Abstract
Several recent studies have provided evidence that use of calcium channel blockers (CCBs), especially amlodipine and nifedipine, can reduce mortality from coronavirus disease 2019 (COVID-19). Moreover, hypocalcemia (a reduced level of serum ionized calcium) has been shown to be strongly positively associated with COVID-19 severity. Both effectiveness of CCBs as antiviral therapy, and positive associations of hypocalcemia with mortality, have been demonstrated for many other viruses as well. We evaluate these findings in the contexts of virus–host evolutionary conflicts over calcium metabolism, and hypocalcemia as either pathology, viral manipulation or host defence against pathogens. Considerable evidence supports the hypothesis that hypocalcemia represents a host defence. Indeed, hypocalcemia may exert antiviral effects in a similar manner as do CCBs, through interference with calcium metabolism in virus-infected cells. Prospective clinical studies that address the efficacy of CCBs and hypocalcemia should provide novel insights into the pathogenicity and treatment of COVID-19 and other viruses.
Keywords: SARS-CoV-2, COVID-19, conflict, hypocalcemia, calcium
INTRODUCTION
Therapeutic agents to reduce morbidity and mortality from human infectious diseases work in one of several main ways. The agent may attack the pathogen directly, through interference with the functions of the proteins that it codes for and the nucleic acids that it requires to survive and replicate. The RNA nucleotide analog remdesivir provides a good example of this approach, as it reduces the ability of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus to copy its genetic material [1]. Such agents can be highly effective but they are prone to the evolution of resistance, due to the strong selection imposed and the usual high numbers of genetically variable pathogens present in any given infection.
Alternatively, a therapeutic agent may modulate some aspect of the human immune system. For example, the corticosteroid dexamethasone, which dampens inflammatory activity, has been demonstrated in one recent study to reduce mortality in severe cases of coronavirus disease 2019 (COVID-19) [2], which are characterized by high inflammation and associated microvascular damage [3, 4]. This ameliorative effect was found among individuals of mean age 59, but not in more-elderly patients. The use of agents that modify the immune system is predicated on the assumption that the body’s immune reaction is, for some reason, dysregulated in a given disease or patient. Such dysregulation has been well established for COVID-19 [3].
A third domain of agents and tactics used in fighting infectious disease is those deployed by the body itself: the changes in the body that represent its own adaptive responses to infection. Examples of such strategies include sequestration of iron [5], fever [6], other elements of acute-phase responses such as inflammation [7] and adaptive immunity. Such tactics can impose substantial costs on the host as well as on pathogens, but with expected net benefits across a population of infected hosts overall, given that the relevant immune-related pathways have evolved. In severe infection, the body escalates its defences, resulting in higher and higher costs to both the pathogens and itself [8], and more-substantial departures from physiological equilibrium. A clinician may then feel compelled to consider the reaction as pathological, inhibit its effects, and seek to restore a patient to pre-infection levels for the relevant factor. Should they, as a matter of course?
In this article, we describe evidence and theory regarding the use of calcium channel blockers (CCBs) as therapeutic agents against COVID-19. We do so in the broader contexts of host–virus evolutionary conflicts, calcium homeostasis, hypocalcemia (low levels of serum calcium ions) as a host defence, a viral tactic or a pathology of infection, and the main forms of therapy described above.
We first provide an overview of the physiological functions of CCBs and their typical clinical uses in the treatment of hypertension. Second, we discuss recent studies suggesting efficacy of some CCBs in treating COVID-19. Third, we briefly describe how and why viruses depend on ionic calcium. Fourth, we explain the evidence regarding antiviral activity of CCBs, in general and against coronaviruses in particular. This exposition is situated in the more-general framework of how calcium metabolism mediates disease and bodily defences. Fifth, we address the hypothesis that hypocalcemia, a physiological state that typifies COVID-19 and other severe infections, represents a beneficial host defence rather than a pathology or an adaptation of pathogens. Moreover, this host defence may mimic CCBs in its impacts on the metabolism of calcium and its deleterious effects on viruses. Finally, we describe the implications of the results for the treatment of COVID-19.
CALCIUM CHANNEL BLOCKERS
CCBs are used predominantly to reduce blood pressure in people with hypertension. They function by blocking the calcium channels that regulate contractility of the smooth muscle lining peripheral arteries, thereby causing vasodilation and lowering of blood pressure.
CCBs are categorized into two groups, dihydropyridines and non-dihydropyridines, that differ in chemical structure and their range of effects [9]. Many dihydropyridines, including amlodipine, nifedipine, and other ‘-pines’, are approved for clinical use, as are two non-dihydropryidines, diltiazem and verapamil. All CCBs reduce levels of intracellular calcium, and the latter two agents also reduce cardiac contractility. Dihydropryidine CCBs, especially amlodipine (which has an especially favorable effect profile that includes low retention of fluids), are among the most-commonly prescribed drugs for hypertension worldwide [10].
