Abstract
OBJECTIVES
Severe acute respiratory syndrome coronavirus 2, a novel coronavirus, affects mainly the pulmonary parenchyma and produces significant morbidity and mortality. During the pandemic, several complications have been shown to be associated with coronavirus disease 2019 (COVID-19). Our goal was to present a series of patients with COVID-19 who underwent chest tube placements due to the development of pleural complications and to make suggestions for the insertion and follow-up management of the chest tube.
METHODS
We retrospectively collected and analysed data on patients with laboratory-confirmed COVID-19 in our hospital between 11 March and 15 May 2020. Patients from this patient group who developed pleural complications requiring chest tube insertion were included in the study.
RESULTS
A total of 542 patients who were suspected of having COVID-19 were hospitalized. The presence of severe acute respiratory syndrome coronavirus 2 was confirmed with laboratory tests in 342 patients between 11 March and 15 May 2020 in our centre. A chest tube was used in 13 (3.8%) of these patients. A high-efficiency particulate air filter mounted double-bottle technique was used to prevent viral transmission.
CONCLUSIONS
In patients with COVID-19, the chest tube can be applied in cases with disease or treatment-related pleural complications. Our case series comprised a small group of patients, which is one of its limitations. Still, our main goal was to present our experience with patients with pleural complications and describe a new drainage technique to prevent viral transmission during chest tube application and follow-up.
Keywords: Chest tube, Coronavirus disease 2019, Pleural complication, Severe acute respiratory syndrome coronavirus 2, Tube thoracostomy
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily affects the lower respiratory system.
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily affects the lower respiratory system. The severity of the clinical presentation ranges from a mild common cold-like illness to severe disseminated viral pneumonia leading to acute respiratory distress syndrome (ARDS) [1–5].
Several complications such as secondary bacterial infections, cardiac arrhythmia, cardiomyopathy, acute kidney injury and liver dysfunction have been shown to be associated with coronavirus disease 2019 (COVID-19); moreover, pleural complications including pneumothorax and pneumomediastinum were also reported [2, 3].
Although there are several guidelines on the application and management of a chest tube during the pandemic, there are no clinical studies in the literature [6–9]. Our goal was to present a series of patients with COVID-19 who underwent chest tube placement due to the development of disease- or procedure-related pleural complications including pneumothorax, parapneumonic effusion, malignant effusion, empyema and haemothorax.
An additional goal was to present our clinical experience and make several suggestions on chest tube placement in patients with COVID-19 in light of the data obtained as a result of the retrospective examinations of these cases.
PATIENTS AND METHODS
This retrospective study was approved by our institutional review board (No. 49109414-604.02).
We retrospectively collected and analysed data on patients who were admitted to our hospital for any reason and who had positive test results for COVID-19 infection at our hospital between 11 March and 15 May 2020. Real-time reverse transcriptase-polymerase chain reaction assays of specimens collected using a nasopharyngeal swab were used to make a diagnosis of infection with SARS-CoV-2.
Treatment and follow-up of the patients with positive test results for SARS-CoV-2 were carried out according to the COVID-19 treatment guideline released from the ministry of health of our country [10]. This guideline includes a combination treatment of hydroxychloroquine 200 mg 2 × 1 po, azithromycin 400 mg po 1 × 1 ± favipiravir (for patients with severe pneumonia).
Patients from this patient group who developed pleural complications requiring chest tube insertion were included in the study. We included complications not only related to COVID-19 or its treatment process but also related to patients' comorbid conditions (e.g. malign pleural effusion). All of the patients in our case series were hospitalized with suspicion of COVID-19, and chest tube implementation was needed during follow-up.
Patient demographic data, comorbidities, clinical symptoms, laboratory and radiological findings and treatment modalities were obtained, and daily chest tube drainage follow-up information was collected by reviewing medical records.
Patients included in the study were examined as separate groups according to the indications for chest tube placement. Risk factors for the development of pleural complications and the effects of the development of complications on the treatment process were investigated.
