Abstract
Background
Long-term health sequelae of coronavirus disease 2019 (COVID-19) may be multiple but have thus far not been systematically studied.
Methods
All patients discharged after COVID-19 from the Radboud University Medical Center, Nijmegen, the Netherlands, were consecutively invited to a multidisciplinary outpatient facility. Also, nonadmitted patients with mild disease but with symptoms persisting >6 weeks could be referred by general practitioners. Patients underwent a standardized assessment including measurements of lung function, chest computed tomography (CT)/X-ray, 6-minute walking test, body composition, and questionnaires on mental, cognitive, health status, and quality of life (QoL).
Results
124 patients (59 ± 14 years, 60% male) were included: 27 with mild, 51 with moderate, 26 with severe, and 20 with critical disease. Lung diffusion capacity was below the lower limit of normal in 42% of discharged patients. 99% of discharged patients had reduced ground-glass opacification on repeat CT imaging, and normal chest X-rays were found in 93% of patients with mild disease. Residual pulmonary parenchymal abnormalities were present in 91% of discharged patients and correlated with reduced lung diffusion capacity. Twenty-two percent had low exercise capacity, 19% low fat-free mass index, and problems in mental and/or cognitive function were found in 36% of patients. Health status was generally poor, particularly in the domains functional impairment (64%), fatigue (69%), and QoL (72%).
Conclusions
This comprehensive health assessment revealed severe problems in several health domains in a substantial number of ex–COVID-19 patients. Longer follow-up studies are warranted to elucidate natural trajectories and to find predictors of complicated long-term trajectories of recovery.
Keywords: COVID-19, fatigue, health status, multidisciplinary, post-acute, sequelae of COVID-19
Three months after recovery from acute coronavirus disease 2019 (COVID-19), this study shows that, while the pulmonary parenchyma is recovering, a substantial number of patients report severe problems in several health domains, including fatigue, functional impairment, and quality of life.
By 5 November 2020, the World Health Organization (WHO) reported more than 47 million confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases worldwide, and the number of daily new cases keeps increasing [1]. Although the mortality rate is considerable, the vast majority of SARS-CoV-2–infected patients recover from the acute phase. Long-term health consequences of this coronavirus disease (COVID-19) are yet largely unknown, but many patients are likely to experience long-lasting morbidity [2]. Indeed, based on observations from diseases that share COVID-19 characteristics such as acute respiratory distress syndrome (ARDS) [3], SARS-CoV [4], and Coxiella burnetii infection (Q fever) [5], it is hypothesized that, in the long-term, a significant number of patients with COVID-19 will suffer from lung function impairment, residual pulmonary parenchymal abnormalities, decreased physical capacity, loss of muscle mass, anxiety, depression, cognitive deficits, post-traumatic stress disorder, fatigue, and poor health status. We aimed to comprehensively assess these health domains in patients 3 months after recovery from acute COVID-19.
METHODS
This prospective observational study reports data on 124 patients who consecutively attended the COVID-19 aftercare facility at Radboud University Medical Center (Radboudumc), Nijmegen, the Netherlands. Within the Netherlands, the center of the outbreak was close to the city of Nijmegen, and the Radboudumc treated patients with moderate-to-critical COVID-19 also including patients who were transferred from other regions because of capacity problems. In the initial phase of the outbreak, SARS-CoV-2 reverse transcription–polymerase chain reaction (RT-PCR) tests were not readily available for all patients with mild symptoms who did not require hospital admission; they were advised to stay at home and self-quarantine until respiratory symptoms resolved. Patients who had been discharged after inpatient treatment for COVID-19 were consecutively invited to the aftercare facility. Also, general practitioner (GP) referrals of RT-PCR–confirmed or patients with clinically suspected SARS-CoV-2 without hospitalization but with symptoms persisting more than 6 weeks were included. A standardized health assessment was performed that included questionnaires, physical measurements, and multidisciplinary consultations followed by interdisciplinary case discussions. The team consisted of a pulmonologist, geriatrician, infectious-diseases specialist, intensive care specialist, nurse practitioner, physiotherapist, psychologist, dietitian, and social worker. Data were collected as part of the ongoing prospective observational POST-COVid-19 recovERY (POSTCOVERY) study, which was approved by the local medical ethics committee of Arnhem-Nijmegen, the Netherlands (ref. 2020-0660), and was not subject to the Medical Research Involving Human Subjects Act.
World Health Organization criteria were applied to divide patients into mild, moderate, severe, or critical disease categories [6]. Age, sex, length of stay, SARS-CoV-2 RT-PCR and serologic results, chest computed tomography (CT) COVID-19 severity scores [7] on admission, and comorbidities were collected from patients’ medical records. Social status, educational level, and smoking status were collected via questionnaires. C-reactive protein (CRP), D-dimer, ferritin, and leukocyte count at follow-up were collected.
