Abstract
Background
Coronavirus disease 2019 (COVID-19) has spread rapidly worldwide since the outbreak originated in Wuhan, China in December 2019. Cardiovascular complications in patients with severe COVID-19 have been reported and are associated with a worse outcome. Coagulopathy is one of the most common life-threatening complication increasing mortality; however, little evidence is available regarding prevention strategies or its treatment in COVID-19 patients.
Case summary
We report a case of a 70-year-old woman admitted to hospital with severe COVID-19 bilateral pneumonia who developed severe coagulopathy with multiple both, venous and arterial, embolisms in major vessels such as bilateral pulmonary embolism, acute thrombus in abdominal aorta, and acute thrombotic occlusion of the right iliac common artery. The patient underwent emergent surgical thrombectomy of the right lower limb; in spite of anticoagulant treatment at therapeutic doses, patient presented poor clinical evolution and an infracondylar amputation of right lower limb was made finally. Subsequently, the patient received low molecular weight heparin (LMWH), antibiotics and antiviral therapy improving her renal function and her pneumonia, so she could be discharged safely.
Discussion
Prothrombotic coagulopathy due to enhanced acute inflammatory response and diffuse intravascular coagulation has been described in severe critical COVID-19 patients. This state of hypercoagulability is associated with organ dysfunction and mortality and may predispose to both, venous and arterial, thromboembolism. Little data are available regarding the best therapeutic and prevention strategies in this scenario, although thrombosis prophylaxis with LMWH has been associated with a better outcome.
Keywords: COVID-19, Pulmonary thromboembolism, Arterial thrombosis, Coagulopathy, Case report
Learning points
Coronavirus disease 2019 (COVID-19) patients have an increased risk of both arterial and venous thromboembolism. Thromboembolic complications in patients with COVID-19 should be suspected and treated early because of the high risk of mortality.
In patients with severe COVID-19 infection, there is an enhanced acute inflammatory response that may induce hypercoagulability and disseminated intravascular coagulation and its management remains unknown.
COVID-19 patients are a diagnostic challenge in a pandemic situation where the number of complementary tests and interventions to reduce exposure tend to be avoided.
Specialities involved other than cardiology
Internal medicine, vascular surgery, radiology.
Introduction
Coronavirus disease 2019 (COVID-19) is a newly recognized infectious disease that has spread rapidly to several countries throughout the world and has led to an increase in hospital admissions. The main cause of admission to the intensive care unit and risk of mortality is pulmonary involvement, with respiratory distress being its most serious manifestation.1,2 Cardiovascular and haematological involvement in COVID-19 are less well known, although more articles are being published.3–5 In this article, we report the first case described to date of a COVID-19 patient admitted for pneumonia who developed thromboembolisms in both arterial and venous territories without a predisposing source of embolism.
Timeline
| Timeline | Course of events |
|---|---|
| Admission |
|
| 3 h after admission |
|
| 4 h after admission |
|
| Day 2 after admission | Acute kidney injury due to rhabdomyolysis and poor clinical response to the surgical thrombectomy and anticoagulant therapy. |
| Day 3 after admission | Surgical right limb amputation. |
| Day 5 | Improvement of the renal function and of the bilateral pneumonia. |
| Day 12 | The patient was safely discharged under anticoagulation treatment with low molecular weight heparin. |
Case presentation
A 70-year-old woman was admitted to the emergency department of our hospital due to pain, paresthesias, and coolness of the right lower limb during the previous 48 h. She also complained of fever within the last 7 days, dry cough and dyspnoea in the last 12 h. Her past medical history includes diabetes mellitus and obesity without known cardiovascular disease or risk factor for deep venous thrombosis or pulmonary embolism (PE). On admission, the patient was hypotensive with a blood pressure of 90/65 mmHg, heart rate of 125 b.p.m., respiratory rate of 28 breaths per minute, a temperature of 38.2°C, and an oxygen saturation of 85% at room air. On cardiovascular examination, the patient was tachycardic, presented normal heart sounds without murmurs and no jugular venous distension was present. Pulmonary examination revealed dispersed crackles bilaterally. The right lower limb was cold with absent distal or proximal pulses and decreased sensitivity and strength.