Surgical technique
Tube thoracostomy is a surgical procedure that may require urgent application and has the risk of facilitating the spread of SARS-CoV-2 via aerosols and droplets. Although it can be applied by members of various specialties, it was applied only by senior surgical residents or thoracic surgeons and with the fewest number of staff members during the pandemic period in our hospital. Throughout the pandemic, all chest tube placements were performed by teams of 3 people (surgeon/senior resident, nurse and surgical technician). This team changed weekly and was responsible for all chest tube insertions, dressings and pleural interventions during its tenure. Personal protective equipment included an FFP3 mask, long-sleeved gown and gloves; eye protection was used with all chest tube placement procedures.
Pleural procedures were performed in a well-ventilated operating theatre or at the bedside in the intensive care unit. No other patients were admitted to the operating room for 25 min after the procedure to ensure sufficient change of air.
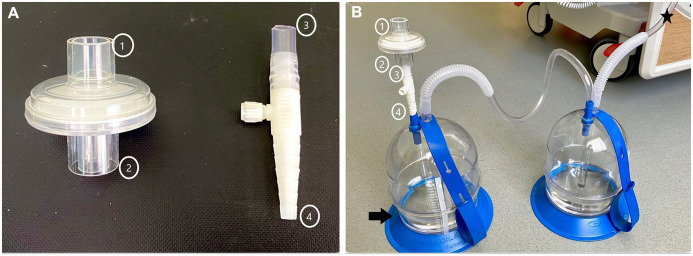
We used a two-bottle technique in all cases (Fig. 1). In this technique, trap (collection) and underwater seal bottles were used. A high-efficiency particulate air (HEPA) viral filter was placed on the tip of the underwater seal bottle filled only with 200 cc (80%) alcohol instead of water alone. The suction tube can be easily connected to the tip (1) of the filter when it is needed. In our centre, we use a suction regulation bottle (manometer bottle) to maintain continuous aspiration, and we have not observed that the HEPA filter makes or requires a change under this pressure.
Figure 1:
We used collection and underwater seal bottles in all cases. (A) The high-efficiency particulate air filter was mounted on the tip of the drainage bottle using an adapter. (B) The system used on the patient. The arrow indicates the underwater seal bottle only filled with 200 cc (80%) alcohol instead of water alone. The asterisk indicates the part of the system that connects to the chest tube.
Follow-up
The thoracic intervention team, which comprises a thoracic surgeon/senior resident, nurse and surgical technician, was established for the evaluation and subsequent follow-up of patients diagnosed with COVID-19 who need surgical pleural intervention. After placement of the chest tube, patients hospitalized in isolated rooms or intensive care units were seen and evaluated twice a day by the thoracic intervention team. This team was also responsible for changing the dressings of patients with chest tubes and terminating the chest tube when necessary. In this way, a small but sufficient number of healthcare professionals had contact with patients diagnosed with COVID-19.
Statistical analyses
Counts and percentages were used to summarize the categorical variables. Medians and interquartile ranges were used to describe continuous variables. SPSS 25.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis.
RESULTS
A total of 542 patients who were suspected of having COVID-19 were hospitalized. The presence of the SARS-CoV-2 virus was confirmed in our centre by laboratory tests of 342 patients between 11 March and 15 May 2020. A chest tube was applied in 13 (3.8%) of these patients. There were 9 men and 4 women. The median age was 54 years (range 3–94). The main characteristics of these 13 patients are summarized in Table 1. Ten (76.9%) patients had 1 or more comorbidities. The indications for chest tube placement were pneumothorax in 6 (46.1%) patients; pleural effusion in 3 (23%) patients; empyema in 3 (23%) patients; and haemothorax in 1 patient (7.6%). In 4 patients, pneumothoraxes developed during mechanical ventilation. In 1 patient, the pneumothorax was spontaneous. Iatrogenic pneumothorax was seen in 1 patient secondary to the endotracheal intubation.
