Abstract
Background
We aimed to describe physician practice patterns in holding or continuing IBD therapy in the setting of COVID-19 infection, using the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease [SECURE-IBD] registry.
Methods
IBD medications that were stopped due to COVID-19 were recorded in the SECURE-IBD registry in addition to demographic and clinical data. We conducted descriptive analyses to understand characteristics associated with stopping IBD medications in response to active COVID-19 infection.
Results
Of 1499 patients, IBD medications were stopped in 518 [34.6%] patients. On bivariate and multivariable analyses, a diagnosis of ulcerative colitis or IBD-unspecified was associated with a lower odds of stopping medication compared with Crohn’s disease (adjusted odds ratio [aOR] 0.6, 95% confidence interval [CI] 0.48, 0.75). When evaluating specific medications, 5-aminosalicylic acid was more likely to be continued [p <0.001] whereas anti-tumour necrosis factor therapy and immunomodulator therapy were more likely to be stopped [global p <0.001]. Other demographic and clinical characteristics did not affect prescription patterns.
Conclusions
IBD medications other than immunomodulators were continued in the majority of IBD patients with COVID-19, in the international SECURE-IBD registry. Future studies are needed to understand the impact of stopping or continuing IBD medications on IBD- and COVID-19 related outcomes.
Keywords: Inflammatory bowel disease, Crohn’s disease, ulcerative colitis, coronavirus disease 2019, IBD therapy
1. Introduction
The impact of holding immunosuppressive and other therapies for inflammatory bowel diseases [IBD] in the context of coronavirus disease 2019 [COVID-19] is unknown. In the interim, expert consensus is to hold corticosteroids, immunosuppressants, and biologics, but not 5-aminosalicylic acid [5-ASA], at the time of suspected or confirmed diagnosis of COVID-19 infection until fever and other symptoms are resolved.1 There are currently no data on physician practice patterns regarding IBD therapy in the setting of COVID-19.
2. Methods
We analysed data from the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease [SECURE-IBD] registry to determine physician practice patterns in IBD patients with confirmed COVID-19. Collection and categorisation of data have been previously reported.2 Reporters to SECURE-IBD were asked if any medications were stopped due to active COVID-19 infection and to indicate which specific drugs were stopped.
We conducted bivariate analyses to understand demographic and clinical characteristics associated with stopping at least one IBD medication due to COVID-19, and subsequently performed multivariable logistic regression to evaluate the independent effects on IBD disease activity [specified a priori] and other patient characteristics that were statistically significant on bivariate analyses. We also analysed country as a variable affecting stopping in multivariate analyses and included any country with ≥50 reported cases in SECURE-IBD. Additionally, among users of each medication class, we compared the proportion of patients who had discontinued treatment after diagnosis of COVID-19; p-values ≤0.05 were considered statistically significant.
3. Results
Of 1499 patients with available medication-related data in the SECURE-IBD registry, IBD medications were stopped in 518 [34.6%] patients and continued in 981 [65.4%] patients. Baseline characteristics of patients who stopped and did not stop at least one medication are reported in Table 1. On bivariate and multivariable analyses, a diagnosis of ulcerative colitis [UC] or IBD-unspecified [IBD-U] was associated with a lower odds of stopping medication compared with Crohn’s disease [CD] (adjusted odds ratio [aOR] 0.6, 95% CI 0.48, 0.75) [Table 2]. Compared with cases from other countries, those from Italy and Brazil were associated with higher odds of stopping IBD medication on bivariate analysis, but this was not significant in the multivariable model. Other demographic variables, physician global assessment of IBD activity, COVID-19 related emergency room visit, or hospitalisation were not associated with stopping IBD therapy in the setting of COVID-19.
Table 1.
Demographic and clinical characteristics of IBD patients in the SECURE-IBD registry based on prescription practice in the setting of COVID-19.