Pulmonary Function and Radiological Imaging
Resting pulse oximetry was performed in a sitting position at least 5 minutes and while breathing room air. Spirometry, single-breath diffusion capacity, and body plethysmography [8–10] were performed and outcomes were expressed according to reference values. The modified Medical Research Council (mMRC) dyspnea scale was assessed [11]. Discharged patients underwent low-dose chest CT imaging at follow-up. Scanning protocol and structured image interpretation by 2 independent chest radiologists are described in the Supplementary Methods. Referred patients with mild disease underwent conventional chest X-ray imaging and the presence of residual COVID-19 abnormalities was collected from the radiological report.
Physical Functioning, Body Composition, and Mental and Cognitive Status
The degree of frailty was assessed with the Clinical Frailty Scale [12]. Scores less than 5 can be considered as not frail, a score of 5 indicates somewhat frail, and scores greater than 5 indicate frailty. A 6-minute walking test (6MWT) [13] was performed and outcomes were expressed according to reference values. Desaturation upon the 6MWT was defined as a decrease of 4% or more of the resting saturation [14]. Anthropometry was assessed by measuring body height and weight and body mass index (BMI) was calculated as body weight/height squared. Body composition was assessed by bioelectrical impedance analysis (Bodystat 500; EuroMedix, Leuven, Belgium) excluding patients with peripheral edema or pacemaker. Fat-free mass index (FFMI) was calculated as fat-free mass/height squared, and age-, sex- and BMI-specific cutoffs were applied to determine the proportion of patients with an FFMI less than the lower limit of normal (LLN) [15]. Anxiety and depression were measured by the Hospital Anxiety and Depression Scale (HADS) [16]. The Telephone Interview of Cognitive Status (TICS) [17] was assessed to screen for cognitive impairment and the Cognitive Failure Questionnaire (CFQ) [18] to measure self-reported cognitive functioning. The Post-Traumatic Stress Syndrome (PTSS) Checklist DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) (PCL-5) [19] and the Impact of Event Scale–Revised (IES-R) [20] were assessed to screen for PTSS and to measure stress reactions after traumatic events, respectively. Generally accepted cutoff scores were applied to determine abnormal mental or cognitive status (HADS-anxiety >10, HADS-depression >10, TICS <34, CFQ >43, PCL-5 >33, and IES-R >33).
Assessment of Health Status
Health status was measured and valued with the Short Form Health survey (SF-36) [21] and the Nijmegen Clinical Screening Instrument (NCSI) [22, 23]. The SF-36 consists of 8 subscales: physical functioning, social functioning, role functioning, role emotional, emotional well-being, energy/fatigue, pain, and general health with higher scores indicating better health status. The NCSI is a battery of subscales from questionnaires that was empirically composed, such that it provides a detailed measure of health status while being short enough to be used in usual care. The NCSI measures 22 aspects of 3 health status domains: symptoms (pain, dyspnea [23], and fatigue [24]), functional impairments (behavioral impairment [25] and subjective impairment [26]), quality of life (QoL; general QoL [27, 28], health-related QoL [23]), and satisfaction with relations [23]), with higher scores indicating worse health status. These 22 aspects are aggregated into 10 dimensions for which cutoffs for normal, mild, and severe problems were applied [22].
Care Delivery
Received treatment by allied health care professionals (AHCPs) between acute COVID-19 and day of outpatient visit was recorded. Advice following interdisciplinary case discussions was collected and grouped into 1 or more of the following categories: additional diagnostics, self-management advice, medication advice, specific advice to the GP, new referral to an AHCP, new referral to another medical specialist, and referral to specialized rehabilitation.
Statistical Analysis
Statistical analyses were performed using SPSS statistical software program, version 25.0 (IBM Corporation). Between-group comparisons for continuous variables were tested by 1-way analysis of variance or Kruskal-Wallis test. Categorical variables were tested with a chi-square test. If a statistically significant difference was obtained, a post hoc test was performed applying Bonferroni correction to account for multiple comparisons. Within-group differences of continuous variables between baseline and follow-up were tested with paired t tests. Missing data were handled by complete case analysis. A P value of less than .05 was considered significant.