CCBS AS TREATMENTS FOR COVID-19
A variety of anti-hypertension medications have been studied for their effects on COVID-19 outcomes. Angiotensin-converting enzyme (ACE) inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs) have been of special interest, because SARS-CoV2 uses ACE2 for viral entry into cells, and these medications upregulate the expression of ACE2 in animal models [11]. However, an observational trial from Italy, published in the NEJM, reported no effects of ACEIs or ARBs on COVID-19 outcomes [12], nor any effects from CCBs (as a class) or other blood pressure medications. A study using the Danish national health registry similarly found no effect of ACEIs or ARBs on COVID-19 mortality [13], a conclusion that was also reached in a recent meta-analysis [14].
Three in vivo studies, each of them retrospective, have specifically evaluated the efficacy of CCBs against COVID-19.
Zhang et al. [15] studied 90 hypertensive patients with COVID-19, of whom 44 had been taking amlodipine, 16 nifedipine, 4 other CCBs, 17 other anti-hypertensive agents and 9 no antihypertensive drug. Case fatality rates were significantly lower in the amlodipine treated group (6.7%, 3 of 44), than in the pooled non-amlodipine group (26.1%, 12 of 46) (P < 0.05 with adjustments for age and sex).
Solaimanzadeh [16] studied 65 hypertensive COVID-19 patients, 24 of whom were taking CCBs (amlodipine or nifedipine), with 41 not on CCBs, during hospitalization. Amlodipine or nifedipine use was associated with significantly lower case fatality rates (14.6% vs 50%, P < 0.01), and lower rates of mechanical ventilation (4.2% vs 39%, P < 0.01).
Reynolds et al. [17] studied 2573 COVID-19 patients with histories of hypertension, 634 of whom had severe illness as indicated by intensive care unit (ICU) admission, ventilation, or death. In this analysis, previous use of CCBs was associated with a ‘slightly higher’ (4%) risk of severe illness, which was considered not to be of clinical significance. The specific CCBs used by patients were not described.
A second line of evidence relevant to CCB effects on SARS-CoV-2 is in vitro studies. Zhang et al. [15] showed that treatment with the CCBs amlodipine or benidipine, but not ACE inhibitors or angiotensin II receptor blockers, showed significant anti-viral effects against SARS-CoV-2 in Vero E6 green monkey cells. Similarly, Straus et al. [18] showed, in Vero E6 cells, and in epithelial kidney cells, that amlodipine, felodipine and nifedipine limited the growth of SARS-CoV-2. Hoagland et al. [19] demonstrated, using stem-cell derived pancreatic organoids, that the CCBs amlodipine and berbamine reduced levels of viral transcription by about three orders of magnitude; amlodipine also caused selective differential expression of type 1 interferon pathway signaling genes, which play central roles in coronavirus–host interactions.
A third source of evidence, here relevant to effects of amlodipine in treatment of COVID-19, comes from an analysis of the genetic risk factors affecting COVID-19 mortality [20]. Allelic variation at seven single nucleotide polymorphisms has been significantly associated with mortality from COVID-19 infection [21, 22]. A phenome-wide association study, which determines what phenotypes these single-nucleotide polymorphisms (SNPs) have been associated with in previous genome-wide association studies, showed that four of the seven SNPs had been linked with use of amlodipine [20]. These findings suggest, with indirect evidence, that use of amlodipine mediates COVID-19 survival.
These convergent sources of data provide biological plausibility for the potential use of CCBs, and perhaps amlodipine in particular, in the treatment or prevention of COVID-19 (see also [23, 24]). Prospective clinical trials are needed, especially given that hypertension has been reported to be a substantial risk factor for COVID-19 mortality [25].
VIRUS DEPENDENCE ON IONIC CALCIUM
Ca++ is necessary for viral entry into host cells, viral gene expression, processing of viral proteins, and viral maturation and release [26–31]. To meet their needs for calcium, many pathogenic viruses induce increased influx of these ions across cell membranes (e.g. [29–31]). These influxes of calcium are often mediated by viroporins that act as viral-encoded calcium channels, facilitating calcium input into the cytoplasm from across the cell plasma membrane or across the membrane of the endoplasmic reticulum, which acts as a store for intracellular ionic calcium [32]. In other host–virus systems, viruses use host-encoded cellular calcium channels to increase Ca++ entry into the cytoplasm (e.g. [27]).