| Characteristica,b | All patients on ≥1 medication | IBD medication stopped | IBD medication not stopped | p-value | |||
|---|---|---|---|---|---|---|---|
| N [mean] | % [SD] | N [mean] | % [SD] | N [mean] | % [SD] | ||
| Total number of patients | 1499 | 518 | 31.8% | 981 | 60.2% | - | |
| Age | 44 | 17.88 | 43 | 16.77 | 44 | 18.43 | 0.276 |
| Female sex | 752 | 48% | 242 | 47% | 485 | 49% | 0.290 |
| Race | |||||||
| White | 1221 | 81% | 420 | 81% | 801 | 82% | 0.787 |
| Black or African American | 96 | 6% | 35 | 7% | 61 | 6% | 0.685 |
| American Indian/Native Alaskan | 2 | 0% | 1 | 0% | 1 | 0% | 0.646 |
| Asian | 87 | 6% | 33 | 6% | 54 | 6% | 0.495 |
| Native Hawaiian/Pacific Islander | 0 | 0% | 0 | 0% | 0 | 0% | - |
| Other | 115 | 8% | 47 | 9% | 68 | 7% | 0.138 |
| Hispanic/Latino | 0.409 | ||||||
| Yes | 262 | 17% | 100 | 19% | 162 | 17% | |
| No | 930 | 62% | 314 | 61% | 616 | 63% | |
| Unknown | 186 | 12% | 66 | 13% | 120 | 12% | |
| Missing | 121 | 8% | 38 | 7% | 83 | 8% | |
| Country | |||||||
| USA | 514 | 34% | 172 | 33% | 342 | 35% | 0.520 |
| Spain | 225 | 15% | 80 | 15% | 145 | 15% | 0.732 |
| France | 90 | 6% | 32 | 6% | 58 | 6% | 0.837 |
| Italy | 74 | 5% | 16 | 3% | 58 | 6% | 0.016 |
| UK | 71 | 5% | 26 | 5% | 45 | 5% | 0.708 |
| Iran | 53 | 4% | 20 | 4% | 33 | 3% | 0.620 |
| Russian | 79 | 5% | 32 | 6% | 47 | 5% | 0.253 |
| Brazil | 50 | 3% | 24 | 5% | 26 | 3% | 0.042 |
| Other | 343 | 23% | 116 | 22% | 227 | 23% | 0.744 |
| Disease type | <0.001 | ||||||
| Crohn’s disease | 821 | 55% | 325 | 63% | 496 | 51% | |
| Ulcerative Colitis | 649 | 43% | 187 | 36% | 462 | 47% | |
| IBD-unspecified | 24 | 2% | 4 | 1% | 20 | 2% | |
| IBD disease activityc | 0.702 | ||||||
| Remission | 854 | 57% | 292 | 56% | 562 | 57% | |
| Mild | 273 | 18% | 92 | 18% | 181 | 18% | |
| Moderate | 240 | 16% | 90 | 17% | 150 | 15% | |
| Severe | 80 | 5% | 24 | 5% | 56 | 6% | |
| Comorbid conditions | |||||||
| Any condition | 555 | 37% | 194 | 37% | 361 | 37% | 0.804 |
| Cardiovascular disease | 109 | 7% | 32 | 6% | 77 | 8% | 0.236 |
| Diabetes | 88 | 6% | 32 | 6% | 56 | 6% | 0.713 |
| Lung disease | 144 | 10% | 42 | 8% | 102 | 10% | 0.153 |
| Hypertension | 194 | 13% | 75 | 14% | 119 | 12% | 0.198 |
| Cancer | 33 | 2% | 10 | 2% | 23 | 2% | 0.603 |
| History of stroke | 19 | 1% | 5 | 1% | 14 | 1% | 0.447 |
| Chronic renal disease | 33 | 2% | 8 | 2% | 25 | 3% | 0.208 |
| Chronic liver disease | 52 | 3% | 17 | 3% | 35 | 4% | 0.774 |
| Other | 214 | 14% | 77 | 15% | 137 | 14% | 0.636 |
| Current smoker | 63 | 4% | 18 | 3% | 45 | 5% | 0.307 |
| BMI | 0.491 | ||||||
| BMI <30 | 925 | 62% | 315 | 61% | 610 | 62% | |
| BMI >=30 | 249 | 17% | 82 | 16% | 167 | 17% | |
| Missing | 325 | 22% | 121 | 23% | 204 | 21% | |
| COVID-19 related emergency room visit | 549 | 37% | 188 | 36% | 361 | 37% | 0.847 |
| COVID-19 related hospitalisation | 422 | 28% | 147 | 28% | 275 | 28% | 0.887 |
Statistically significant associations are in bold.
IBD, inflammatory bowel disease; SD, standard deviation; BMI, body mass index.
aUnless otherwise specified, percentages do not include missing values or ‘unknown’. For all characteristics, unless noted above, less than 4% of data were missing and unknown, respectively, for each category.
bPercentages and n from each subcategory may not add up to the exact number of total reported cases, due to missing values and/or non-mutually exclusive variables.
cBy physician global assessment [PGA] at time of COVID-19 infection.