RESULTS
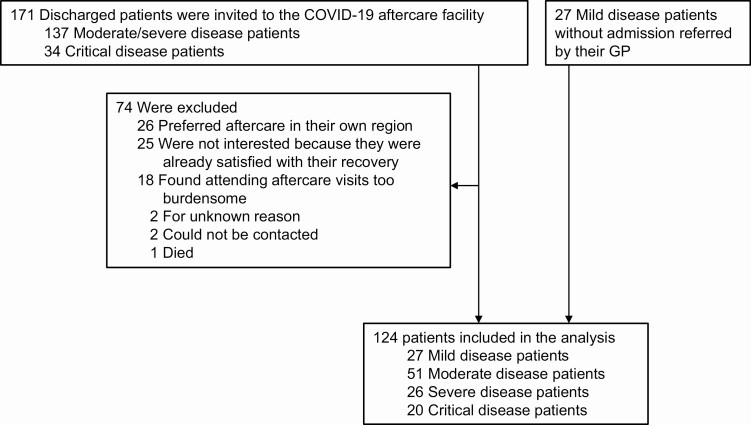
Between 23 April and 15 July 2020, 171 consecutively discharged patients with COVID-19 were invited to the aftercare facility, of whom 97 (57%) attended and were included in the analysis. Reasons for nonattendance are presented in Figure 1. Compared with discharged patients included in the analysis, nonattendees had similar mean age, sex distribution, mean length of stay, and distribution of COVID-19 severity grades (all P > .05; data not shown). All 27 referred patients with mild disease were included. Collectively, this study describes the results of 124 patients.
Figure 1.
Flow chart of patient inclusion. Abbreviations: COVID-19, coronavirus disease 2019; GP, general practitioner.
Table 1 provides demographics, acute COVID-19 characteristics, comorbidities, and the timing of outpatient follow-up. Patients with critical disease were younger compared with patients with moderate-to-severe disease and were predominantly male. Referred patients with mild disease were younger than patients with moderate-to-critical disease and were predominantly female. Increasing COVID-19 severity grade was paralleled by increases in CT severity scores, length of stay, and prevalence of pulmonary embolism. Patients with critical disease had a mean (SD) of 15 (8) intensive care unit (ICU) treatment days. Twenty-nine percent of patients with moderate-to-critical disease had no comorbidities, while this was 78% in referred patients with mild disease. Patients with critical disease had less comorbidity than patients with moderate and severe disease. The assessment was performed at a mean (SD) of 13.0 (2.2) weeks after onset of SARS-CoV-2 symptoms and 9.1 (1.6) weeks after discharge. At follow-up, median CRP, D-dimer, ferritin, and mean leukocyte count showed normal levels in all of the study groups (Supplementary Table 1).
Table 1.
Demographics, Acute COVID-19 Characteristics, Comorbidity, and Timing of Outpatient Follow-up
| Patients With Missing Data, n | All Patients (N = 124) | Patients With Critical Disease (n = 20) (a) | Patients With Severe Disease (n = 26) (b) | Patients With Moderate Disease (n = 51) (c) | Referred Patients With Mild Disease (n = 27) (d) | P | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age, mean (SD), y | 0 | 59 (14) | 57 (10) | 63 (13) | 61 (14) | 52 (14) | .010 b-d, c-d |
| Male sex, n (%) | 0 | 74 (60) | 16 (80) | 19 (73) | 31 (61) | 8 (30) | .001 |
| Social status, n (%) | 0 | ||||||
| Living with spouse | 93 (75) | 17 (85) | 19 (73) | 14 (80) | 16 (59) | .140 | |
| Living alone | 31 (25) | 3 (15) | 7 (27) | 10 (20) | 11 (41) | ||
| Educational level, n (%) | 0 | ||||||
| Low | 30 (24) | 1 (5) | 12 (46) | 14 (28) | 3 (11) | .005 | |
| Middle | 34 (27) | 5 (25) | 5 (19) | 18 (35) | 6 (22) | ||
| High | 60 (48) | 14 (70) | 9 (35) | 19 (37) | 18 (67) | ||
| Employed, n (%) | 0 | 71 (57) | 16 (80) | 11 (42) | 24 (47) | 20 (74) | .008 |
| Smoking status, n (%) | 0 | ||||||
| Current | 2 (2) | 0 (0) | 1 (4) | 0 (0) | 1 (4) | .378 | |
| Former | 74 (60) | 12 (60) | 19 (73) | 30 (41) | 13 (48) | ||
| Never | 48 (39) | 8 (40) | 6 (23) | 21 (41) | 13 (48) | ||
| Acute COVID-19 characteristics | |||||||
| Laboratory-confirmed SARS-CoV-2, n (%) | 4 | 107 (86) | 20 (100) | 24 (92) | 49 (96) | 14 (52) | <.001 |
| Length of stay, median (IQR), days | 0 | 8 (5–14) | 20 (17–32) | 10 (7–10) | 5 (3–9) | NA | <.001 a-b, a-c, b-c |
| CT severity score at admission, mean (SD) | 11 | 13 (5) | 18 (4) | 14 (4) | 10 (5) | NA | <.001 a-b, a-c, b-c |
| Pulmonary embolism, n (%) | 0 | 9 (7) | 6 (30) | 2 (8) | 1 (2) | 0 (0) | <.001 |
| Comorbidity, n (%) | 0 | ||||||
| None, n (%) | 49 (40) | 7 (35) | 5 (19) | 16 (31) | 21 (78) | <.001 | |