Viruses thus cause selective alterations to calcium signaling in host cells as central aspects of their strategies for efficient replication [33, 34], with the alterations normally involving increased intracellular levels [26, 27]. These findings suggest that conflicts between hosts and viruses may frequently involve calcium. Pathogen manipulation of host calcium metabolism also apparently extends to bacterial infection; for example, Bosson et al. [35, 36] showed that CCBs improved survival in two animal models of sepsis.
MECHANISMS OF CCB EFFECTS
How do CCBs work in the context of viral infection, and how might they impact the pathogenicity of COVID-19? Ionic calcium regulates many fundamental intracellular processes through its activity as a second messenger for transduction of signals [26]. Agents that block calcium transport across membranes, such as CCBs, may affect SARS-CoV-2 and the symptoms of COVID-19 by any of several mechanisms.
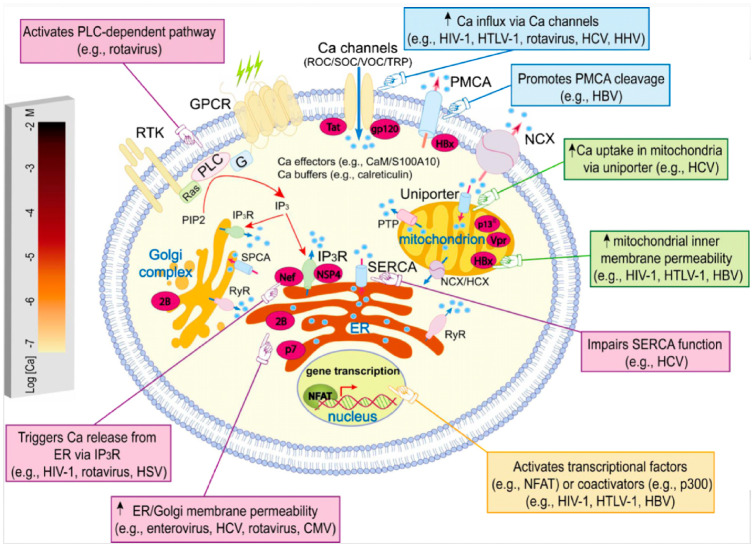
CCBs reduce levels of intracellular calcium [37], presumably countering this and other calcium-manipulating adaptations of viruses (e. g. [38]). Table 1 illustrates the range of viral manipulation and exploitation of host calcium across diverse viruses, and provides examples of CCB effects that alleviate viral infections through interference with these mechanisms. Examples of specific virus-induced alterations to host calcium metabolism are also illustrated in Fig. 1.
Table 1.
Examples of Ca++ effects in viral infection, and their inhibition by CCBs or other calcium blocking or reducing agents
| Virus | Alterations to Ca++ | Comments | Refs. |
|---|---|---|---|
| Porcine coronavirus PDCoV | Infection leads to upregulation of intracellular Ca++ concentrations | Treatment with CCB (diltiazem) inhibited viral replication | 31 |
| Murine coronavirus | Infection induced rapid calcium increase in about 5% of cells (apparently those infected by multiple viruses) | CCB verapimil inhibited viral replication | 39 |
| SARS CoV and MERS | Ca++ required for viral entry | CCBs inhibit SARS CoV infection in vitro | 40 |
| Recovirus | Increased cytosolic Ca++ levels mediated by viroporin NS1-2 shown to facilitate viral replication | Virus yield reduced by experimental Ca++ reduction | 41 |
| Dengue virus | Infected cells show increased permeability to Ca++, mediated by virus | Ca++ increases favored viral replication; virus yield reduced by Ca++ reduction | 42 |
| Hepatitis B virus | HBx protein stimulates Ca++ entry into cells | Reduction of Ca++ impaired viral replication | 43 |
| West Nile virus | Infection leads to rapid Ca++ influx into cells, via calcium channels | Treatment with CCBs (verapamil, diltiazem, nifedipine) decreased viral yield | 28 |
| Dengue, hepatitus C and Zika | Cellular ion channel TRPV4 mediates Ca++ influx | Blocking of TRPV4 channel reduced viral infectivity | 30 |
| Herpes virus | Infection induces rapid and transient increased in intracellular Ca++ | Ca++ alteration mediates viral entry into cells | 44 |
| Phelbovirus | Infectivity mediated by Ca++ influx into cells | CCBs (benidipine or nifedipine) reduced intracellular Ca++ and improved survival in mouse model and in human retrospective study | 45 |
| Influenza A | Infection triggers influx of Ca++ | CCBs (verapimil and diltiazem) inhibit viral infection | 46–48 |
| Rotavirus | Influx of Ca++ early in infection due to viroporin NSP4 | Viroporin-defective mutant lacked Ca++ conductivity | 32, 49 |
| Filoviruses (Ebola and Marburg) | Viral entry into cells requires Ca++ permeable ion channel TPC2 | Calcium channel blocker verapamil (and other channel blockers) inhibit viral cell entry | 50, 51 |
| Coxsackievirus | Influx of Ca++ early in infection due to viroporin 2B | Viroporin with mutations showed low infectivity | 32, 52 |
| Cytomegalovirus | Virus induces early influx of Ca++ from extracellular environment, perhaps via viroporin US21 | CCBs nifedipine, verapamil and manidipine inhibit virus | 53, 54 |
Figure 1.
