double-blind | Hospitalized patients with severe or critical COVID-19 (194) | Mortality at 28 days | 37.1% for methylprednisolone vs 38.2% (P = .629) | Not reported | No difference in overall mortality | A |
| Dexamethasone [58] | Brazil | Open-label, RCT | Hospitalized patients with moderate to severe COVID-19 (151) | Ventilator-free days during first 28 days | More ventilator-free days for dexamethasone (difference 2.26; 95% CI, .2 to 4.38; P = .04); no difference in all-cause mortality at 28 days (56.3% vs 61.5%; HR, 0.97; 95% CI, .72 to 1.31; P = .85) | 21.9% of dexamethasone and 29.1% of usual care had secondary infections | Dexamethasone was associated with more days off of a ventilator; however, in this study, a mortality benefit was not seen | A |
| Methylprednisolone [59] | Iran | RCT, single-blind | Hospitalized patients with SpO2 <90%, elevated CRP, and elevated interleukin-6, though excluded if acute respiratory distress syndrome, SpO2 <75%, positive procalcitonin or positive troponin (34) | Time to clinical improvement and discharge or death, whichever came first | Methylprednisolone significantly associated with reduced time to primary outcome (11.6 ± 4.8 days vs 17.6 ± 9.8 days, P = .006); mortality rate lower for methylprednisolone group (5.9% vs 42.9%, P < .001) | Not well defined | In a small study with a highly specific group, methylprednisolone showed a benefit | A |
| Methylprednisolone [60] | United States (Michigan) | Single pre-test post-test quasiexperimental study | Hospitalized patients requiring supplemental oxygen (132) | Composite of escalation to ICU or all-cause in-hospital mortality | Primary composite endpoint occurred in 34.9% vs 54.3% (P = .005), favoring early steroid group; after multivariable adjustment, early corticosteroids were independently associated with a reduction in composite outcome at day 14 (OR, 0.4; 95% CI, .22 to .77) | Not reported | Early steroid use was associated with improved outcomes in this nonrandomized trial | B |
| Methylprednisolone [61] | Spain | Retrospective cohort study | Hospitalized patients (396) | In-hospital mortality | Patients treated with steroids had lower mortality than those treated with standard of care (13.9% vs 23.9%; HR, 0.51; 95% CI, .27 to .96; P = .044) | Not reported | Steroid use associated with lower mortality in this nonrandomized trial; the finding persisted after propensity score matching | B |
| Corticosteroids [62] | United States (New York City) | Retrospective cohort study | Hospitalized patients; compared those who received steroids within 48 hours of admission compared with those who never received steroids (140) | Composite of in-hospital mortality or in-hospital mechanical ventilation | Early glucocorticoids were not associated with decreased in-hospital mortality, though among subgroup with CRP >20 mg/dL was associated with reduced mortality or mechanical ventilation (adjusted OR, 0.20; 95% CI, .06 to .67) | Not reported | Steroid use was not associated with improved outcomes overall; among those with elevated CRP, steroid use was associated with improved outcomes | B |
| Corticosteroids [63] | China | Retrospective cohort study | Hospitalized patients (158) | In-hospital mortality | Patients who received corticosteroids had higher mortality (45.6% vs 11.5%, P < .0001); after propensity matching; there was no difference in mortality | There were more nosocomial infections among those treated with steroids (7.0% vs 2.9%, P = .02) | This nonrandomized trial found no benefit of steroids for treatment of COVID-19 | B |
| Corticosteroids [64] | Italy | Retrospective cohort study | Hospitalized patients with severe COVID-19 (170) | Mortality at day 30 from hospital admission | 35% in corticosteroid group and 31% in nonsteroid group died within 30 days of hospital admission; multivariable analysis adjusted OR, 0.59; 95% CI, .20 to 1.74; P = .33 | 17% of overall cohort had bacterial superinfections; hazard was higher for those who received steroids but not statistically significant (HR, 1.55; 95% CI, .95 to 2.55; P = .08) | This nonrandomized trial found no mortality benefit of corticosteroids for severe COVID-19 | B |
| Corticosteroids [55] | China | Retrospective cohort study | Hospitalized patients (126) | Hospital length of stay | After matching, among nonsevere group, steroid use associated with increased length of stay (19.0 days vs 11.5 days, P < .001); among severe group, no significant difference in length of stay (14.0 days vs 16.0 days, P = .883) | Unable to report infection rates, but antibiotic use higher among those who received steroids (P < .001) | This nonrandomized trial found no benefit of steroid use for COVID-19 and found longer hospital stay for nonsevere patients who received steroids compared with matched nonsteroid recipients | B |
| Corticosteroids [65] | United States (New York City) | Retrospective cohort study | Hospitalized patients with severe COVID-19 (SpO2/fiO2 <440) (60) | Composite outcome of ICU transfer, intubate, or death | In adjusted analysis, those who received steroids were less likely to have had a primary outcome (adjusted HR, 0.15; 95% CI, .07 to .33; P < .001) | Not reported | In this nonrandomized study of patients with severe COVID-19, steroid administration was associated with improved outcomes | B |
| Corticosteroids [66] | China | Retrospective cohort study | Hospitalized patients with severe (requiring supplemental oxygen) or critical (shock, mechanical ventilation, or ICU-level care) COVID-19 (531) | In-hospital mortality | In multivariable analysis, steroid use was independently associated with in-hospital mortality (HR, 1.77; 95% CI, 1.08 to 2.89; P = .023) | Not reported | In this nonrandomized study of severe and critically ill patients with COVID-19, steroid use was associated with an increased risk of death | B |
| Methylprednisolone [67] | China | Retrospective cohort study | Hospitalized patients with severe or critical COVID-19 (140) | Progression from severe to critical illness | In multivariate analysis, methylprednisolone was associated with less risk of progression to critical illness (OR, 0.054; 95% CI, .017 to .173; P < .001); in a subgroup analysis, the finding held for individuals aged <65 years but not for those aged >65 years | Not reported | In this nonrandomized study, steroid use was associated with less progression to critical illness | B |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; fiO2, fraction of inspired oxygen; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio; RCT, randomized, controlled trial; Ref, reference; SpO2, peripheral capillary oxygen saturation.
an = number of patients in study who received immunomodulatory therapy.
bStrength of evidence graded as: A = from a randomized, controlled trial; B = from a nonrandomized study.