| No. of comorbidities, median (IQR) | 1 (0–2) | 1 (0–2) | 2 (1–3) | 1 (0–3) | 0 (0-0) | <.001 a-b, a-d, b-d, c-d | |
| Cardiovascular | 30 (24) | 5 (25) | 9 (35) | 14 (28) | 2 (7) | .109 | |
| Oncologic | 25 (20) | 2 (10) | 12 (46) | 8 (16) | 3 (11) | .003 | |
| Chronic lung disease | 23 (19) | 1 (5) | 9 (35) | 12 (24) | 1 (4) | .008 | |
| Asthma | 12 (10) | 1 (0) | 4 (15) | 7 (14) | 1 (4) | .145 | |
| COPD/emphysema | 7 (6) | 0 (0) | 3 (12) | 4 (8) | 0 (0) | .173 | |
| Other lung disease | 4 (3) | 0 (0) | 2 (8) | 2 (4) | 0 (0) | .348 | |
| Immunocompromised | 18 (15) | 1 (5) | 5 (19) | 12 (24) | 0 (0) | .018 | |
| Hypertension | 34 (28) | 9 (45) | 9 (35) | 13 (26) | 3 (11) | .060 | |
| Diabetes mellitus | 17 (14) | 0 (0) | 7 (27) | 9 (18) | 1 (4) | .018 | |
| Chronic kidney failure | 10 (8) | 0 (0) | 4 (15) | 6 (12) | 0 (0) | .072 | |
| Timing of outpatient follow-up | |||||||
| Time since first SARS-CoV-2 complaints, mean (SD), weeks | 8 | 13,0 (2, 2) | 14,0 (2,0) | 12,8 (1, 2) | 12,0 (2, 1) | 14,7 (2, 2) | <.001 a-b, b-d, c-d |
| Time since discharge, mean (SD), weeks | 0 | 10,0 (1, 7) | 9,0 (1, 6) | 10,2 (1.0) | 10,2 (1, 9) | NA | .037 a-b, a-c |
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CT, computed tomography; IQR, interquartile range; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
apatients with critical disease; bpatients with severe disease; cpatients with moderate disease; dreferred patients with mild disease.
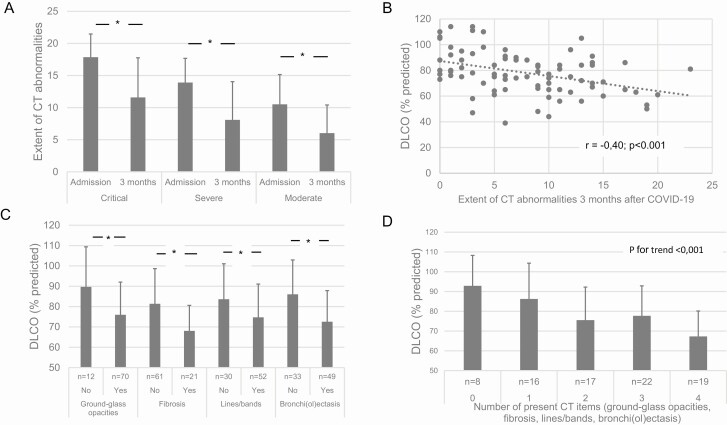
Table 2 provides the data on dyspnea, lung function, and CT imaging at follow-up. Referred patients with mild disease reported higher mMRC than patients with moderate-to-critical disease. Mean (SD) resting oxygen saturation was 96% (1%) and mean spirometric indices were normal across study groups. Mean diffusion capacity of the lungs for carbon monoxide (DLCO) was significantly lower in patients with moderate-to-critical disease compared with referred patients with mild disease who had normal mean DLCO. Patients with critical disease had the lowest mean total lung capacity (TLC) and residual volume. Differences in lung function outcomes were unaffected when the 7 patients with chronic obstructive pulmonary disease/emphysema were excluded (data not shown). Chest X-rays in referred patients with mild disease were normal in 93% of cases; 2 patients (7%) had mild signs of bronchial disease. On follow-up CT imaging of discharged patients, 99% of patients had decreased ground-glass opacities (GGOs); in 1%, GGO was unchanged. Also, the extent of residual pulmonary parenchymal abnormalities was significantly lower compared with at admission (Figure 2A). Nine percent of patients had no residual CT pulmonary abnormalities, while residual GGOs, bronchi(ol)ectasis, lines or bands, and radiological signs of fibrosis were present in 86%, 60%, 64%, and 26% of patients, respectively. No patient had signs of active organizing pneumonia and 1 (moderate disease) patient had minor residual crazy paving. The extent of residual abnormal pulmonary parenchymal involvement was significantly correlated with DLCO (Figure 2B). More specifically, presence and number of residual CT items were associated with lower DLCO (Figure 2C and 2D). The presence of signs of residual fibrosis was also associated with older age (68 [12] vs 59 [12] years; P = .004), lower TLC (91 [13] vs 99 [15] %predicted; P = .031), and more frequent desaturation upon the 6MWT (χ 2 = 4.49; P = .034). Neither individual CT items nor number of present residual CT items nor extent of residual abnormalities were associated with Borg dyspnea scores upon 6MWT or mMRC dyspnea scores (data not shown).