Examples of how viruses disrupt and exploit calcium signaling in host cells. Calcium ions are represented as blue circles. Reprinted from Zhou et al. [27] with permission
The coronaviruses SARS-CoV and Middle East respiratory syndrome (MERS)-CoV are known to use calcium ions to orchestrate entry into host cells, via a fusion peptide derived from the spike protein [55, 56]. Experimental depletion of intracellular or extracellular Ca++ (or both) eliminates or reduces viral entry [40, 56]. The close similarity of SARS-CoV and MERS-CoV to SARS-CoV-2 [57] suggests that the same or similar mechanisms apply to the current pandemic virus. SARS-CoV and other coronavirus also produce an envelope (E) protein that can assemble into viroporins, whose activity leads to increased inflammation, damage to host cells and edema [58, 59].
A second mechanism of potential antiviral CCB effects is indicated by the observation that amlodipine and other CCBs exert anti-inflammatory and anti-coagulatory effects in humans [60, 61] and animal or cell models [62–66]. These mechanisms are important because, as noted above, high levels of inflammation and microvascular coagulation are considered as major causes of COVID-19 morbidity and mortality [3, 4].
Third, Solaimanzadeh [16] suggests that the vasodilatory effects of CCBs in the lungs and vascular system may mitigate the effects of high inflammation, hypercoagulation, edema and local vasoconstriction, facilitating oxygen diffusion and host cell survival.
The hypotheses described above for the effects of CCBs in COVID-19 and other viral diseases are not mutually exclusive. Determining which are most relevant has key implications, however, for COVID-19 treatments that involve alterations to the metabolism of calcium in viruses and hosts.
HYPOCALCEMIA: PATHOLOGY, VIRAL TACTIC OR HOST DEFENCE?
In many viral diseases, concentrations of serum calcium decrease substantially without medical intervention. In particular, many such diseases are characterized by so-called hypocalcemia, defined as serum levels of ionic Ca++ below some threshold. Severe hypocalcemia can cause cramps, numbness, cardiac arrhythmia, seizures, delirium, hypotension and death (e.g. [67]). If it is deleterious to the host, why does hypocalcemia frequently accompany infectious disease?
In COVID-19, hypocalcemia is highly prominent, being reported in 60% or more of patients at hospital admission [68–70]. It is associated with hospitalization itself (as the strongest of nine risk factors reported in [68]), longer hospitalizations [70], and ventilation, ICU admission, and mortality [69]. The degree of hypocalcemia thus represents a robust metric of disease aggressiveness [68, 69], and calcium supplementation has been suggested [68, 71].
A decision to treat hypocalcemia, in COVID-19 or other infectious diseases, is based on the assumption that this physiological phenotype is more deleterious for the patient than are normal levels of calcium. This assumption is unwarranted without further evidence. Hypocalcemia, like many other signs and symptoms of a disease state, may represent either: (i) a pathological effect of disease that is beneficial to neither the virus nor the host; (ii) a tactic of the virus to enhance its own growth, survival and transmission, that is deleterious to the host; or (iii) a defence of the host against the virus, that is instigated by the host to make the environment of the virus less hospitable [72, 73]. In this latter case, hypocalcemia would exert negative physiological effects on the host, but would be relatively more deleterious for the virus, providing a net benefit to hosts in terms of survival [8]. Under this scenario, the degree of hypocalcemia is also expected to be positively associated with disease severity, as is typically observed [68, 69, 73]. Hypocalcemia also tends to resolve spontaneously among hospitalized patients [74], as predicted if it represents a conditionally adaptive state. Finally, the defence hypothesis predicts that hypocalcemia is induced by the host, rather than by the virus.
What are the implications of these hypotheses for therapy? By hypothesis (1) above, replacement of serum calcium should tend to normalize host physiology and generate better disease outcomes. Under hypothesis (2), low serum calcium is beneficial to the virus, so replacing calcium should harm the virus, again improving outcomes. But under hypothesis (3), calcium replacement should be harmful to the host, or neutral if the body can still sustain a low-calcium equilibrium [73]. For COVID-19, data are not available on the effects of calcium supplementation in hypocalcemic patients. However, in other cases of critical illness, calcium supplementation has been shown to increase mortality rates, in humans [75–77] and in animal models (e. g. [78, 79]). There is also no evidence that calcium supplementation reduces mortality among patients in the ICU [73, 80].