An electrocardiogram revealed sinus tachycardia at a rate of 125 b.p.m. with no relevant repolarization abnormalities. Urgent blood test results revealed leucocytosis (28 000/mm3, normal range: <10 000/mm3) with lymphopenia (0.85/mm3, normal range: 1.3–4.10), thrombocytopenia (87 000, normal range: 150 000–450 000), and a significantly high D-dimer value (72 000 ng/mL, normal range: <500 ng/mL). Other clotting laboratory results were within normal index, such as: fibrinogen level (350 mg/dL, normal range: 150–450mg/dL), prothrombin time (13.8 s, normal range: 9.2–14.5 s), activated partial thromboplastin time (30.2 s, normal range: 26.9–43.7 s), and international normalized ratio (1.02, normal range: 0.8–1.26). Creatinine kinase (CK) levels were markedly high (14 000 U/L, normal range: <145 U/I) and renal function was impaired (creatinine 2.38 mg/dL, normal range: <0.9 mg/dL) with an estimated glomerular filtration rate of 20 mL/min/1.73 m2 likely secondary to rhabdomyolysis. Cardiac troponin and N-terminal pro-B type natriuretic peptide values at admission were normal. Baseline blood gas results elicited a pH of 7.48, oxygen saturation of 85%, partial oxygen pressure of 45 mmHg (normal range > 60 mmHg), and partial CO2 pressure of 32 mmHg (normal range 35–45 mmHg).
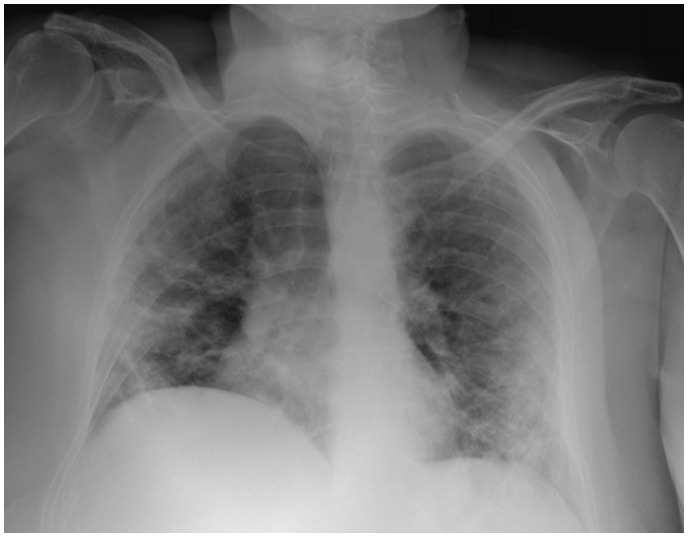
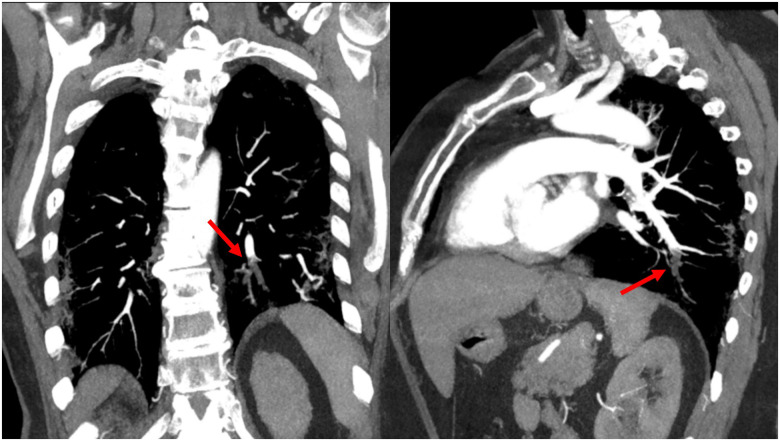
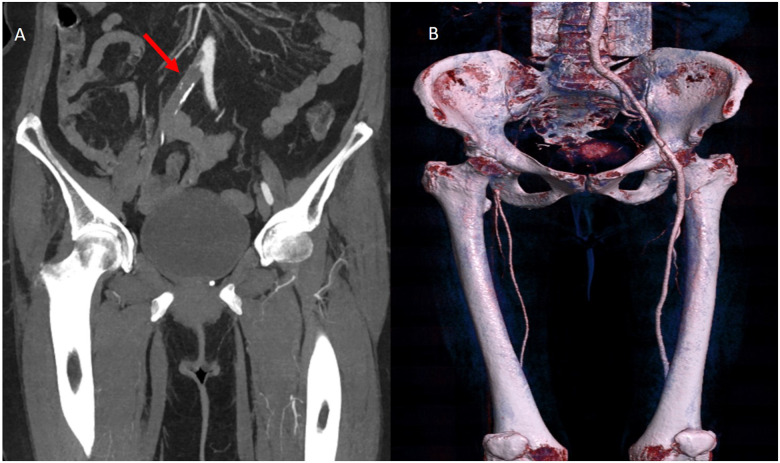
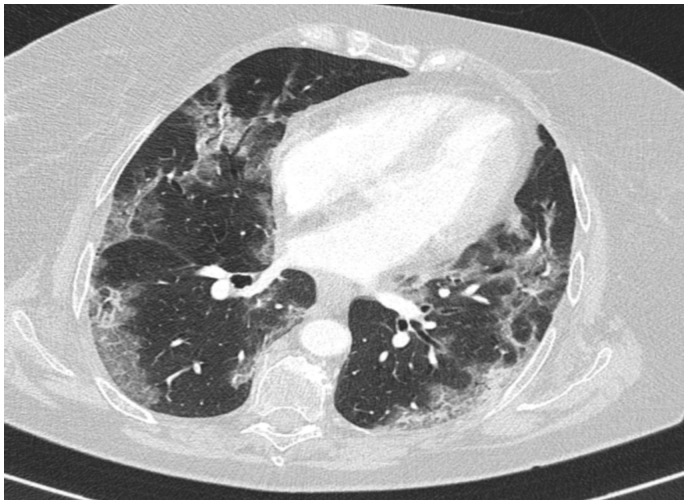
Reverse-transcriptase–polymerase-chain-reaction performed at the emergency department confirmed coronavirus infection (SARS COV-2). Urgent chest radiograph (X-ray) revealed peripheral consolidations and ground-glass opacities consistent with coronavirus infection (see Figure 1). Due to the clinical suspicion of acute arterial thrombosis, an urgent venous and arterial Doppler ultrasound was performed