Table 1:
Characteristic features of the patients
| Case | Sex | Age | Indication | Localization | Drainage time (days) | Comorbidity | The need for MV | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 67 | Iat pnx | Right | 7 | DM, HT | Yes | Alive |
| 2 | M | 68 | Pnx | Right | 2 | Neurological | Yes | Died |
| 3 | M | 54 | Pnx | Right | 5 | None | No | Alive |
| 4 | M | 64 | Pnx | Right | 7 | Larynx ca | Yes | Died |
| 5 | M | 64 | Spn pnx | Left | 6 | Bronchial ca | No | Alive |
| 6 | M | 45 | Pnx + Emp | Bilateral | 20 | None | Yes | Died |
| 7 | F | 45 | Empyema | Left | 13 | CRF | No | Alive |
| 8 | M | 31 | Empyema | Right | 24 | Neurological | Yes | Alive |
| 9 | F | 45 | Empyema | Right | 10 | DM | Yes | Alive |
| 10 | M | 94 | PE | Left | 4 | CRF, CHF | Yes | Died |
| 11 | M | 69 | MPE | Right | 7 | Bronchial ca | Yes | Died |
| 12 | F | 51 | MPE | Left | 4 | Cervical ca | No | Alive |
| 13 | M | 48 | Haemothorax | Right | 10 | None | Yes | Died |
ca: cancer; CHF: congestive heart failure; CRF: chronic renal failure; DM: diabetes mellitus; Emp: empyema; HT: hypertension; Iat: iatrogenic; MPE: malignant pleural effusion; MV: mechanical ventilation; PE: pleural effusion; pnx: pneumothorax; Spn: spontaneous.
During follow-up, empyema and contralateral pneumothorax were seen in 1 patient who had a chest tube due to pneumothorax.
Massive haemothorax was seen in 1 patient who was on extracorporeal membrane oxygenation (ECMO).
The median drainage time was 7 (2–24) days, and the median hospital stay was 21 (4–25) days.
In patients with empyema, mean drainage time and hospital stay were longer than with patients with pneumothorax and pleural effusion (P = 0.024 and P = 0.040, respectively) (Table 2).
Table 2:
Comparison of mean drainage times and hospital stays according to types of pleural complications
| Drainage time (days), mean ± SD | Hospital stay (days), mean ± SD | |
|---|---|---|
| Pneumothorax (n = 5) | 5.4 ± 2.0 | 18.4 ± 7.2 |
| Pleural effusiona (n = 4) | 6.2 ± 2.8 | 12.5 ± 5.8 |
| Empyemab (n = 4) | 16.7 ± 6.3 | 23.0 ± 1.8 |
| P-value | 0.024 | 0.04 |
The patient with haemothorax was included in this group.
The patient who was followed up due to pneumothorax but later developed empyema was included in this group.
SD: standard deviation.
In patients with empyema, a microbiological examination of the pleural fluid revealed beta-haemolytic streptococcus in 3 patients and Acinetobacter baumannii in 1 patient.
Eight (61.5%) of 13 patients required follow-up in the intensive care unit. Six patients were in the intensive care unit before application of the chest tube. Two patients, 1 with empyema and the other with malignant pleural effusion, were taken to the intensive care unit in the days following insertion of the chest tube. During the study period, 6 (46.1%) patients died of respiratory failure secondary to ARDS. Two patients who were taken to the intensive care unit after the insertion of a chest tube were extubated, and their chest tubes were removed during follow-up.
DISCUSSION
Novel pathological studies revealed exudative diffuse alveolar damage, capillary congestion and hyaline membrane formations in the early phase and interstitial and intra-alveolar proliferation of fibroblasts and fibrin deposition in the late phase of the COVID-19 infection. Moreover, most autopsy reports showed disseminated fibrotic changes in the lung parenchyma. Although these pathological changes are similar to those of ARDS, the clinical course is not the same in every patient, and pleural complications can develop due to these COVID-19-related pathophysiological changes, treatment and interventions applied or comorbid conditions of the patients [11, 12].
Several studies showed that high positive end-expiratory pressure (PEEP) would be associated with alveolar rupture and pneumothorax. Therefore, it is important to see the prevailing pathophysiological picture and distinguish the ‘L-type’ and ‘H-type’ pneumonia in patients diagnosed with COVID-19 in order to manage the ventilator successfully [13, 14].