Table 2.
Multivariable analysis to determine factors associated with odds of stopping IBD medication in IBD patients with COVID-19.
| Effect | Crude odds ratio | 95% Wald | p-value | Adjusted odds ratio | 95% Wald | p-value | ||
|---|---|---|---|---|---|---|---|---|
| confidence limits | confidence limits | |||||||
| Active vs remission | 1.024 | 0.822 | 1.277 | 0.8296 | 0.971 | 0.772 | 1.221 | 0.8003 |
| Unknown vs remission | 1.203 | 0.676 | 2.14 | 0.5298 | 1.261 | 0.702 | 2.266 | 0.4375 |
| UC/IBDU vs CD | 0.607 | 0.489 | 0.755 | <0.0001 | 0.597 | 0.478 | 0.747 | <0.0001 |
| USA vs non-USA | 0.929 | 0.742 | 1.163 | 0.5203 | 0.943 | 0.704 | 1.264 | 0.6965 |
| Spain vs non-Spain | 1.053 | 0.783 | 1.417 | 0.7311 | 1.13 | 0.791 | 1.615 | 0.5015 |
| France vs non-France | 1.048 | 0.671 | 1.636 | 0.8361 | 1.029 | 0.63 | 1.68 | 0.9092 |
| Italy vs non-Italy | 0.507 | 0.289 | 0.892 | 0.0183 | 0.595 | 0.326 | 1.087 | 0.0916 |
| UK vs non-UK | 1.099 | 0.67 | 1.803 | 0.7081 | 1.154 | 0.674 | 1.975 | 0.6014 |
| Iran vs non-Iran | 1.154 | 0.655 | 2.032 | 0.6205 | 1.403 | 0.764 | 2.577 | 0.2748 |
| Russia vs non-Russia | 1.308 | 0.824 | 2.078 | 0.2544 | 1.477 | 0.882 | 2.472 | 0.1381 |
| Brazil vs non-Brazil | 1.785 | 1.014 | 3.141 | 0.0447 | 1.731 | 0.947 | 3.167 | 0.0748 |
Statistically significant associations are in bold.
UC, ulcerative colitis; IBD-U, IBD-unspecified; CD, Crohn’s disease.
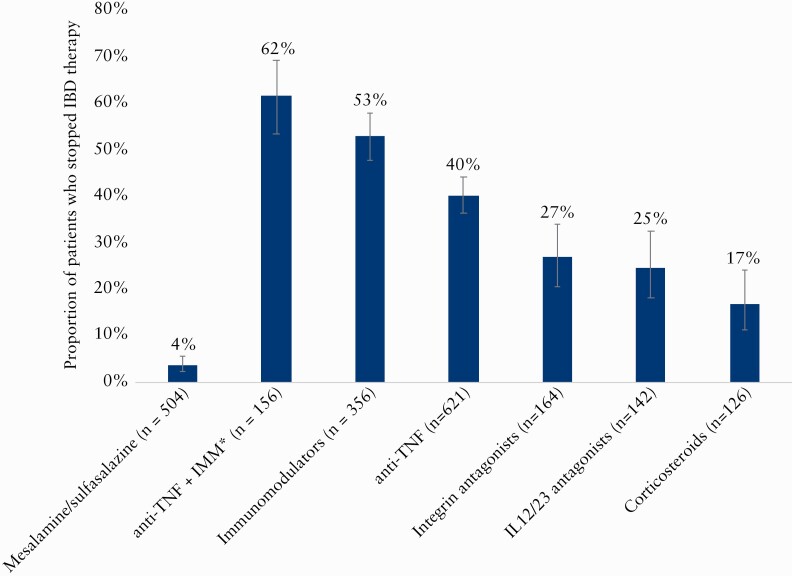
When evaluating specific medications, 5-ASA was more likely to be continued [p <0.001] whereas anti-tumour necrosis factor [anti-TNF] therapy and immunomodulator therapy 96-mercaptopurine [6MP], azathioprine, methotrexate) were more likely to be stopped [global p <0.001] [Figure 1]. Of 156 patients on combination therapy with an anti-TNF and an immunomodulator, the anti-TNF alone was stopped in 10 [6.4%], immunomodulator alone in 33 [21.2%], and both medications in 53 [34%] patients. Sixty [38%] patients continued on combination therapy.