Table 2.
Dyspnea, Pulmonary Function, and Chest Computed Tomography Imaging Results 3 Months After Recovery From Acute COVID-19
| Patients With Missing Data, n | All Patients (N = 124) | Patients With Critical Disease (n = 20) (a) | Patients With Severe Disease (n = 26) (b) | Patients With Moderate Disease (n = 51) (c) | Referred Patients With Mild Disease (n = 27) (d) | P | |
|---|---|---|---|---|---|---|---|
| Dyspnea | |||||||
| mMRC, median (IQR) | 0 | 1 (0–2) | 1 (0–1) | 1 (0–2) | 1 (0–1) | 2 (1–2) | <.001 a-d, c-d |
| Pulmonary function | |||||||
| Resting oxygen saturation, mean (SD), % | 15 | 96 (1) | 96 (1) | 96 (1) | 96 (2) | 97 (1) | .387 |
| VCmax, mean (SD), %predicted | 2 | 99 (16) | 98 (15) | 92 (17) | 102 (18) | 100 (9) | .111 |
| VCmax <LLN, n (%) | 8 (7) | 1 (5) | 2 (8) | 5 (10) | 0 (0) | .378 | |
| FEV1, mean (SD), % predicted | 2 | 97 (16) | 101 (16) | 91 (24) | 97 (19) | 99 (13) | .254 |
| FEV1<LLN, n (%) | 2 | 12 (10) | 1 (5) | 3 (12) | 6 (12) | 2 (7) | .774 |
| FEV1/VCmax, mean (SD), % | 2 | 76 (11) | 81 (6) | 75 (12) | 75 (10) | 76 (16) | .253 |
| FEV1/VCmax<LLN, n (%) | 2 | 13 (11) | 0 (0) | 4 (15) | 6 (12) | 3 (11) | .372 |
| DLCO, mean (SD), %predicted | 2 | 81 (17) | 77 (14) | 75 (17) | 80 (17) | 93 (10) | <.001 a-d, b-d, c-d |
| DLCO <LLN, n (%) | 2 | 41 (34) | 11 (55) | 14 (54) | 16 (33) | 0 (0) | <.001 |
| TLC, mean (SD), % predicted | 2 | 99 (14) | 94 (16) | 95 (14) | 101 (14) | 104 (9) | .013 a-d |
| TLC <LLN, n (%) | 5 | 15 (13) | 4 (20) | 3 (12) | 7 (15) | 1 (4) | .355 |
| RV, mean (SD), % predicted | 5 | 100 (22) | 86 (19) | 101 (25) | 101 (21) | 107 (20) | .009 a-c, a-d |
| RV <LLN, n (%) | 5 | 10 (8) | 2 (15) | 1 (4) | 4 (9) | 2 (7) | .599 |
| Imaging | |||||||
| Available CT at follow-up, n (%) | 13 | 84 (87) | 17 (85) | 22 (85) | 45 (88) | NA | - |
| Extent of residual CT abnormalities, median (IQR), arbitrary unit | 13 | 8 (6) | 12 (6) | 8 (6) | 6 (4) | NA | .019 a-c |
| Type of residual CT abnormalities present, n (%) | 13 | ||||||
| Ground-glass opacity | 73 (86) | 16 (89) | 18 (86) | 39 (85) | NA | .914 | |
| Bronchi(ol)ectasis | 51 (60) | 12 (67) | 10 (48) | 29 (63) | NA | .396 | |
| Lines and bands | 54 (64) | 15 (83) | 13 (62) | 26 (57) | NA | .132 | |
| Fibrosis | 22 (26) | 9 (50) | 5 (24) | 8 (17) | NA | .027 | |
| Number of residual CT abnormalities, n (%) | 13 | ||||||
| 0 | 8 (9) | 2 (10) | 2 (10) | 4 (9) | NA | .267 | |
| 1 | 17 (20) | 0 (0) | 5 (24) | 12 (26) | NA | ||
| 2 | 17 (20) | 3 (17) | 6 (29) | 8 (17) | NA | ||
| 3 | 23 (27) | 6 (33) | 3 (14) | 14 (30) | NA | ||
| 4 | 20 (24) | 7 (39) | 5 (24) | 8 (17) | NA |
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FRC, functional residual capacity; IQR, interquartile range; LLN, lower limit of normal (ie, fifth percentile); mMRC, modified Medical Research Council; NA, not available; RV, residual volume; SD, standard deviation; TLC, total lung capacity; VCmax, maximal vital capacity.
apatients with critical disease; bpatients with severe disease; cpatients with moderate disease; dreferred patients with mild disease.