In our case series, 4 of 6 patients who developed pneumothorax were intubated patients who underwent high PEEP (14–15 cm H2O). These patients were those with H-type pneumonia who needed high PEEP therapy. In 1 patient, pneumothorax developed secondary to tracheal laceration, and spontaneous pneumothorax was seen in 1 patient.
Detection of pneumothorax may be difficult in critically ill patients. Therefore, sudden changes in respiratory parameters, especially in patients receiving high PEEP therapy, should suggest the possibility of pneumothorax.
We preferred to keep the chest tube clamped for 24 h before removing it in patients with COVID-19 and pneumothorax. No recurrent pneumothorax was observed in these patients.
According to the novel case series and our clinical experience, isolated pleural effusion is extremely uncommon in patients with COVID-19. In our case series, a total of 7 patients had pleural effusions. Three of them had empyema; 2 had malignant effusions; 1 had parapneumonic effusion; and 1 had haemothorax.
It has been shown that viral infections facilitate secondary infections by disrupting mucociliary clearance, weakening neutrophil functions and affecting the functioning of immunological pathways [15–18].
Secondary bacterial infections can also be seen in patients with COVID-19. Zhou et al. [16] demonstrated that 50% of patients with COVID-19 who died had secondary bacterial infections. Wang et al. [17] showed that the most common complications in patients with COVID-19 were bacterial infections (42.8%).
We detected empyema in 4 patients and performed tube thoracostomy. In 1 of these patients, empyema developed secondary to prolonged air leakage after pneumothorax. Despite the complete drainage of the empyema, regular irrigation of the pleural cavity with antibiotic solutions and appropriate antibiotherapy, the mean drainage time was relatively long (16.7 ± 6.3 days).
Prevention is the ideal solution for secondary infections in COVID-19. Several studies have shown the positive effect of empiric antibiotherapy in patients with COVID-19. However, it is extremely important to know institutional antibiograms, local drug resistance and hospital epidemiological prototypes when choosing empiric regimens [18].
Malignant pleural effusion was detected in 2 patients with COVID-19. The respiratory parameters of these patients were poor, and rapid progression of the effusions was observed despite recurrent thoracentesis. Therefore, the chest tube was inserted, and total lung expansion was obtained. Because of the poor general condition of these patients, talc slurry was not administered.
Massive haemothorax was observed in a patient undergoing ECMO with an indication of severe respiratory failure due to ARDS. Haemothorax was unrelated to ECMO cannulation, and there was no history of cardiopulmonary resuscitation. Left-sided tube thoracostomy was performed, and drainage of the haemothorax was obtained.
Low-dose heparin infusion (100 U/kg heparin) was discontinued, and intravenous tranexamic acid (500 mg q8h) was applied. A total of 500 cc of haemorrhagic drainage was observed in the first 2 h. Subsequently, a total of 1000 cc of haemorrhagic fluid drainage occurred in 24 h, and total lung expansion was obtained. No deterioration was observed in the patient's vital signs with adequate volume replacement. Therefore, no surgical intervention was needed.
Several guidelines and recommendations regarding a chest tube thoracostomy and patient follow-up during the pandemic have been published. Common points in these guidelines include avoiding unnecessary surgical interventions and working with a small but sufficient number of medical staff [6–8]. SARS-CoV-2 is not currently considered to be airborne, but several procedures like chest tube placement would be associated with aerosol generation. Therefore, pleural procedures must be performed in a well-ventilated room, and personal protective equipment must be worn [19, 20].
Another important point is the risk of droplet spread from the chest tube. Traditional underwater seal drainage bottles have a port that allows the air to escape and, if necessary, to connect to the low-pressure wall suction. In the presence of air leakage, aerosolization can occur inside the drainage bottle, and air escaping through the port would increase the risk of viral transmission. This issue has been noted before, and various suggestions have been made in the literature [7–9]:
Bilkhu et al. [7] suggested connecting the chest drain to the wall suction and attaching a viral filter to the suction port of a chest drainage bottle or using digital drain circuits to reduce droplet spread from the chest tube.