Figure1.
Prescription practice in the setting of COVID-19 based on IBD medication class#. Medication categories are not mutually exclusive unless otherwise noted. #At time of COVID-19 infection; *stopping either anti-TNF, IMM, or both. TNF = tumour necrosis factor; IMM, immunomodulator; IL, interleukin.
4. Discussion
In this brief report, we describe physician practice patterns in holding IBD medications, in an international registry of IBD patients with confirmed COVID-19. IBD medications were more likely to be continued in those with UC or IBD-U than with CD. This is likely due, at least in part, to a higher proportion of UC patients on 5-ASA therapy, which was the most likely medication to be continued. Conversely, anti-TNFs and immunomodulators, used alone or in combination, were the most frequently stopped classes of medications. These practice patterns are largely concordant with expert guidance on IBD medication management in setting of COVID-19.1
However, IBD medications were continued for nearly two-thirds of patients, and combination therapy with an anti-TNF and immunomodulator in nearly 40% of patients. It is important to note that biweekly or less frequent dosing of certain biologics could affect decision and feasibility to stop. Emergency room visit or hospitalisation due to COVID-19 did not impact on IBD medication management. Although variation in the discontinuation of IBD medications was significant by country in bivariate analyses, these associations did not remain statistically significant in a multivariable model. Notable trends that may have been limited by sample size include lower odds of medication discontinuation in patients from Italy and higher odds of discontinuation in patients from Brazil. These findings suggest the need to further study international variation in practice patterns and patient outcomes. In the absence of these data, we suggest following guidance laid out per expert consensus.1
Strengths of this study include the use of a large, international registry with a diverse adult and paediatric IBD patient population. Limitations include the considerable risk of reporting bias in this voluntary registry of IBD patients with COVID-19 patients. Another limitation is missing data, although this was <4% for all variables except ethnicity. In the present study, we were not able to evaluate the impact of holding or continuing medications on COVID-19 outcomes, due to issues related to unmeasured confounding of COVID-19 severity and lack of data regarding duration of holding medication and timing of medication restarting.
Acknowledgements
We acknowledge the physicians and other health care providers worldwide who have reported cases to the SECURE-IBD database, and the organisations who supported or promoted the SECURE-IBD database [reporter names available at [www.covidibd.org/reporter-acknowledgment]. Partnering organisations are available at [https://covidibd.org/our-partners]].
In summary, we found that IBD medications other than immunomodulators were continued in the majority of IBD patients with COVID-19, in the international SECURE-IBD registry. Future studies are needed to understand the impact of stopping or continuing IBD medications on IBD-related outcomes as well as COVID-19 related outcomes. The data underlying this article are available in the article and in its online supplementary material.
Funding
This work was funded by the Helmsley Charitable Trust [2003–04445], National Center for Advancing Translational Sciences [UL1TR002489], a T32DK007634 [EJB], and a K23KD111995-01A1 [RCU]. Additional funding was provided by Pfizer, Takeda, Janssen, Abbvie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm.
Conflict of Interest
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. MA receives research support from the Dickler Family Fund, New York Community Trust, and Helmsley Charitable Trust Fund for SECURE-IBD. EJB is supported by an Institutional Training Grant from the National Institutes of Health [T32DK007634]. JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals, and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, and Viela bio; and holds stock options in Intestinal Biotech Development and Genfit. MDK has consulted for Abbvie, Janssen, Pfizer, and Takeda, is a shareholder in Johnson & Johnson, and has received research support from Abbvie and Janssen. RCU has served as a consultant and/or advisory board member for Eli Lilly, Janssen, Pfizer, and Takeda. He has received research support from AbbVie, Boehringer Ingelheim, and Pfizer. He is supported by a Career Development Award from the National Institutes of Health [K23KD111995‐01A1].
Author Contributions
MA: study concept and design, interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content; EJB: study concept and design, acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content; XZ: acquisition, analysis, and interpretation of data; JFC: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content; MDK: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content; RCU: study concept and design, acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content.
References
- 1. Rubin DT, Abreu MT, Rai V, Siegel CA. Management of patients with Crohn’s disease and ulcerative colitis during the coronavirus disease-2019 pandemic: results of an International Meeting. Gastroenterology 2020;159:6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brenner EJ, Ungaro RC, Gearry RB, et al. . Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020. PMID: 32425234. [DOI] [PMC free article] [PubMed] [Google Scholar]