Figure 2.
A–D, Extent and type of residual pulmonary parenchyma abnormalities 3 months after recovery from acute COVID-19 and association with DLCO. Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; DLCO, lung diffusion capacity of carbon monoxide. *P < .05.
Table 3 provides outcomes on physical function, body composition, and mental and cognitive status. Clinical Frailty Scale outcomes showed, on average, nonfrail scores. Although the mean 6-minute walking distance (6MWD) was normal, 22% of patients had a 6MWD less than 80% of predicted. Sixteen percent of patients desaturated upon the 6MWT, with similar proportions in patients with critical, severe, and moderate disease versus 4% in the referred mild disease group. Desaturators had lower DLCO than nondesaturators (65 [16] vs 85 [14] %predicted; P < .001). Mean FFMI was normal, while 19% of all patients had an FFMI less than the LLN. Abnormal HADS-anxiety, HADS-depression, TICS, CFQ, PCL-5, and IES-R scores were observed in 10%, 12%, 15%, 17%, 7%, and 10% of patients, respectively, with no statistically significant differences between the study groups.
Table 3.
Physical Functioning, Body Composition, and Mental and Cognitive Status 3 Months After Recovery From Acute COVID-19
| Patients With Missing Data, n | All Patients (N = 124) | Patients With Critical Disease (n = 20) | Patients With Severe Disease (n = 26) | Patients With Moderate Disease (n = 51) | Referred Patients With Mild Disease (n = 27) | P | |
|---|---|---|---|---|---|---|---|
| Physical functioning | |||||||
| CFS, n (%) | 3 | ||||||
| Not frail | 104 (84) | 18 (90) | 21 (81) | 43 (84) | 22 (92) | .577 | |
| Somewhat frail | 6 (5) | 0 (0) | 2 (12) | 2 (4) | 1 (4) | ||
| Frail | 11 (9) | 2 (10) | 2 (8) | 6 (12) | 1 (4) | ||
| 6MWD, mean (SD), %predicted | 9 | 92 (18) | 99 (16) | 83 (17) | 91 (17) | 95 (22) | .134 |
| 6MWD <80%predicted, n (%) | 25 (22) | 1 (5) | 8 (32) | 13 (28) | 3 (12) | .068 | |
| Desaturation ≥4% upon 6MWT, n (%) | 14 | 20 (16) | 4 (22) | 4 (17) | 11 (25) | 1 (4) | .194 |
| Body composition | |||||||
| BMI, mean (SD), kg/m2 | 0 | 28.3 (5.4) | 27.2 (3.2) | 29.6 (4.6) | 27.9 (4.8) | 28.8 (7.8) | .387 |
| FFMI, mean (SD), kg/m2 | 7 | 19.4 (2.6) | 19.2 (1.7) | 20.2 (2.3) | 19.4 (2.7) | 18.6 (3.1) | .157 |
| FFMI <LLN, n (%) | 23 (19) | 4 (21) | 7 (27) | 5 (11) | 7 (27) | .260 | |
| Mental and cognitive status, n (%) | |||||||
| HADS-anxiety >10 | 0 | 12 (10) | 2 (10) | 2 (8) | 6 (12) | 2 (7) | .912 |
| HADS-depression >10 | 0 | 14 (12) | 2 (10) | 2 (8) | 4 (8) | 6 (22) | .241 |
| TICS <34 | 0 | 19 (15) | 1 (5) | 6 (23) | 9 (18) | 3 (11) | .330 |
| CFQ >43 | 0 | 21 (17) | 3 (17) | 8 (17) | 6 (12) | 4 (15) | .210 |
| PCL-5 >33 | 0 | 9 (7) | 1 (5) | 3 (12) | 3 (6) | 2 (7) | .800 |
| IES-R >33 | 2 | 12 (10) | 0 (0) | 3 (12) | 7 (14) | 2 (7) | .339 |
| Normal scores on all mental and cognitive status questionnaires | 2 | 79 (64) | 14 (70) | 14 (54) | 35 (69) | 16 (59) | .532 |
Abbreviations: BMI, body mass index; CFQ, Cognitive Failure Questionnaire; CFS, Clinical Frailty Scale; COVID-19, coronavirus disease 2019; FFMI, fat-free mass index; HADS, Hospital Anxiety and Depression Scale; IES-R, Impact of Event Scale–Revisited; LLN, lower limit of normal (ie, fifth percentile); PCL-5, Post-traumatic Stress Checklist According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; SD, standard deviation; TICS, Telephone Interview of Cognitive Status; 6MWD, 6-minute walking distance; 6MWT, 6-minute walking test.