Barr et al. [9] described the ‘rocket tubing’ method to prevent viral transmission through the port of the drainage bottle.
Coronaviruses are ∼65–125 nm in size, and unlike standard filters, HEPA filters have a minimum 99.97% efficiency rating for removing particles ≥30 nm [21, 22]. Therefore, we used the HEPA filter mounted bottle system, including the trap and the underwater seal bottle.
The trap bottle was filled only with 200 cc (80%) of alcohol instead of water alone, and a HEPA filter was placed on the tip of the underwater seal bottle. Pieracci et al. recommended adding dilute sodium hypochlorite (household bleach) to the water seal chamber [6, 10]. Although there is no evidence for which one is more protective, we preferred adding alcohol into the trap bottle instead of sodium hypochlorite because it is easily accessible and applicable in our clinic.
To prevent viral dissemination, pleural catheters, which may be inserted with the Seldinger technique, are preferred instead of traditional chest tube systems, especially in loculated pleural effusions. In our centre, only the 8-Fr size of these types of catheters is available. Consequently, they often become occluded in a short time after the placement, and repeated surgical procedures can be required. Therefore, we did not use this type of catheter with any patients during the study period; however, it should be kept in mind as an alternative method for the drainage of pleural effusions.
In our centre, we organized a pleural intervention team of 3 people: a surgeon, a nurse and a surgical technician. The primary purpose was to limit and regulate the contact of healthcare professionals with patients diagnosed with COVID-19.
During the pandemic, COVID-19 (or related symptoms) was not observed in any of the healthcare professionals in our surgical team.
Limitations
This study has several limitations. First, it is a retrospective case series, and there is no control group. Second, our study includes a heterogeneous group of patients; therefore, it is difficult to make comprehensive inferences. Besides, the number of patients in our case series is small, so the conclusions of the study are limited. However, the primary purpose of our research was to provide knowledge and share our experiences under pandemic conditions rather than to produce statistically significant results.
CONCLUSION
The chest tube can be applied with various indications in patients diagnosed with COVID-19.
The addition of pleural complications such as pneumothorax, empyema or haemothorax can create a serious burden for patients whose respiratory parameters are already on the border.
Broad participation in the studies by multiple centres is needed to identify statistically significant risk factors and to offer ways to prevent pleural complications.
Another important point is to prevent aerosol and droplet transmission in patients with COVID-19 during the placement of the chest tube and during the follow-up period.
COVID-19 transmission was not detected in the members of our surgical team who dealt with pleural complications during the study period. Therefore, we believe that the HEPA filter mounted double-bottle technique described above is an effective and feasible method for preventing viral transmission during the follow-up of patients with chest tubes during the pandemic. However, the effectiveness and success of this technique need to be supported by other studies.
ACKNOWLEDGEMENTS
The authors thank all COVID-19 Working Group members at the University of Health Sciences Turkey, Dr Suat Seren Chest Diseases and Surgery, Medical Practice and Research Center: Berrin Akkol, Nimet Aksel, Ceyda Anar, Gülsüm Arı, Sena Ataman, Çağrı Atasoy, Aysu Ayrancı, Günseli Balcı, Özgür Batum, Mualla Elif Bayındır, Aylin Bayram, Eda Bayramiç, Can Biçmen, Semra Bilaçeroğlu, Seda Bilgen, Melih Büyükşirin, Emel Cireli, Melis Çaktu, Kadri Çırak, Pınar Çimen, Sinan Çolak, Hasan Demir, Emine Sena Dikmen, Özlem Ediboğlu, Ece Elburus, İsmail Erikçi, Sinem Ermin, Mücahit Fidan, Mine Gayaf, Gamze Göker, Mutlu Onur Güçsav, Filiz Güldaval, Burçin Hakoğlu, Lütfü Can Hepduman, Osman Hilmioğlu, Gülistan Karadeniz, Fatmanur Kazankaya, Merve Keskin, S.Cenk Kıraklı, Ali Korkmaz, Berna Kömürcüoğlu, Nil Kuranoğlu, Aydan Mertoğlu, Zeynep Öndeş, Hilal Özdemir, Serir Özkan, Gülru Polat, Bilge Salık, Hülya Şahin, Yosun Şan, Güneş Şenol, İmren Taşkıran, Dursun Tatar, Zühre Sarp Taymaz, Serpil Tekgül, İhsan Topaloğlu, Fevziye Tuksavul, Betül Tunçel, Merve Türk, Damla Serçe Unat, Özgür Uslu, Fatma Demirci Üçsular, Yelda Varol, Enver Yalnız, Özlem Yalnız, İlkin Yetişkin, Celalettin Yılmaz, Nisel Yılmaz, Ufuk Yılmaz.