The RECOVERY trial showed a significant mortality benefit for dexamethasone in COVID-19, with the biggest effect in the subgroup that received mechanical ventilation where risk of death was decreased by one-third (29.3% vs 41.4%, with a relative risk of death of 0.64; 95% confidence interval [CI], .51 to .81) [21]. The mortality benefit was more modest for individuals who received supplemental oxygen but did not require mechanical ventilation (23.3% vs 26.2%; rate ratio, 0.82; 95% CI, .72 to .94). Importantly, among those who did not receive supplemental oxygen, there was no benefit seen and, indeed, a trend toward harm (17.8% vs 14.0%; rate ratio, 1.19; 95% CI, .91 to 1.55). The mean time from symptom onset for those not on supplemental oxygen was 6 days compared with 8 days for those with supplemental oxygen and 13 days for those who required mechanical ventilation. In fact, when the subgroup started on steroids before 7 days of symptoms is considered, no mortality benefit was seen. The heterogeneity seen in the results of this trial suggests that a one-size-fits-all approach is not appropriate for treatment of COVID-19. Based on data from the RECOVERY trial, there is strong evidence for steroid administration, preferably dexamethasone, for individuals with COVID-19 who require supplemental oxygen or mechanical ventilation, particularly if they are beyond 7 days of symptom onset. Steroids should be avoided for individuals who do not require supplemental oxygen.
In addition to steroids, numerous other immunomodulatory agents have been used for COVID-19. In an early report from China, it was noted that a IL-6 receptor blocker (tocilizumab) was used to treat 21 patients with severe or critical disease, and rapid and profound improvements in oxygenation, inflammatory markers, and clinical status were reported, generating tremendous interest [68]. Our systematic evaluation identified 412 tocilizumab and 14 sarilumab peer-reviewed articles related to COVID-19, of which 13 are included here (Table 2). A preprint and 2 press releases of major RCTs were also included. The results from the nonrandomized studies are mixed. Importantly, peer-reviewed results from 1 double-blind RCT and 2 open-label RCTs are now available, and additional RCT results are available by preprint and press release. The results from the RCTs are largely concordant, with neither benefit nor an increased risk of secondary infections. One trial reported a benefit because a composite primary endpoint was met, but mortality at 28 days was numerically higher in the tocilizumab arm [69]. These accumulating negative results suggest that COVID-19 is not a true cytokine, specifically IL-6–mediated, storm but rather the result of more complex immune dysregulation. Additional peer-reviewed data are forthcoming; however, at this time, there is no evidence to support the use of IL-6 receptor inhibition for treatment of COVID-19.
Table 2.
Major Studies Reporting Interleukin-6 Receptor Inhibition With Tocilizumab or Sarilumab for Coronavirus Disease 2019
| Reference | Country | Comedications | Study Design | Target Population (n)a | Endpoint Measured | Outcome and Multivariable Analysis | Infectious Complications | Conclusion or Recommendation | Strength of Evidenceb |
|---|---|---|---|---|---|---|---|---|---|
| 70 | United States (Boston) | Steroids (10%), RDV (~33%), HCQ (4%) | RCT, double-blind | Hospitalized patients with 2 of the following: fever, pulmonary infiltrates, need for supplemental oxygen and 1 of the following: elevated CRP, D-dimer, ferritin, or lactate dehydrogenase (LDH) (161) | Intubation and mortality at day 28 | 10.6% in TCZ group vs 12.5% in placebo group had been intubated or died by day 28 (HR, 0.83; 95% CI, .38 to 1.81; P = .64) | There were fewer infectious complications in the TCZ group (8.1% vs 17.1%, P = .03) | This double-blind RCT does not support using TCZ for patients with severe COVID-19 | A |
| 71 | France | Azithromycin (~20%), HCQ (~8%), steroids (~30%, more in usual care arm) | RCT, open-label | Hospitalized patients with moderate, severe, or critical COVID-19 (63) | Need for ventilation and mortality | Suggestion of benefit for TCZ at day 14; however, mortality at day 28 11.1% for TCZ vs 11.9% for stand of care (aHR, 0.92; 95% CI, .33 to 2.53) | Secondary infections reported in 3.7% for TCZ vs 20.9% for standard-of-care group | This open-label RCT found no mortality benefit for TCZ at 28 days | A |
| 72 | Italy | Azithromycin (~20%), DRV/c or LPV/r (~40%) | RCT, open-label | Hospitalized patients with PaO2/fiO2 of 200–300 and fever or elevated CRP (60) | Admission to ICU or death by day 14 | 28.3% for TCZ vs 27.0% met primary outcome; mortality at 30 days was 3.3% for TCZ vs 1.6% | Secondary infections reported in 1.7% of TCZ vs 6.3% | This open-label RCT found no benefit of TCZ | A |
| 69 | Multinational | Steroids and various antivirals used in 80% | RCT, double-blind | Hospitalized patients with SpO2 ≤94% on ambient air (249) | Composite of ventilation or mortality by day 28 | Composite outcome occurred in 12.0% for TCZ vs 19.3% (HR, 0.56; 95% CI, .33 to 0.97; P = .036); mortality at day 28 was numerically higher in the TCZ arm (10.4% vs 8.6%, weighted difference, 2%; 95% CI, –5.2% to 7.8% | Serious infections reported in 5.2% in TCZ and 7.1% placebo | This double-blind RCT met its primary composite endpoint; however, there was numerically higher mortality at 28 days in the TCZ arm | A (report not peer reviewed) |
| 73 | United States and Europe | No details to date | RCT, double-blind | Hospitalized patients with severe COVID-19 (~225) | Improved clinical status at day 28 and mortality | No difference in clinical status at day 28 ( odds ratio, 1.19; 95% CI, .81 to 1.76; P = .36); mortality 19.7% vs 19.4% with a difference of 0.3% (95% CI, –7.6% to 8.2%; P = .94) | No difference in secondary infections between the groups (38.3% vs 40.6%) | This double-blind RCT found no benefit of TCZ for severe COVID-19 | A (though data not peer reviewed) |
| 74 | United States (multiple sites) | No details to date | RCT, double-blind | Hospitalized patients with severe-COVID-19 (~1200) | Improved clinical status and mortality | Per press report: “did not meet its primary and key secondary endpoints” | Not reported to date | This double-blind RCT found no benefit for sarilumab | A (though data not peer reviewed) |