Evolved host defence tactics that harm the self, as well as a pathogen, carry risks in that, for any given patient with severe disease, the defence can become sufficiently pronounced to increase morbidity or mortality [8]. In such situations, clinical decisions regarding treatment need to become more nuanced. For example, in a retrospective study of patients with sepsis, He et al. [77] found the lowest mortality among individuals with mild hypocalcemia, which they considered as protective. These patients were harmed by calcium supplementation. In contrast, patients with severe hypocalcemia showed benefits from supplementation, as expected if their defence system was in these cases imposing undue costs. Prospective clinical trials are needed for robust tests of the costs and benefits of calcium supplementation in patients with different degrees of hypocalcemia.
Cases of extreme and deleterious host defences, such as a substantial level of hypocalcemia, are also not unexpected, given that: (i) levels of expression of such defences should be adapted to ancestral human environments, and to the range and intensity of pathogens to which humans were formerly exposed, and (ii) some pathogens, such as SARS-CoV-2, are novel to humans such that some degree of initial host-pathogen adaptive mismatch is expected. As such, a substantial degree of hypocalcemia in COVID-19 may, like high levels of inflammation, be excessive and deleterious in many patients.
The important question then becomes, if CCBs, and hypocalcemia, may be beneficial against COVID-19 at least in some cases, then might their physiological effects be similar or the same? Do both CCBs and hypocalcemia interfere with the calcium metabolism of pathogens, and thereby inhibit their replication? Hypocalcemia, and CCBs, have, as noted above, both been reported to mediate reductions in intracellular calcium [37, 81], and amlodipine reduces intracellular calcium (in neurons) in a cellular model of Batten disease [82]. The effects of CCBs and hypocalcemia in COVID-19 require targeted studies that take account of host-pathogen conflicts over calcium, and the possibility that hypocalcemia represents a conditional host defence rather than a unilaterally deleterious state.
FUTURE DIRECTIONS
The decision of whether and how to manage hypocalcemia in COVID-19 patients remains an open question deserving further study. Hypocalcemia is associated with disease severity in COVID-19, prompting some clinicians to advocate for calcium supplementation [68, 71]. However, hypocalcemia may well be protective, as suggested by the evidence described above, in which case calcium supplementation will not improve COVID-19 outcomes, and may indeed worsen them. Since equipoise exists in deciding whether to treat low calcium in COVID-19, calcium supplementation in COVID-19 patients should occur in the setting of well-designed randomized controlled trials.
Evidence from retrospective observational trials, and a recent phenome-wide association study, point to the therapeutic potential of CCBs in general, and amlodipine in particular, against COVID-19. Recent preclinical work also suggests that the CCB diltiazem, in conjunction with remdesivir, may provide notable benefits [83]; this CCB, and others, are in clinical trials for COVID-19 efficacy. Diltiazem is also effective against several other viruses, including a porcine coronavirus (Table 1). Large scale prospective trials will be useful in answering the question of whether these and other CCBs have protective effects for those taking these medications chronically. Whether to initiate CCBs for patients with COVID-19 is another unanswered question. In this context, the potential benefits of starting CCBs should be weighed against their potential harms, including lowering blood pressure and inhibiting hypoxic pulmonary vasoconstriction, which might negatively affect oxygenation during pneumonia [84]. This hypothesis requires direct tests in cellular and animal models, as well as trials in human patients.
CONCLUSIONS
The development of effective therapies for COVID-19 will benefit from several evolutionary medical considerations, including the evolutionary dynamics and mechanisms of host–virus conflicts over calcium, the recognition that some symptoms of the disease may represent host defences rather than pathologies or viral adaptations, and the fact that SARS-CoV-2 is novel to humans, such that maladaptive phenotypes are not unexpected in both viruses and hosts. Calcium ions are central to coronavirus replication, and available evidence suggests that both CCBs, and hypocalcemia, may interfere with viral replication by reducing levels of intracellular calcium.
Acknowledgements
We thank A. Mooers, Charlie Nunn and two anonymous reviewers for helpful comments, and Dr Jenny Yang for permission to use Fig. 1.
Conflict of interest: None declared.