Conflict of interest: The authors declare that there is no conflict of interest.
Author contributions
Kenan Can Ceylan: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation. Guntug Batihan: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing—original draft. Serkan Yazgan: Conceptualization; Data curation; Investigation; Supervision; Writing—review & editing. Soner Gürsoy: Conceptualization; Data curation; Supervision; Validation; Writing—original draft; Writing—review & editing. Sami Cenk Kıraklı: Conceptualization; Investigation; Methodology; Supervision. Sena Ataman: Conceptualization; Data curation; Investigation; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Emmanouil Ioannis Kapetanakis, Rita Daniela Francesca Marasco and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- ECMO
Extracorporeal membrane oxygenation
- HEPA
High-efficiency particulate air
- PEEP
Positive end-expiratory pressure
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
REFERENCES
- 1.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19): situation report, 74. World Health Organization. [Google Scholar]
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care 2020;24:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higny J, Feye F, Foret F. COVID-19 pandemic: overview of protective-ventilation strategy in ARDS patients. Acta Clin Belg 2020;27:1–3. [DOI] [PubMed] [Google Scholar]
- 5. Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care 2020;24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pieracci FM, Burlew CC, Spain D, Livingston DH, Bulger EM, Davis KA et al. Tube thoracostomy during the COVID-19 pandemic: guidance and recommendations from the AAST Acute Care Surgery and Critical Care Committees. Trauma Surg Acute Care Open 2020;5:e000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bilkhu R, Viviano A, Saftic I, Billè A. COVID-19: Chest Drains with Air Leak—The Silent ‘Super Spreader’? CTSNet, Inc. 2020. Dataset. doi:10.25373/ctsnet.12089130.v1.
- 8.Hallifax R, Wrightson JM, Bibby A, Walker S, Stanton A, Fonseka DD. Pleural Services during the COVID-19 Pandemic (V2.0). British Thoracic Society, 2020.
- 9. Barr J, Internullo E, West D, Krishnadas R, Batchelor T, Saftic I. COVID-19: Safe Thoracic Surgery. CTSNet, Inc. Media. 2020. doi:10.25373/ctsnet.12200786.v1.
- 10. Demirbilek Y, Pehlivantürk G, Özgüler ZÖ, Alp Meşe E. COVID-19 outbreak control, example of ministry of health of Turkey. Turk J Med Sci 2020;50:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain A, Doyle DJ. Stages or phenotypes? A critical look at COVID-19 pathophysiology. Intensive Care Med 2020;46:1494–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu CW, Sun SF. Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med 2014;3:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ioannidis G, Lazaridis G, Baka S, Mpoukovinas I, Karavasiliset V, Lampakial S. Barotrauma and pneumothorax. J Thorac Dis 2015;7:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hendaus MA, Jomha FA. Covid-19 induced superimposed bacterial infection. J Biomol Struct Dyn 2020;25:1–10. [DOI] [PubMed] [Google Scholar]
- 16. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L, He W, Yu X, Hu D, Bao M, Liu H et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; doi:10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Setti L, Passarini F, Gennaro GD, Barbieri P, Perrone MG, Borelli M et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health 2020;23:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohanski MA, Palmer JN, Cohen NA. Aerosol or droplet: critical definitions in the COVID-19 era. Int Forum Allergy Rhinol 2020;10:968–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res 2020;24:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vijayan VK, Paramesh H, Salvi SS, Dalal AAK. Enhancing indoor air quality—the air filter advantage. Lung India 2015;32:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]