| 75 | Italy | HCQ and LPV/r | Retrospective cohort study | Hospitalized patients with RR ≥30, SpO2 ≤93% on ambient air, or PaO2/fiO2 ≤300; critical patients excluded (62) | Survival rate | 3.2% vs 47.8% mortality favoring TCZ (aHR, 0.035; 95% CI, .004 to .347; P = .004) | No secondary infections reported in either group | This nonrandomized study in patients with severe COVID-19 found TCZ was associated with decreased mortality | B |
| 76 | Italy | HCQ + LPV/r or RDV | Retrospective cohort study | Hospitalized patients with bilateral pulmonary infiltrates and CRP >1 mg/dL, interleukin-6 >40 pg/mL, D-dimer >1.5 µg/mL, or ferritin >500 ng/mL with severe or critical COVID-19 (74) | Survival rate | TCZ use associated with improved survival (HR, 0.499; 95% CI, .262 to .952; P = .035); benefit highest in critical illness, no severe disease | 32.4% of TCZ patients had secondary infections, but no comparison reported for standard-of-care group | This nonrandomized study found TCZ was associated with decreased mortality; many secondary infections were reported, but no comparison was available with the standard-of-care group | B |
| 77 | United States (Michigan) | 25% received steroids, 23% HCQ, 3% RDV | Retrospective cohort study | Intubated patients (78) | Survival probability after intubation | Mortality at day 28 lower for TCZ-treated patients at 18% vs 36% (P = .01; aHR, 0.54; 95% CI, .35 to .84) | TCZ-treated patients more likely to have superinfection (54% vs 26%, P < .001) | This nonrandomized study found TCZ use was associated with decreased mortality but increased rate of superinfections in a critically ill cohort | B |
| 78 | United States (New York City) | HCQ + azithromycin in >90%, steroids ~40%, RDV ~10% | Retrospective case-control study | Hospitalized patients with severe or critical COVID-19 (96) | Overall mortality rate | Mortality rates 52% vs 62% (P = .09); excluding intubated patients 6% vs 27% (P = .024), favoring TCZ | Bacteremia more common in control group (23.7% vs 12.5%, P = .04), fungemia similar (3% vs 4%, P = .7) | This nonrandomized study found TCZ was associated with a lower mortality rate among nonintubated patients with COVID-19 | B |
| 79 | Spain | HCQ (98%), LPV/r (82%), azithromycin (74%), interferon-ß (28%), steroids (19%) | Retrospective cohort study | Hospitalized patients with fever or need for supplemental oxygen and elevated CRP, D-dimer, or ferritin (88) | Intubation or death | 11.4% vs 20.1% of patients required intubation or died, favoring TCZ; HR after matching cases was 0.22 (95% CI, .05 to .96; P = .04) | Rates of secondary bacterial infections were similar (12.5% vs 10.3%, P = .57) | This nonrandomized study found TCZ was associated with lower rates of intubation or death with similar rates of secondary bacterial infections | B |
| 80 | Italy | HCQ + LPV/r | Retrospective cohort study | Hospitalized patients with bilateral pulmonary opacities and RR ≥30, SpO2 ≤93% on ambient air, or PaO2/fiO2 ≤300 (90) | Survival rate | 7.7% vs 50% mortality favoring TCZ, aHR for death was 0.057 (95% CI, .017 to .187; P < .001) | No secondary infections observed | This nonrandomized study found TCZ was associated with lower mortality in patients with COVID-19 | B |
| 81 | United States (New Jersey) | Steroids (66%), HCQ + azithromycin (>90%) | Retrospective cohort study | Hospitalized patients with COVID-19 in the ICU (134) | Survival rate | 46% vs 56% mortality favoring TCZ (aHR, 0.76; 95% CI, .57 to 1.00) | 13% vs 11% bacteremia | This nonrandomized study found a trend toward improved mortality when TCZ was given for critical COVID-19 | B |
| 82 | United States (Chicago) | RDV in around one-third; TCZ patients more likely to get HCQ than controls (57% vs 20%, P = .001) | Retrospective cohort study | Hospitalized patients with severe COVID-19 with progressive hypoxemia with elevated D-dimer >2 mg/L, CRP >100 mg/dL, or ferritin > 600 µg/L | Secondary infections and mortality | Mortality was higher among those who received TCZ (39% vs 23%, P = .03) | Late-onset infections were more commonly seen in the TCZ group (23% vs 8%, P = .013) | This nonrandomized trial found TCZ was associated with increased mortality and increased late-onset infections | B |
| 83 | Italy | LPV/r (or DRV/c) and HCQ | Retrospective case-control study | Hospitalized patients with worsening oxygen requirement, elevated CRP, and another in a list of abnormal laboratory results (64) | Mortality rates | Mortality was not associated with TCZ treatment (aHR, 0.82; 95% CI, .42 to 1.58; P = .55) | The rate of secondary infections was not different between the groups, 31% for TCZ vs 39% (HR, 0.71; 95% CI, .38 to 1.32, P = 0.28) | This nonrandomized trial found no mortality benefit for TCZ, with a similar amount of secondary infectious complications | B |
| [84] | India | HCQ, ivermectin, oseltamivir, methylprednisolone | Retrospective cohort study | Hospitalized patients with SpO2 ≤94% despite supplemental oxygen or PaO2/fiO2 ≤200 | Death | TCZ independently associated with reduced death (aHR, 0.62; 95% CI, .38 to .99) | Not reported | This nonrandomized trial found improved mortality among those who received TCZ | B |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DRV/c, darunavir with cobicistat; fiO2, fraction of inspired oxygen; HCQ, hydroxychloroquine; HR, hazard ratio; ICU, intensive care unit; LPV/r, lopinavir with ritonavir; PaO2, partial pressure of oxygen; RCT, randomized, controlled trial; RDV, remdesivir; RR, respiratory rate; SpO2, peripheral capillary oxygen saturation; TCZ, tocilizumab.
an = number of patients in study who received immunomodulatory therapy.
bStrength of evidence graded as: A = from a randomized, controlled trial; B = from a nonrandomized study.
Use of other immunomodulatory agents is described in fewer publications. These agents include anakinra (3 studies), baricitinib (2 studies), and ruxolitinib (1 study) (Table 3). Treatment of COVID-19 with the IL-1 receptor antagonism anakinra has been studied, with no RCTs published to date. Two small nonrandomized series have suggested a mortality benefit with this agent, but there are currently no data to support use of this agent outside of a clinical trial [85, 86].
