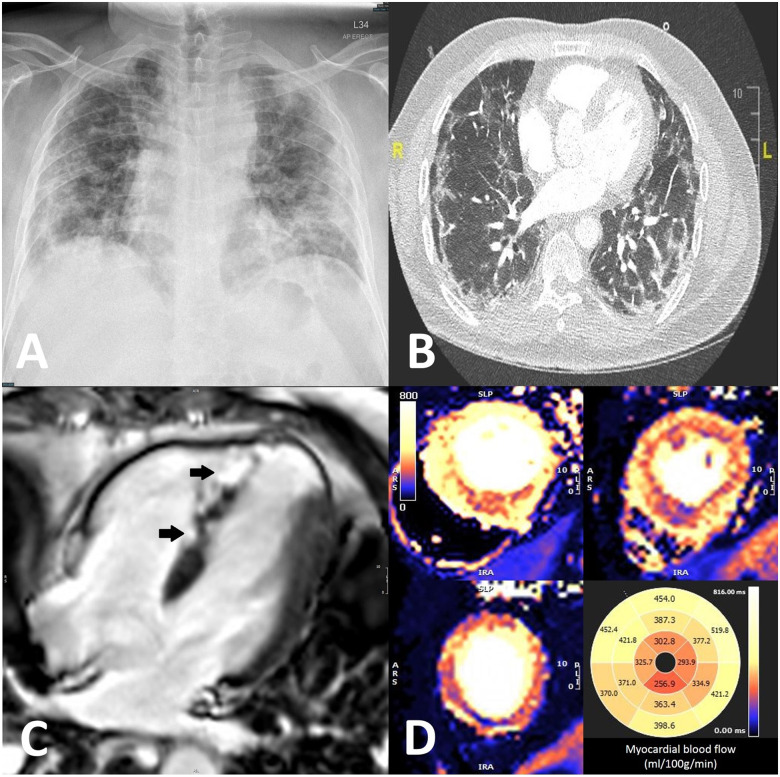
A previously fit 49-year-old male of South Asian ethnicity, presented to our emergency department at the height of the COVID-19 pandemic with a 2-day history of dyspnoea and a dry cough, preceded by an acute diarrhoeal illness. Clinical examination revealed a temperature of 38.6°C, blood pressure of 150/105 mmHg, heart rate of 129 b.p.m., and a respiratory rate of 40/min with oxygen saturations of 96% on air. A chest radiograph showed bilateral patchy lung opacities (Figure 1A). Initial laboratory tests showed an elevated white cell count of 11.5 [normal range (NR) 4–11 × 109 L], neutrophil count of 9.27 (NR 1.5–7.5 × 109 L), C-reactive protein of 129 (NR < 5mg/L), and a D-dimer of 7.7 (NR < 0.5 µg/mL). International normalized ratio and cardiac troponin were normal. The patient was treated empirically with intravenous clarithromycin 500 mg twice daily, co-amoxiclav 1.2 g three times daily, dexamethasone 6 mg once daily, and continuous positive airway pressure ventilation, along with anti-thrombotic prophylaxis on the intensive care unit. The patient underwent a SARS-CoV-2 polymerase chain reaction test which demonstrated that he had active COVID-19. On Day 6, the patient developed left arm swelling. Ultrasound confirmed a thrombus in the left internal jugular vein and axillary vein and was subsequently anticoagulated with dalteparin 12 500 units once daily. On Day 10, the patient developed central chest pain. Electrocardiogram showed anterior ST-segment elevation and Q-waves (Supplementary material online, Figure S1), high-sensitive troponin-I was 27 266 (NR < 14 ng/L). An echocardiogram showed moderate left ventricular systolic dysfunction with apical akinesia. A computed tomography pulmonary angiogram showed subpleural organizing areas of consolidation with patchy peripheral ground-glass opacities with multiple bilateral pulmonary emboli (Figure 1B). Cardiac magnetic resonance imaging was undertaken to establish the aetiology of the cardiac injury. T1 mapping showed septal oedema with preserved ejection fraction (63%) but with apical akinesia and late gadolinium enhancement confirming apical myocardial infarction (MI) and patchy micro-infarction in the mid septum on the epicardial right ventricular side (Figure 1C, Video 1). Adenosine stress perfusion, to assess reversible myocardial ischaemia revealed marked hyperaemic myocardial blood flow (up to 5 mL/g/min) with patchy apical and mid septal hypoperfusion (Figure 1D) suggesting microvascular disease. Subsequent coronary angiography revealed unobstructed coronary arteries (Supplementary material online, Figures S2 and S3). This case highlights the increasingly recognised macrovascular (venous and pulmonary arterial thrombosis, apical MI) and microvascular complications (patchy micro MI with non-matching stress hypoperfusion). The cardiac complications occurred despite 4 days of anticoagulation and there is increasing evidence that the vascular sequalae of COVID-19 are related to immune complex deposition and localized vascular inflammation rather than thromboembolism.
Figure 1.
(A) Chest radiograph on Day 1 showing diffuse bilateral pulmonary opacities. (B) Computed tomography pulmonary angiogram on Day 10 showed patchy peripheral ground-glass opacities with multiple bilateral pulmonary emboli. (C) Late gadolinium enhancement showing patchy micro-infarction in the mid septum on the epicardial right ventricular side (arrows). (D) Marked hyperaemic myocardial blood flow (up to 5 mL/g/min) with patchy apical and mid septal hypoperfusion.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.