REFERENCES
- 1. Rochwerg B, Agarwal A, Zeng L. et al. Remdesivir for severe Covid-19: a clinical practice guideline. BMJ 2020;370:m2924. [DOI] [PubMed] [Google Scholar]
- 2. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leisman DE, Deutschman CS, Legrand M.. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med 2020;46:1105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merad M, Martin JC.. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barber MF, Elde NC.. Buried treasure: evolutionary perspectives on microbial iron piracy. Trends Genet 2015;31:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kluger MJ. Fever: Its Biology, Evolution, and Function. Princeton University Press, 2015. [Google Scholar]
- 7. Okin D, Medzhitov R.. Evolution of inflammatory diseases. Curr Biol 2012;22:R733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeGrand EK, Alcock J.. Turning up the heat: immune brinksmanship in the acute-phase response. Q Rev Biol 2012;87:3–18. [DOI] [PubMed] [Google Scholar]
- 9. DeDea L. How do dihydropyridine and nondihydropyridine CCBs differ? J Amer Acad Phys Assist 2012;25:15. [DOI] [PubMed] [Google Scholar]
- 10. Wang AL, Iadecola C, Wang G.. New generations of dihydropyridines for treatment of hypertension. J Geriat Cardiol 2017;14:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sriram K, Insel PA.. Risks of ACE inhibitor and ARB usage in COVID‐19: evaluating the evidence. Clin Pharmacol Ther 2020;108:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancia G, Rea F, Ludergnani M. et al. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–40. May [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fosbøl EL, Butt JH, Østergaard L. et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020;324:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Teng Y, Shang L. et al. The effect of prior ACEI/ARB treatment on COVID-19 susceptibility and outcome: a systematic review and meta-analysis. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Sun Y, Zeng HL. et al. Calcium channel blocker amlodipine besylate is associated with reduced case fatality rate of COVID-19 patients with hypertension. medRxiv2020. preprint. [DOI] [PMC free article] [PubMed]
- 16. Solaimanzadeh I. Nifedipine and amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID-19. Cureus 2020;12:e8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reynolds HR, Adhikari S, Pulgarin C. et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;382:2441–8. May [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Straus MR, Bidon M, Tang T. et al. 2020. FDA approved calcium channel blockers inhibit SARS CoV 2 infectivity in epithelial lung cells. BioRxiv preprint.
- 19. Hoagland DA, Clarke DJ, Møller R. et al. 2020. Modulating the transcriptional landscape of SARS-CoV-2 as an effective method for developing antiviral compounds. BioRxiv preprint.
- 20. Crespi B. Evolutionary medical insights into the SARS-CoV-2 pandemic. EMPH 2020;doi:10.1093/emph/eoaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellinghaus D, Degenhardt F, Bujanda L. et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020;doi:10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu C, Gam R, Pandurangan AP. et al. Genetic risk factors for death with SARS-CoV-2 from the UK Biobank. MedRxiv 2020;doi:10.1101/2020.07.01.20144592. [Google Scholar]
- 23. Danta CC. Calcium channel blockers: a possible potential therapeutic strategy for the treatment of Alzheimer’s dementia patients with SARS-CoV-2 infection. ACS Chem NeuroSci 2020;11:2145–8. [DOI] [PubMed] [Google Scholar]
- 24. Balasubramanyam M. COVID-19: is it time to revisit the research on calcium channel drug targets? E MJ Diabet 2020;doi:10.33590/emjdiabet/200608. [Google Scholar]
- 25. Roncon L, Zuin M, Zuliani G, Rigatelli G.. Patients with arterial hypertension and COVID-19 are at higher risk of ICU admission. Br J Anaesth 2020;125:e254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou Y, Xue S, Yang JJ. (2013) Calcium and viruses. In: Kretsinger RH, Uversky VN, Permyakov EA (eds.). Encyclopedia of Metalloproteins. New York, NY: Springer. 10.1007/978-1-4614-1533-6_58. [DOI] [Google Scholar]
- 27. Zhou Y, Frey TK, Yang JJ.. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 2009;46:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scherbik SV, Brinton MA.. Virus-induced Ca2+ influx extends survival of West Nile virus-infected cells. J Virol 2010;84:8721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueda M, Daidoji T, Du A. et al. Highly pathogenic H5N1 avian influenza virus induces extracellular Ca2+ influx, leading to apoptosis in avian cells. J Virol 2010;84:3068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doñate-Macián P, Jungfleisch J, Pérez-Vilaró G. et al. The TRPV4 channel links calcium influx to DDX3X activity and viral infectivity. Nat Commun 2018;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bai D, Fang L, Xia S. et al. Porcine deltacoronavirus (PDCoV) modulates calcium influx to favor viral replication. Virology 2020;539:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hyser JM, Estes MK.. Pathophysiological consequences of calcium-conducting viroporins. Ann Rev Virol 2015;2:473–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chami M, Oulès B, Paterlini-Bréchot P.. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim Biophys Acta 2006;1763:1344–62. [DOI] [PubMed] [Google Scholar]