Table 3.
Summary of Additional Immunomodulatory Coronavirus Disease 2019 COVID-19 Series
| Agent [Ref] | Country | Study Design | Target Population (n)a | Endpoint Measured | Outcome and Multivariable Analysis | Infectious Complications | Conclusion or Recommendation | Strength of Evidenceb |
|---|---|---|---|---|---|---|---|---|
| Anakinra [85] | Italy | Retrospective cohort study | Hospitalized patients with moderate to severe COVID-19 with hyperinflammation, with C-reactive protein ≥100 mg/dL or ferritin ≥900 ng/mL (29) | Survival rates | Mortality was 10% in the anakinra group and 44% in the standard treatment group (P = .009) | Bacteremia in 14% anakinra vs 13% standard treatment | In this small nonrandomized study, anakinra was associated with decreased mortality among patients with severe COVID-19 and laboratory evidence of inflammation | B |
| Anakinra [86] | France | Retrospective cohort study | Hospitalized patients with severe COVID-19 (52) | Composite of intensive care unit admission, need for mechanical ventilation, or death | Composite less common in those who received anakinra compared with historical controls (25% vs 73%; HR, 0.22; 95% CI, .11 to .41; P < .0001) | No secondary bacterial infections documented | In this nonrandomized study, anakinra was associated with reduced mortality compared with a historical control | B |
| Anakinra [87] | United States (Los Angeles) | Retrospective cohort study | Hospitalized patients with COVID-19 with progressive hypoxemia and bilateral pulmonary infiltrates (52) | Survival rates | Mortality was lower in anakinra group (22%) than TCZ group (46.2%) after adjustment (adjusted HR, 0.46; 95% CI, .18 to 1.20; P = .11) | Not reported | In this nonrandomized study that compared anakinra with TCZ administration, there was no statistically significant difference in mortality between the 2 agents | B |
| Baricitinib [88] | Global (National Institutes of Health) | RCT, double-blind | Hospitalized patients with COVID-19 (~500) | Time to clinical recovery | Study met primary endpoint | Not reported | In this double-blind, randomized, controlled trial, baricitinib improved time to clinical recovery when added to remdesivir | A |
| Baricitinib [89] | Italy | Retrospective cohort study | Hospitalized patients with moderate COVID-19 with radiographic pneumonia, SpO2 >92% on room air, and PaO2/fiO2 100–300 (113) | Mortality rate at 2 weeks | Lower mortality in baricitinib arm (0% vs 6.4%, P = .010) | Not reported | In this nonrandomized study, baricitinib was associated with improved mortality at 2 weeks compared with historical controls; polymerase chain reaction positivity was significantly lower at day 14 for those who received baricitinib (12.5% vs 40%) | B |
| Baricitinib [90] | Spain | Prospective cohort study | Hospitalized patients with severe COVID-19 with PaO2/fiO2 <200 (62) | Improved SpO2/fiO2 | A greater improvement in SpO2/fiO2 was seen for those who received baricitinib | Two bacteremias in control group, none in baricitinib group | In this nonrandomized study, baricitinib improved oxygenation when added to steroids and multiple other “standard therapies” compared with those therapies alone | B |
| Ruxolitinib [91] | China | RCT, single-blind | Hospitalized patients with severe COVID-19 (20) | Time to improved clinical status, mortality | Patients who received ruxolitinib had a numerically shorter time to clinical improvement (12 days vs 15 days; HR, 1.67; 95% CI, .84 to 3.34; P = .15); mortality at day 28 was 0% for ruxolitinib vs 14.3%, but cumulative incidence of death was the same between the groups | Two secondary infections in control group and none in ruxolitinib group | This small RCT found numerically faster but not statistically significant clinical improvement for those who received ruxolitinib | A |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; fiO2, fraction of inspired oxygen; HR, hazard ratio; RCT, randomized, controlled trial; Ref, reference; SpO2, peripheral capillary oxygen saturation; TCZ, tocilizumab.
an = number of patients in study who received immunomodulatory therapy.
b Strength of evidence graded as: A = from a randomized, controlled trial, B = from a nonrandomized study.
Baricitinib and ruxolitinib are Janus kinase (JAK) inhibitors. Severe COVID-19 is associated with an imbalanced JAK and STAT pathway, with increased relative activity of STAT3 compared with STAT1, contributing to an ineffective antiviral response and a proinflammatory phenotype. Inhibition of JAK-dependent signaling can attenuate overactive STAT3 activity and theoretically ameliorate the immune dysregulation in severe COVID-19 [36, 92]. Baricitinib administration was associated with normalization in the cytokine profile and restoration of circulating lymphocytes levels within a small cohort of hospitalized patients with COVID-19 with fewer than 9 days of symptoms [92]. The results of the ACTT-2 trial were released via a press release, reporting faster time to clinical recovery when baricitinib was added to remdesivir. There are fewer robust data at this time for ruxolitinib. Based on the press release, it is likely that baricitinib will have a role in the treatment of patients with COVID-19, but more details are required from peer-reviewed data. Other kinase inhibitors that are showing preliminary good effect in the reduction of inflammatory parameters and improved oxygenation are selective blockers of Bruton’s tyrosine kinase such as acalabrutinib [93, 94].
Interferon therapy is another immunomodulatory approach being studied for treatment of COVID-19. SARS-CoV-2 is sensitive to type I interferons in vitro, with markedly decreased viral replication [95]. SARS-CoV-2 evades the interferon response, and insufficient interferon stimulation is seen in patients with severe COVID-19 [96]. Taken together, this observation has led to the hypothesis that early type I interferon administration might help limit viral replication. The MERS-CoV Infection Treated with a Combination of Lopinavir- Ritonavir and Interferon Beta-1b (MIRACLE) trial for MERS, which is the first RCT published for treatment of either SARS or MERS, found that interferon-ß1b was associated with lower mortality in a prespecified subgroup when it was given within 7 days of symptom onset but had no effect later in the illness course [97]. It is important to note that viral load dynamics are different between MERS-CoV and SARS-CoV-2, with upper respiratory tract viral load peaking at around 7–10 days for MERS-CoV and earlier for SARS-CoV-2 infection [98]. Given the earlier viral phase for SARS-CoV-2 and the fact that most people present 4–7 days after symptom onset when viral loads are already declining, it remains to be seen whether interferons have a role in the treatment of COVID-19 [99, 100].