and showed absent flow at the level of the right popliteal artery. Computed tomographic angiography (CTA) revealed segmental bilateral pulmonary emboli at the level of the right lower lobe and left lower lobe posterior arteries (see Figure 2); an acute thrombus in the abdominal aorta prior to renal artery and a complete thrombotic occlusion of the right common iliac artery (see Figures 3 and 4). Chest CTA also showed peripheral distribution of ground-glass patches in both lung fields and ‘crazy paving’ patterns typical of COVID-19 infection at the lung parenchyma (see Figure 5). An urgent echocardiogram was performed at that moment, showing a normal left ventricle with preserved ejection fraction (60% measured by Simpsons biplane method), right cavities with normal size and function, no relevant valve disease, and no indirect signs of pulmonary hypertension. At that time, in an attempt to preserve the right lower extremity, an emergent iliac and a femoral-popliteal surgical thrombectomy was performed by the vascular surgery department of our centre. Unfortunately, blood flow of the right lower limb could not be successfully restored.
Figure 1.
Portable urgent chest X-ray, peripheral consolidations compatible with coronavirus infection.
Figure 2.
A pulmonary angiography showing acute bilateral pulmonary embolism (red arrow).
Figure 3.
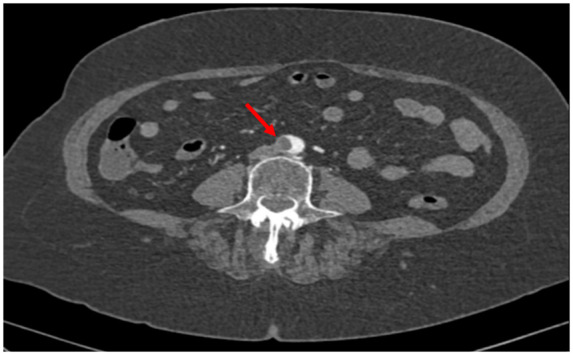
Computed tomography angiography abdominal. Acute thrombus in abdominal aorta (red arrow).
Figure 4.
(A) Computed tomography angiography, complete total occlusion of the right common iliac artery (red arrow). (B) Volume rendering technique showing absence of flow at iliac level.
Figure 5.
Lung parenchyma computed tomographic. Ground glass patches of peripheral distribution in both lung fields and ‘crazy paving’ patterns typical of COVID19 infection.