- 34. Chen X, Cao R, Zhong W.. Host calcium channels and pumps in viral infections. Cells 2019;9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bosson S, Kuenzig M, Schwartz SI.. Verapamil improves cardiac function and increases survival in canine E. coli endotoxin shock. Circ Shock 1985;16:307–16. [PubMed] [Google Scholar]
- 36. Bosson S, Kuenzig M, Schwartz SI.. Increased survival with calcium antagonists in antibiotic-treated bacteremia. Circ Shock 1986;19:69–74. [PubMed] [Google Scholar]
- 37. DeWitt CR, Waksman JC.. Pharmacology, pathophysiology and management of calcium channel blocker and β-blocker toxicity. Toxicol Rev 2004;23:223–38. [DOI] [PubMed] [Google Scholar]
- 38. Alam MI, Mostafa A, Kanrai P. et al. Verapamil has antiviral activities that target different steps of the influenza virus replication cycle. J Antivir Antiretrovir 2016;8:121–30. [Google Scholar]
- 39. Kraeft SK, Chen DS, Li HP. et al. Mouse hepatitis virus infection induces an early, transient calcium influx in mouse astrocytoma cells. Exp Cell Res 1997;237:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai AL, Millet JK, Daniel S. et al. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J Molec Biol 2017;429:3875–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strtak AC, Perry JL, Sharp MN. et al. Recovirus NS1-2 has viroporin activity that induces aberrant cellular calcium signaling to facilitate virus replication. mSphere 2019;4:e00506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dionicio CL, Pena F, Constantino-Jonapa LA. et al. Dengue virus induced changes in Ca2+ homeostasis in human hepatic cells that favor the viral replicative cycle. Virus Res 2018;245:17–28. [DOI] [PubMed] [Google Scholar]
- 43. Yang B, Bouchard MJ.. The hepatitis B virus X protein elevates cytosolic calcium signals by modulating mitochondrial calcium uptake. J Virol 2012;86:313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheshenko N, Del Rosario B, Woda C. et al. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol 2003;163:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li H, Zhang LK, Li SF. et al. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res 2019;29:739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujioka Y, Tsuda M, Nanbo A. et al. A Ca 2+-dependent signalling circuit regulates influenza A virus internalization and infection. Nat Commun 2013;4:1–3. [DOI] [PubMed] [Google Scholar]
- 47. Fujioka Y, Nishide S, Ose T. et al. A sialylated voltage-dependent Ca2+ channel binds hemagglutinin and mediates influenza A virus entry into mammalian cells. Cell Host Microbe 2018;23:809–18. [DOI] [PubMed] [Google Scholar]
- 48. Nugent KM, Shanley JD.. Verapamil inhibits influenza A virus replication. Archiv Virol 1984;81:163–70. [DOI] [PubMed] [Google Scholar]
- 49. Pham T, Perry JL, Dosey TL. et al. The rotavirus NSP4 viroporin domain is a calcium-conducting ion channel. Sci Rep 2017;7:43487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gehring G, Rohrmann K, Atenchong N. et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother 2014;69:2123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sakurai Y, Kolokoltsov AA, Chen CC. et al. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015;347:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Kuppeveld FJ, Hoenderop JG, Smeets RL. et al. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J 1997;16:3519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mercorelli B, Luganini A, Celegato M. et al. Repurposing the clinically approved calcium antagonist manidipine dihydrochloride as a new early inhibitor of human cytomegalovirus targeting the Immediate-Early 2 (IE2) protein. Antiviral Res 2018;150:130–6. [DOI] [PubMed] [Google Scholar]
- 54. Luganini A, Di Nardo G, Munaron L. et al. Human cytomegalovirus US21 protein is a viroporin that modulates calcium homeostasis and protects cells against apoptosis. Proc Natl Acad Sci USA 2018;115:E12370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Millet JK, Whittaker GR.. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virol 2018;517:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Straus MR, Tang T, Lai AL. et al. Ca2+ ions promote fusion of Middle East Respiratory Syndrome coronavirus with host cells and increase infectivity. J Virol 2020;94:e00426–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Petrosillo N, Viceconte G, Ergonul O. et al. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM. et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015;485:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schoeman D, Fielding BC.. Coronavirus envelope protein: current knowledge. Virol J 2019;16:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hernandez R, Carvajal AR, Armas-de MH. et al. The effects of the calcium antagonist amlodipine on blood pressure and platelet aggregation in hypertensive patients. Postgrad Med J 1991;67:S38–40. [PubMed] [Google Scholar]