Of 418 papers related to SARS-CoV-2 and interferons, 8 are included here (Table 4). An open-label RCT evaluated treatment with triple therapy (interferon-β1b, ribavirin, and lopinavir/ritonavir) against lopinavir/ritonavir monotherapy and found that the interferon-treated group had faster viral clearance from nasopharyngeal swabs of 7 days vs 12 days (P = .001) [101]. This striking result is notable since no other randomized treatment study has demonstrated such impact, including a remdesivir study [14], and suggests that specific immune augmentation may have a potent anti–SARS-CoV-2 viral effect. Preliminary data from the large World Health Organization–sponsored solidarity trial suggest interferon-ß1b administration was not associated with a change in mortality; however, there is no information about the timing of administration [19]. Currently there are insufficient data to support interferon use for COVID-19 outside of a clinical trial, and further study, particularly early in the disease, is needed. Given the finding of autoantibodies to some type I interferons (most commonly interferon-α) in severe COVID-19, interferon-ß formulations may be more likely to have effect than interferon-α [39].
Table 4.
Series Reporting Data for Interferon for Treatment of Coronavirus Disease 2019
| Type of IFN [Ref] | Country | Comedications | Study Design | Target Population (n)a | Endpoint Measured | Outcome and Multivariable Analysis | Infectious Complications | Conclusion or Recommendation | Strength of Evidenceb |
|---|---|---|---|---|---|---|---|---|---|
| IFN-β [101] | Hong Kong | LPV/r + ribavirin, 7% steroids | RCT, open-label | Hospitalized patients with COVID-19 and National Early Warning Score 2, ≥1, with symptoms ≤14 days (86) | Time to negative PCR, mortality | Combination therapy associated with significantly shorter median time to PCR negativity (7 days vs 12 days; HR, 4.37; 95% CI, 1.86 to 10.24; P = .001); no patients died in either arm | Not reported | In this RCT where treatments were started around day 5 after symptom onset in a relatively mild cohort, combination therapy with IFN- β1b, ribavirin, and LPV/r showed faster viral clearance compared with LPV/r only | A |
| IFN- β [102] | Iran | HCQ + LPV/r or ATV/r, 62% received steroids | RCT, open label | Hospitalized patients with severe COVID-19 with hypoxemia, hypotension, renal failure, neurologic change, thrombocytopenia, or severe gastrointestinal symptoms | Time to clinical improvement | No difference in time to clinical improvement between the groups, 9.7 days for IFN vs 8.3 days (P = .95; HR, 1.10; 95% CI, .64 to 1.87); however, day 28 mortality lower in IFN group (19% vs 43.6%, P = .015) when adjusted for IVIG and steroid administration effect remained (aHR, 0.375; 95% CI, .16 to .87; P = .024) | There were numerically more nosocomial infections in the IFN group (26.2% vs 12.8%, P = .09) | In this RCT, IFN- β1a did not increase time to clinical improvement but was associated with lower mortality even after controlling for steroid use; IFN was started a mean of 11.7 days after symptom onset | A |
| IFN-β [103] | Iran | LPV/r or ATV/r + HCQ, steroids in nearly 30% | RCT, open-label | Hospitalized patients with severe COVID-19 (33) | Time to improved clinical status | Time to clinical improvement was shorter for the IFN group (9 days vs 11 days; P = .002; aHR, 3.41; 95% CI, 1.33 to 8.72) | Nosocomial infections in 3% vs 18% favoring IFN | In this small RCT, IFN-β1b was associated with reduced mortality among a cohort with severe COVID-19; started at mean 7 days of symptom onset | A |
| IFN-β [18] | Multinational (World Health Organization) | LPV/r or “local standard of care” | RCT, open-label | Hospitalized patients with COVID-19 (2050) | Mortality | 12.9% deaths for IFN vs 11.0% for controls, no difference | Not reported | In this open-label RCT, IFN-β1a was not associated with improved outcomes; there are no data yet available about when in the illness course the treatment was given | A |
| IFN-α [104] | China | Arbidol | Retrospective cohort study | Hospitalized patients with moderate COVID-19 (53) | Time to negative upper respiratory tract PCR test | IFN was associated with accelerated viral clearance from the upper respiratory tract by ~7 days (P = .002) | Not reported | In this nonrandomized study, IFN-α2b therapy was associated with more rapid viral clearance from the upper respiratory tract | B |
| IFN-α [105] | Cuba | LPV/r + chloroquine | Prospective cohort study | Hospitalized patients with COVID-19 (761) | Time to discharge and mortality | Mortality reported much lower in IFN group | Not reported | In this highly confounded nonrandomized study where there were significant age and comorbidity differences between the groups, IFN-α2b was associated with improved outcomes | B |
| IFN-α [106] | China | Nearly 80% received LPV/r, 60% steroids, around 40% IVIG | Retrospective case-control study | Hospitalized patients with COVID-19 (68) | Time to negative upper respiratory tract PCR | Time to negative PCR was not shorter for IFN after propensity matching (12 days vs 15 days, P = .206) | Not reported | In this nonrandomized study, IFN-α2b did not have an effect on time to negative upper respiratory tract PCR | B |
| IFN-α [96] | China | LPV/r or arbidol | Retrospective cohort study | Hospitalized patients with COVID-19 (242) | Mortality | Early IFN therapy was associated with lower mortality (aHR, 0.10; 95% CI, .02 to .50); among the 26 who received late IFN, there was increased mortality (aHR, 2.30; 95% CI, .64 to 8.27 compared with no IFN therapy | Not reported | In this nonrandomized study, early IFN-α2b (defined as given within 48 hours of admission) was associated with reduced mortality | B |
Abbreviations: aHR, adjusted hazard ratio; ATV/r, atazanavir with ritonavir; CI, confidence interval; COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine; HR, hazard ratio; IFN, interferon; IVIG, intravenous immunoglobulin; LPV/r, lopinavir with ritonavir; PCR, polymerase chain reaction; RCT, randomized, controlled trial; Ref, reference.
an = number of patients in study who received immunomodulatory therapy.
bStrength of evidence graded as: A = from a randomized, controlled trial; B = from a nonrandomized study.