After surgical thrombectomy, the patient was transferred to a specific COVID-19 hospital floor and received the following in accordance with our centre protocols: Azithromycin 500 mg, Ceftriaxone 2 g once a day, lopinavir-ritonavir 400 mg twice a day, and 400 mg hydroxychloroquine twice a day. Anticoagulation treatment with low molecular weight heparin (LMWH) was initiated and adjusted to renal function and weight (enoxaparin 60 mg per day). On the following day after admission, the patient presented with a poor clinical response and deterioration of renal function reaching creatinine levels of 3.36 mg/dL and elevated CK levels (25 000 U/L). Finally, after discussing the case in a multidisciplinary session the following day, the patient underwent infracondylar amputation of the right lower limb without any other major complications. After 3 days, the patient experienced clinical and analytical improvement with normalization of renal function. She recovered from pneumonia with subsequent blood cultures, nasopharyngeal, and sputum samples showing no evidence of active infections; and could be safely discharged after 12 days since admission with anticoagulation treatment of Enoxaparin 60 mg twice daily. No bleeding complications presented during her admission.
Discussion
The lung is the main organ affected in patients with COVID-19 and acute respiratory distress syndrome is the main cause of mortality.1,2 Among the risk factors for developing severe disease include presence of predisposing heart disease or any other cardiovascular risk factors such as diabetes or hypertension.6 Apart from pulmonary involvement, coagulopathy and cardiovascular implications of COVID-19 are relatively common, and also reflective of acting as an important contributor in morbidity and mortality.3–5 In critical patients with severe COVID-19 pneumonia, there is an enhanced acute inflammatory response that may induce hypercoagulability and disseminated intravascular coagulation.7 According to the International Society on Thrombosis and Haemostasis (ISTH), disseminated intravascular coagulopathy (DIC) in an infection scenario is a ‘coagulation disorder induced by infection, but also represents an acute systemic inflammatory response’.7 Our patient presented with thrombocytopenia (87 000, normal range: 150 000–450 000), and elevated levels of D-dimer (72 000 ng/mL, normal range <500 ng/mL). However, other clotting parameters and fibrin degradation products (FDPs) along with her peripheral blood film were all normal. Therefore, her presentation does not strictly meet the criteria for DIC according to ISTH. Common clinical manifestations of heart failure or PE can be masked by lung involvement. In an effort to avoid excessive exposure, physicians may not be able to perform a thorough physical examination including assessment of distal pulses. Clinical suspicion and use of biomarkers should be the cornerstone in this scenario. Among with the analytical coagulation parameters that are abnormal in patients with COVID-19, high levels of D-dimer and FDPs should concern physicians about an underlying thrombotic complication.
Recent studies show that PE is the most frequent thromboembolic complication in patients with COVID-19.8,9 The pathophysiological reason for this thromboembolic risk remains unknown. Patients with severe COVID-19 are usually elderly and remain immobile during admission contributing to the higher risk for venous thromboembolism. However, our patient suffered both arterial and venous thromboembolism. Therefore, coagulopathy due to an inflammatory status could have also played an important role in the thrombus formation.
Little data are available regarding the optimal management of these patients. LMWH therapy seems to be associated with a better prognosis in patients with coagulopathy and high levels of D-dimer;10 whereby therapeutic doses should be considered in severe cases. In our centre, all patients with high D-dimer levels (≥3000 ng/mL, normal range: <500 ng/mL) are considered to be at high risk and receive prophylactic dose of LMWH adjusted to renal function and weight. The duration of treatment once the patient has recovered from the acute phase remains unknown. To the best of our knowledge, this is the first case report published in the literature of a concomitant venous and arterial thromboembolism in the acute phase of COVID-19 infection.
Conclusion
Thromboembolic events are common in severe COVID-19 patients and are associated with a poor outcome, requiring a high level of clinical suspicion and careful management. This could occur in both arterial and venous territories, and although optimal management is unclear, therapeutic doses of LMWH should be considered in these patients.
Lead author biography

Samuel Del Castillo García was born in Escalonilla, Toledo, Spain in 1990. He graduated in medicine in 2014 from Universidad Europea de Madrid, and currently, he is resident doctor at Complejo Asistencial Universitario de León, in León, Spain. His area of interest includes cardiac imaging and clinical cardiology.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors acknowledge the Radiology department for the acquisition of the images and we would like also to acknowledge the invaluable help of Gretel Echarte Morales in the writing process of this manuscript.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J. et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonow RO, Fonarow GC, O’Gara PT, Yancy CW.. Association of Coronavirus Disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol 2020;5:51–53. [DOI] [PubMed] [Google Scholar]
- 4. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. et al. Coronavirus Disease 2019 (COVID-19) and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 5. Giannis D, Ziogas IA, Gianni P.. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T. et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iba T, Levy JH, Warkentin TE, Thachil J, Poll T, Levi M; the Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost 2019;17:1989–1994. [DOI] [PubMed] [Google Scholar]
- 8. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z.. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.