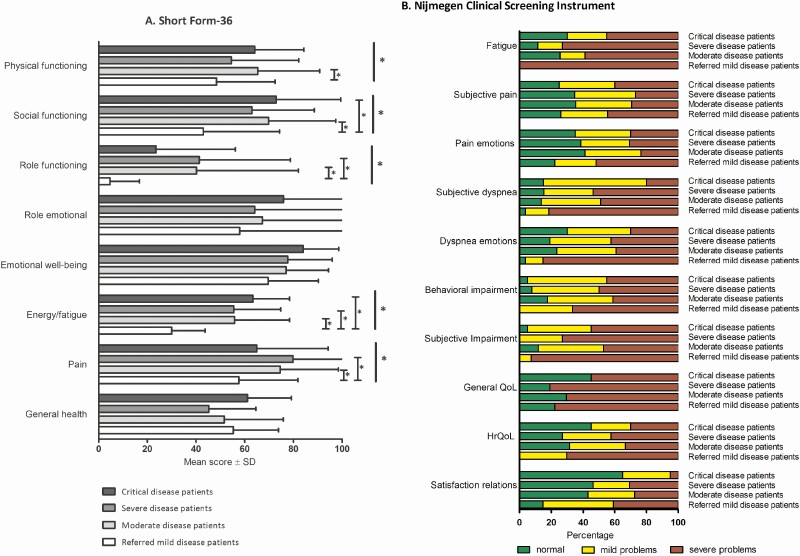
Figure 3 shows health status data. Scores on all domains of the SF-36 were lowered, especially on the domains functioning, energy/fatigue, and general health. Across all NCSI health status domains, substantial proportions of patients reported severe problems. This was most pronounced in the domains fatigue (69%), functional impairments in daily life (64%), and general quality of life (72%). Overall, patients with critical, severe, and moderate disease had comparable scores on the SF-36 and NCSI health domains. Referred patients with mild disease reported significantly worse health status on most subscales of the SF-36 and on the subdomains of the NCSI as compared with discharged patients with moderate-to-critical disease.
Figure 3.
Health status on domains of the SF-36 (A) and on subdomains of the Nijmegen Clinical Screening Instrument (B) 3 months after recovery from acute COVID-19. *P < .05. Abbreviations: COVID-19, coronavirus disease 2019; QoL, quality of life; HrQoL, health-related quality of life; SF-36, Short Form-36.
Care Delivery
In the time between recovery from acute SARS-CoV-2 infection and outpatient assessment day, 26% of the study cohort had received treatment by at least 1 AHCP, in particular patients with critical disease (85%), and most frequently by a physiotherapist (Supplementary Table 2). Following interdisciplinary case discussions, additional diagnostics were indicated in 37% of patients and 85% received self-management advice. Medication advice was given in 20% of patients, and in 46% specific advice was given to the respective GPs. New referrals to AHCPs were indicated in 40% of cases, particularly in referred patients with mild disease. Sixteen percent of patients were referred to another medical specialist, and 4% of the cohort was referred to specialized rehabilitation.
DISCUSSION
We report the outcomes of a comprehensive health assessment in patients 3 months after COVID-19. At this point in time, the pulmonary parenchyma has recovered significantly: 99% of patients had lower GGO density and consistent decreases in the extent of affected pulmonary parenchyma were found across COVID-19 severity grades. Residual pulmonary parenchymal abnormalities, however, were present in more than 90% of discharged patients and correlated with the lower lung diffusion capacity after 3 months. Residual signs suggesting pulmonary fibrosis were found most frequently in those who had critical COVID-19 (50%). This was associated with lower TLC, older age, and exercise-induced desaturation. Our data on decreased DLCO and mildly decreased TLC in recovered patients with COVID-19 are in line with previous observations in SARS-CoV [29] and ARDS [30] survivors and with a previous report in COVID-19 survivors from China [31]. Our data on radiologically assessed pulmonary recovery from COVID-19 are also in line with emerging reports [32, 33]. The study from Liu et al [32] showed that 4 weeks after discharge for moderate COVID-19, significant improvements in the pulmonary parenchyma were observed by CT imaging. Contrary to these authors who found that 65% of patients had full resorption of COVID-19 pulmonary abnormalities after 4 weeks, we found only 9% of our cohort to be free of residual disease after 3 months. Potential explanations are that we included patients with COVID-19 with severe and critical disease in addition to those with moderate disease, and that we used a different CT technique with high-resolution 0.5-mm slides, allowing for more detailed analysis.
Given the level of respiratory insufficiency, relatively long lengths of stay, and mechanical ventilation in those with critical disease (ie, factors known to decrease physical capacity and muscle mass [34]), it was hypothesized that we would find low exercise capacity and FFMI at follow-up in our cohort of patients with COVID-19. This hypothesis was further substantiated by the recent publication in an Italian COVID-19 cohort showing low levels of physical functioning at discharge [35]. However, mean 6MWD and FFMI proved to be normal. We speculate that received treatment and natural recovery in the time between acute infection and outpatient visit contributed to this observation. It should, however, be noted that still 22% of our patients had a low 6MWD and 19% had a low FFMI, calling for continued attention to these potentially modifiable factors.