INFECTIOUS, NONINFECTIOUS, AND IMMUNOLOGIC UNINTENDED CONSEQUENCES OF IMMUNOMODULATORY THERAPY
Some immunomodulatory agents are associated with an increased risk of secondary infections. Notably, tocilizumab in the setting of CAR-T–related CRS is not associated with increased infection risk compared with patients who receive similar salvage chemotherapies without this agent [107, 108]. To date, few published series have reported systematically on the incidence of secondary and nosocomial infections for patients receiving immunomodulatory treatment. Notably, secondary infection rates have not been reported in the RECOVERY trial for dexamethasone [21]. In addition to common nosocomial infections that include bacteremia and pneumonia, case reports document sometimes fatal secondary infections, including from Herpes simplex virus (HSV) reactivation, disseminated strongyloidiasis, and invasive fungal infections [109–112]. Monitoring for reactivation of other latent infections such as hepatitis B and tuberculosis is also critical [113, 114].
Noninfectious complications, including osteonecrosis related to steroids and bowel perforation after IL-6 inhibitor administration, have been noted [115, 116]. A larger unknown is the possible long-term immunologic consequences of immunomodulatory therapy. Cases of SARS-CoV-2 reinfection are now being reported around the globe and may be common around 12 months after initial infection [117–119]. Given the associations of a coordinated immune response and recent common coronavirus infection with less severe COVID-19, therapies that inhibit a protective immune response may keep people at risk for future severe COVID-19, particularly if reinfection is inevitable [6, 7]. All of these issues will have to be explored further in future RCTs.
CONCLUSIONS
Severe COVID-19 is marked by a protracted course with evidence of immune dysregulation and, at times, multisystem organ dysfunction. Several proposed strategies for treatment include antiviral agents and immunomodulatory therapeutics. Since SARS-CoV-2 viral loads peak around the time of symptom onset and patients with severe immune dysregulation often present 5–7 days later, an approach that is exclusively antiviral may not be sufficient for all patients. Antivirals and stimulators of innate antiviral response (ie, interferons) may be most likely to show benefit early in the disease course when viral loads are highest, likely within 7 days of symptom onset and sooner if possible. While early hypotheses proposed that the second phase of the severe COVID-19 illness course might be similar to cytokine release syndrome, immune profiling has revealed a complex immune dysregulation with a central role for the type I interferon response. Strategies to attenuate this imbalanced response, including steroids and targeted therapies are all being actively studied.
Given the marked heterogeneity of COVID-19 clinical presentations, therapeutic approaches will likely need to be tailored to individual patients, and a one-size-fits-all approach may not provide optimal benefit. Potential therapeutic approaches will need to identify the right therapy, dose, patient, and proper timing in relation to the disease course. To define these specific treatments, data from well-performed RCTs are needed that include details about timing of administration of agents in the COVID-19 illness course.
Notes
Financial support. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI132638 to M. K. M).
Potential conflicts of interest. All authors participated in the Bacc Bay Trial for Tocilizumab in COVID-19, a randomized, controlled trial [70] supported by Genentech. A. Y. K. reports scientific advisory board fees from Biomarin, Inc, outside the submitted work. J. H. S. reports personal fees from Principia Biopharma, Viela Bio, Sanofi, Chemocentryx, Celgene, AbbVie, Chugai, Grunenthal, Glaxo Smith Kline, InflaRx, INSmed, Regeneron, Roche, and Roivant and grants from Viela Bio and Roche outside the submitted work. M. K. M. reports consultation fees from Vericel, SmartPharm Therapeutics, Pulsethera, Gen Mark Diagnostics, Globe Life Sciences, and Day Zero Diagnostics; grant support from Thermo Fisher Scientific and Genentech; medical editing/writing fees from UpToDate; scientific advisory board fees from Celularity; and editing fees from the Infectious Diseases Society of America outside the submitted work. M. K. M. also reports patents 14/110 443 and 15/999 463 pending. T. G. N. reports personal fees from BMS, Paraxel, Intrinsic Imaging, AbbVie (scientific advisory board fees), and H3 Biomedicine and grants from Astra Zeneca outside the submitted work. M. J. F. reports consulting fees from Novartis, Kite/Gilead, and BMS outside the submitted work.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 2. Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for Covid-19: systematic review, meta-analysis, and public policy implications. medRxiv 2020. doi: 10.1101/2020.07.23.20160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenyon C. COVID-19 infection fatality rate associated with incidence—a population-level analysis of 19 Spanish autonomous communities. Biology 2020; 9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang W, Kandula S, Huynh M, et al. . Estimating the infection fatality risk of COVID-19 in New York City during the spring 2020 pandemic wave. medRxiv 2020. doi: 10.1101/2020.06.27.20141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grifoni A, Weiskopf D, Ramirez SI, et al. . Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181:1489–501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sagar M, Reifler K, Rossi M, et al. . Recent endemic coronavirus infection is associated with less severe COVID-19. J Clin Invest 2020:143380. doi: 10.1172/JCI143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. . Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020; 183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borczuk AC, Salvatore SP, Seshan SV, et al. . COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol 2020: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lauer SA, Grantz KH, Bi Q, et al. . The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan W-J, Ni Z-Y, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020; 383:1757–66. [DOI] [PubMed] [Google Scholar]
- 12. Magleby R, Westblade LF, Trzebucki A, et al. . Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019 [manuscript published online ahead of print 30 June 2020]. Clin Infect Dis 2020:ciaa851. doi: 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westblade LF, Brar G, Pinheiro LC, et al. . SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020; 38:661–671.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Zhang D, Du G, et al. . Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson BN, Feldmann F, Schwarz B, et al. . Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020; 585:273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med 2020; 383:994. [DOI] [PubMed] [Google Scholar]
- 17. Spinner CD, Gottlieb RL, Criner GJ, et al. ; GS-US-540-5774 Investigators . Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324:1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan H, Peto R, Abdool Karim Q, et al. . Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results. medRxiv 2020. doi: 10.1101/2020.10.15.20209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. To KK-W, Tsang OT-Y, Leung W-S, et al. . Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Yan LM, Wan L, et al. . Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horby P, Lim WS, Emberson JR, et al. . Dexamethasone in hospitalized patients with Covid-19—preliminary report [manuscript published online ahead of print 17 July 2020]. N Engl J Med 2020:NEJMoa2021436. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 22. Tursi A, Vetrone LM, Papa A. Anti-TNF-α agents in inflammatory bowel disease and course of COVID-19. Inflamm Bowel Dis 2020; 26:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gianfrancesco M, Hyrich KL, Al-Adely S, et al. ; COVID-19 Global Rheumatology Alliance . Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2020; 79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herold T, Jurinovic V, Arnreich C, et al. . Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146:128–36.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen X, Zhao B, Qu Y, et al. . Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020; 71:1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin A, He Z-B, Zhang S, Zhang J-G, Zhang X, Yan W-H. Early risk factors for the duration of SARS-CoV-2 viral positivity in COVID-19 patients. Clin Infect Dis 2020; 71:2061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrilli CM, Jones SA, Yang J, et al. . Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Lu X, Chen H, et al. . Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020; 201:1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368:473–4. [DOI] [PubMed] [Google Scholar]