- 61. Kim HJ, Han SJ, Kim DJ. et al. Effects of valsartan and amlodipine on oxidative stress in type 2 diabetic patients with hypertension: a randomized, multicenter study. Korean J Internal Med 2017;32:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kataoka C, Egashira K, Ishibashi M, Inoue S. et al. Novel anti-inflammatory actions of amlodipine in a rat model of arteriosclerosis induced by long-term inhibition of nitric oxide synthesis. Am J Physiol Heart Circ Physiol 2004;286:H768–74. [DOI] [PubMed] [Google Scholar]
- 63. Chou TC, Yang SP, Pei D.. Amlodipine inhibits pro-inflammatory cytokines and free radical production and inducible nitric oxide synthase expression in lipopolysaccharide/interferon-γ-stimulated cultured vascular smooth muscle cells. Jpn J Pharmacol 2002;89:157–63. [DOI] [PubMed] [Google Scholar]
- 64. Das R, Burke T, Van Wagoner DR, Plow EF.. L-type calcium channel blockers exert an antiinflammatory effect by suppressing expression of plasminogen receptors on macrophages. Circ Res 2009;105:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Silva IV, de Figueiredo RC, Rios DR.. Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int J Mol Sci 2019;20:3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang J, Si D, Zhao Y. et al. S-amlodipine improves endothelial dysfunction via the RANK/RANKL/OPG system by regulating microRNA-155 in hypertension. Biomed Pharmacother 2019;114:108799. [DOI] [PubMed] [Google Scholar]
- 67. Llach F, Weidmann P, Reinhart R. et al. Effect of acute and long-standing hypocalcemia on blood pressure and plasma renin activity in man. J Clin Endocrin Metab 1974;38:841–7. [DOI] [PubMed] [Google Scholar]
- 68. Di Filippo L, Formenti AM, Rovere-Querini P. et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine 2020;12:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu J, Han P, Wu J. et al. 2020. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health 2020;13:1224–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu Y, Hou B, Liu J. et al. Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered, retrospective study. Front Med (Lausanne) 2020;7:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. di Filippo L, Formenti AM, Giustina A.. Hypocalcemia: the quest for the cause of a major biochemical feature of COVID-19. Endocrine 2020;22:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Williams GC, Nesse RM.. The dawn of Darwinian medicine. Q Rev Biol 1991;66:1–22. [DOI] [PubMed] [Google Scholar]
- 73. Aberegg SK. Ionized calcium in the ICU: should it be measured and corrected? Chest 2016;149:846–55. [DOI] [PubMed] [Google Scholar]
- 74. Steele T, Kolamunnage-Dona R, Downey C. et al. Assessment and clinical course of hypocalcemia in critical illness. Crit Care 2013;17:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Collage RD, Howell GM, Zhang X. et al. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase (CaMKK) signaling. Crit Care Med 2013;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dotson B, Larabell P, Patel JU. et al. Calcium administration is associated with adverse outcomes in critically ill patients receiving parenteral nutrition: results from a natural experiment created by a calcium gluconate shortage. J Hum Pharmacol Drug Ther 2016;36:1185–90. [DOI] [PubMed] [Google Scholar]
- 77. He W, An Y, Huang L. et al. Calcium supplementation prolongs the time of hospitalization and has a double effect on mortality in septic patients: a retrospective study from MIMIC-III. Preprint. Research Square doi: 10.21203/rs.3.rs-16486/v1. [Google Scholar]
- 78. Malcolm DS, Zaloga GP, Holaday JW.. Calcium administration increases the mortality of endotoxic shock in rats. Crit Care Med 1989;17:900–3. [DOI] [PubMed] [Google Scholar]
- 79. Zaloga GP, Sager A, Black KW, Prielipp R.. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock 1992;37:226–9. [PubMed] [Google Scholar]
- 80. Forsythe RM, Wessel CB, Billiar TR. et al. Parenteral calcium for intensive care unit patients. Cochrane Database Syst Rev 2008;4:CD006163. [DOI] [PubMed] [Google Scholar]
- 81. Goltzman D, Mannstadt M, Marcocci C. 501–13. Physiology of the calcium-parathyroid hormone-vitamin D axis. In: Vitamin D in Clinical Medicine 2018, Vol. 50. Karger Publishers, 1–13. [DOI] [PubMed] [Google Scholar]
- 82. Warnock A, Tan L, Li C. et al. Amlodipine prevents apoptotic cell death by correction of elevated intracellular calcium in a primary neuronal model of Batten disease (CLN3 disease). Biochem Biophys Res Comm 2013;436:645–9. [DOI] [PubMed] [Google Scholar]
- 83. Pizzorno A, Padey B, Julien T. et al. Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia. bioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 84. Naeije R, Brimioulle S.. Physiology in medicine: importance of hypoxic pulmonary vasoconstriction in maintaining arterial oxygenation during acute respiratory failure. Crit Care 2001;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]