- 32. Alvi RM, Frigault MJ, Fradley MG, et al. . Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol 2019; 74:3099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA 2020; 324:1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leisman DE, Ronner L, Pinotti R, et al. . Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. . Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181:1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spinelli FR, Conti F, Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol 2020; 5:eabc5367. [DOI] [PubMed] [Google Scholar]
- 37. Mazzoni A, Salvati L, Maggi L, et al. . Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest 2020; 130:4694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Q, Bastard P, Liu Z, et al. . Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020; 370:eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bastard P, Rosen LB, Zhang Q, et al. . Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xia H, Cao Z, Xie X, et al. . Evasion of type I interferon by SARS-CoV-2. Cell Rep 2020; 33:108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lei X, Dong X, Ma R, et al. . Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun 2020; 11:3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death Differ 2020: 1–17. doi: 10.1038/s41418-020-00633-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones VG, Mills M, Suarez D, et al. . COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr 2020; 10:537–40. [DOI] [PubMed] [Google Scholar]
- 44. Verdoni L, Mazza A, Gervasoni A, et al. . An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team . Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morris SB, Schwartz NG, Patel P, et al. . Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carter MJ, Fish M, Jennings A, et al. . Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020; 26:1701–7. [DOI] [PubMed] [Google Scholar]
- 49. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zayed Y, Barbarawi M, Ismail E, et al. . Use of glucocorticoids in patients with acute respiratory distress syndrome: a meta-analysis and trial sequential analysis. J Intensive Care 2020; 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care 2019; 23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ding C, Feng X, Chen Y, et al. . Effect of corticosteroid therapy on the duration of SARS-CoV-2 clearance in patients with mild COVID-19: a retrospective cohort study. Infect Dis Ther 2020; 9:943-52. doi: 10.1007/s40121-020-00337-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang R, Zhu C, Wang J, et al. . Corticosteroid therapy is associated with the delay of SARS-CoV-2 clearance in COVID-19 patients. Eur J Pharmacol 2020; 889:173556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu Z, Lv Y, Xu C, et al. . Clinical use of short-course and low-dose corticosteroids in patients with non-severe COVID-19 during pneumonia progression. Front Public Health 2020; 8:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ma Y, Zeng H, Zhan Z, et al. . Corticosteroid use in the treatment of COVID-19: a multicenter retrospective study in Hunan, China. Front Pharmacol 2020; 11:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dequin PF, Heming N, Meziani F, et al. . Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020; 324:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jeronimo CMP, Farias MEL, Val FFA, et al. . Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial [manuscript published online ahead of print 12 August 2020]. Clin Infect Dis 2020;ciaa1177. doi: 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tomazini BM, Maia IS, Cavalcanti AB, et al. . Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; 324:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edalatifard M, Akhtari M, Salehi M, et al. . Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial [manuscript published online ahead of print 17 September 2020]. Eur Respir J 2020;2002808. doi: 10.1183/13993003.02808-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fadel R, Morrison AR, Vahia A, et al. . Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020; 71:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, et al. . A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother 2020; 64:e01168–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Keller MJ, Kitsis EA, Arora S, et al. . Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med 2020; 15:489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Z, Li X, Fan G, et al. . Low-to-moderate dose corticosteroids treatment in hospitalized adults with COVID-19 [manuscript published online ahead of print 29 September 2020]. Clin Microbiol Infect 2020; S1198-743X(20)30599-1. doi: 10.1016/j.cmi.2020.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bartoletti M, Marconi L, Scudeller L, et al. . Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study [manuscript published online ahead of print 22 September 2020]. Clin Microbiol Infect 2020; S1198-743X(20)30563-2. doi: 10.1016/j.cmi.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Majmundar M, Kansara T, Lenik JM, et al. . Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York metropolitan region. PLoS One 2020; 15:e0238827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu J, Huang J, Zhu G, et al. . Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan, China. J Clin Endocrinol Metab 2020; 105:dgaa627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang R, Xiong Y, Ke H, Chen T, Gao S. The role of methylprednisolone on preventing disease progression for hospitalized patients with severe COVID-19. Eur J Clin Invest 2020; 50:e13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu X, Han M, Li T, et al. . Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020; 117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salama C, Han J, Yau L, et al. . Tocilizumab in nonventilated patients hospitalized with Covid-19 pneumonia. medRxiv 2020. doi: 10.1101/2020.10.21.20210203. [DOI] [Google Scholar]
- 70. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. . Efficacy of tocilizumab in patients hospitalized with Covid-19 [manuscript published online ahead of print 21 October 2020]. N Engl J Med 2020; NEJMoa2028836. doi: 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial [manuscript published online ahead of print 20 October 2020]. JAMA Intern Med 2020; e206820. doi: 10.1001/jamainternmed.2020.6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salvarani C, Dolci G, Massari M, et al. . Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial [manuscript published online ahead of print 20 October 2020]. JAMA Intern Med 2020; e206615. doi: 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. 2020. Available at: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm. Accessed 17 October 2020.
- 74.Regeneron and Sanofi provide update on Kevzara (Sarilumab) phase 3 U.S. trial in COVID-19 patients. 2020. Available at: https://newsroom.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-kevzarar-sarilumab-phase-3. Accessed 17 October 2020.
- 75. Capra R, De Rossi N, Mattioli F, et al. . Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med 2020; 76:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rossotti R, Travi G, Ughi N, et al. . Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect 2020; 81:e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Somers EC, Eschenauer GA, Troost JP, et al. . Tocilizumab for treatment of mechanically ventilated patients with COVID-19 [manuscript published online ahead of print 11 July 2020]. Clin Infect Dis 2020;ciaa954. doi: 10.1093/cid/ciaa954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rojas-Marte G, Khalid M, Mukhtar O, et al. . Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM 2020; 113:546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rodríguez-Baño J, Pachón J, Carratalà J, et al. . Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) [manuscript published online ahead of print 27 August 2020]. Clin Microbiol Infect 2020; S1198-743X(20)30492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De Rossi N, Scarpazza C, Filippini C, et al. ; Montichiari COVID-19 Study Group . Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. EClinicalMedicine 2020; 25:100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ip A, Berry DA, Hansen E, et al. . Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—an observational study. PLoS One 2020; 15:e0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pettit NN, Nguyen CT, Mutlu GM, et al. . Late onset infectious complications and safety of tocilizumab in the management of COVID-19 [manuscript published online ahead of print 13 August 2020]. J Med Virol 2020. doi: 10.1002/jmv.26429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Canziani LM, Trovati S, Brunetta E, et al. ; Humanitas and Gavazzeni/Castelli COVID-19 Task Forces . Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J Autoimmun 2020; 114:102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gokhale Y, Mehta R, Karnik N, Kulkarni U, Gokhale S. Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinicalMedicine 2020; 24:100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cavalli G, De Luca G, Campochiaro C, et al. . Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020; 2:e325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huet T, Beaussier H, Voisin O, et al. . Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2020; 2:e393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Langer-Gould A, Smith JB, Gonzales EG, et al. . Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis 2020; 99:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baricitinib in combination with remdesivir reduces time to recovery in hospi talized patients with COVID-19 in NIAID-sponsored ACTT-2 Trial. 2020. Available at: https://www.prnewswire.com/news-releases/baricitinib-in-combination-with-remdesivir-reduces-time-to-recovery-in-hospitalized-patients-with-covid-19-in-niaid-sponsored-actt-2-trial-301129865.html. Accessed 17 October 2020.
- 89. Cantini F, Niccoli L, Nannini C, et al. . Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect 2020; 81:647–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study [manuscript published online ahead of print 6 October 2020]. Rheumatology 2020; keaa587. doi: 10.1093/rheumatology/keaa58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cao Y, Wei J, Zou L, et al. . Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020; 146:137–46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bronte V, Ugel S, Tinazzi E, et al. . Baricitinib restrains the immune dysregulation in severe COVID-19 patients. J Clin Invest 2020; 130:6409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Treon SP, Castillo JJ, Skarbnik AP, et al. . The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood 2020; 135:1912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roschewski M, Lionakis MS, Sharman JP, et al. . Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol 2020; 5:eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 2020; 178:104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang N, Zhan Y, Zhu L, et al. . Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe 2020; 28:455–64.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Arabi YM, Asiri AY, Assiri AM, et al. ; Saudi Critical Care Trials Group . Interferon beta-1b and lopinavir-ritonavir for Middle East respiratory syndrome. N Engl J Med 2020; 383:1645–56. [DOI] [PubMed] [Google Scholar]
- 98. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV-1 and MERS-CoV viral load dynamics, duration of viral shedding and infectiousness: a living systematic review and meta-analysis. medRxiv 2020. doi: 10.1101/2020.07.25.20162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C Investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McCarthy CP, Murphy S, Jones-O’Connor M, et al. . Early clinical and sociodemographic experience with patients hospitalized with COVID-19 at a large American healthcare system. EClinicalMedicine 2020; 26:100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hung IF, Lung KC, Tso EY, et al. . Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395:1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Davoudi-Monfared E, Rahmani H, Khalili H, et al. . A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother 2020; 64:e01061–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rahmani H, Davoudi-Monfared E, Nourian A, et al. . Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol 2020; 88:106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhou Q, Chen V, Shannon CP, et al. . Interferon-α2b treatment for COVID-19. Front Immunol 2020; 11:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pereda R, González D, Rivero HB, et al. . Therapeutic effectiveness of interferon-α2b against COVID-19: the Cuban experience. J Interferon Cytokine Res 2020; 40:438–42. [DOI] [PubMed] [Google Scholar]
- 106. Hao SR, Yan R, Zhang SY, et al. . Interferon-α2b spray inhalation did not shorten virus shedding time of SARS-CoV-2 in hospitalized patients: a preliminary matched case-control study. J Zhejiang Univ Sci B 2020; 21:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Frigault MJ, Nikiforow S, Mansour MK, et al. . Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood 2020; 136:137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hill JA, Li D, Hay KA, et al. . Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018; 131: 121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Busani S, Bedini A, Biagioni E, et al. . Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies [manuscript published online ahead of print 25 August 2020]. Clin Infect Dis 2020;ciaa1246. doi: 10.1093/cid/ciaa1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lier AJ, Tuan JJ, Davis MW, et al. . Case report: disseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg 2020; 103:1590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Marchese V, Crosato V, Gulletta M, et al. . Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection 2020: 1–4. doi: 10.1007/s15010-020-01522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. White PL, Dhillon R, Cordey A, et al. . A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit [manuscript published online ahead of print 29 August 2020]. Clin Infect Dis 2020; ciaa1298. doi: 10.1093/cid/ciaa1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rodríguez-Tajes S, Miralpeix A, Costa J, et al. . Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy [manuscript published online ahead of print 24 September 2020]. J Viral Hepat 2020. doi: 10.1111/jvh.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tham SM, Lim WY, Lee CK, et al. . Four patients with COVID-19 and tuberculosis, Singapore, April-May 2020. Emerg Infect Dis 2020; 26:2764–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang B, Zhang S. Corticosteroid-induced osteonecrosis in COVID-19: a call for caution. J Bone Miner Res 2020; 35:1828–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bruce-Hickman D, Sajeed SM, Pang YH, Seow CS, Chen W, Gulati Kansal M. Bowel ulceration following tocilizumab administration in a COVID-19 patient. BMJ Open Gastroenterol 2020; 7:e000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. To KK-W, Hung IF-N, Ip JD, et al. . Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing [manuscript published online ahead of print 25 August 2020]. Clin Infect Dis 2020; ciaa1275. doi: 10.1093/cid/ciaa1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mulder M, van der Vegt DSJM, Oude Munnink BB, et al. . Reinfection of SARS-CoV-2 in an immunocompromised patient: a case report [manuscript published online ahead of print 9 October 2020]. Clin Infect Dis 2020; ciaa1538. doi: 10.1093/cid/ciaa1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Edridge AWD, Kaczorowska J, Hoste ACR, et al. . Seasonal coronavirus protective immunity is short-lasting. Nat Med 2020; 26:1691–3. [DOI] [PubMed] [Google Scholar]