Approximately one-third of the patients in our cohort had abnormal outcomes on mental status or cognitive function 3 months after COVID-19. This should raise awareness among healthcare professionals in COVID-19 aftercare. Post-traumatic stress syndrome generally develops over time and, from this perspective, formally only can be diagnosed after at least 6 months [36], requiring longer follow-up. A pathophysiological role of inflammation underlying psychological and psychiatric symptoms after COVID-19 has been suggested [37]. Considering our data that COVID-19 severity grade does not appear to be associated with differences in mental or cognitive status, while, in particular, patients with critical disease are characterized by a cytokine storm during the acute moment [38], a multifactorial cause of these phenomena requires more investigation.
Our results indicate that a substantial proportion of patients still experience severe problems in various health domains 3 months after COVID-19. Referred patients with mild disease displayed a female predominance and more frequently severe problems than discharged patients with moderate-to-critical disease, particularly in the domains of physical functioning, fatigue, and quality of life. Since we found no major radiological, lung function, inflammatory, or exercise capacity abnormalities in these referred patients with mild disease after 3 months, explanations for their poor health status remain unclear at this point. Since impaired health status may become chronic as has been found, for example, ARDS [30] and Q fever [5], our observations warrant confirmation in larger cohorts and further long-term investigation.
The strength of our study is the systematic approach and comprehensive assessment of health status in patients with different severity-grade COVID-19, allowing for a broad view on COVID-19 sequelae. In addition to survival bias, several factors should be taken into consideration in the interpretation of the current results. Selection bias has occurred since some patients refused hospitalization or ICU admission, or a non-ICU policy was agreed upon following regular shared decision-making conversations. Results may be influenced by the lack of data on patients who refused assessment because they were already satisfied with their recovery (15%) or found it too burdensome (11%). Also, the referred patients with mild disease included in this analysis form a highly selected subgroup who had health status impairment already for a longer period of time and are therefore not representative of the COVID-19 population with mild disease as a whole. Thirteen (48%) of the patients with COVID-19 with mild disease and 4 (4%) of the patients with moderate-to-critical disease were not laboratory confirmed but fulfilled clinical criteria and epidemiological linkage [39]. Considering that symptomatic patients can become seronegative already in the early convalescent phase [40], we chose to retain these patients with probable COVID-19 in the analysis. Our study is limited by its single-center design and relatively small number of patients. Inherent in studying long-term effects of an acute outbreak such as COVID-19, standardized baseline measurements of health status and physical measures from before the acute phase are lacking. Several factors, however, suggest that COVID-19 was responsible for the current findings. Indeed, 40% had no relevant comorbidity, only 2% were active smokers, and it was clear from the consultations that the majority of patients marked the infection as the initiating cause of their current health problems.
With the SARS-CoV-2 pandemic still ongoing, we are only at the beginning of understanding long-term sequelae of COVID-19. The current observational study described ex–COVID-19 patients at an average of 3 months after the infection, and showed that while the pulmonary parenchyma has markedly recovered, residual abnormalities were frequently present and were associated with lower lung function. Furthermore, a substantial number of patients suffered from severe problems in different health domains, requiring continued attention from healthcare providers. Longer follow-up studies are warranted to elucidate natural trajectories of COVID-19 recovery, to find predictors of complicated long-term trajectories, and to develop strategies to decrease long-term COVID-19 morbidity.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. B. v. d. B., J. B. P., Y. S., H. S., C. P. B.-R., M. H. R., J. V., and M. v. d. H. developed the concept of the study. B. v. d. B., J. B. P., and M. B. were responsible for data curation. B. v. d. B. and J. B. P. analyzed the data. J. V., M. P., and M. v. d. H. provided supervision. B. v. d. B. and J. B. P. wrote the original draft of the manuscript. M. B., Y. S., C. P. B.-R., H. S., H. W. H. v. H., H. v. H., M. v. d. B., H. v. d. H., M. H. R., M. P., J. V., and M. v. d. H. reviewed and edited the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Acknowledgments. The authors are grateful to the following individuals: Alicia Ijssel-de Schepper, Annemieke Koopman, Bas Robberts, Didy Jacobsen, Ed van Mackelenberg, Elke Pothof, Elke Verberkt, Esther van Voorthuizen, Eveline van der Kolk, Grietje Marten-van Stijn, Hanneke van Duijnhoven, Janette Rahamat-Langendoen, Jeanine Antons, Joëlle Bastiaanse, Joke Kalkman, Jolanda van Haren-Willems, Kirsten de Haas, Koen Siefkes, Laura Elbers-van de Ven, Laurens Bisschops, Laurie van Eyndhoven, Lianne Vermeeren, Lieven de Zwart, Maartje Salemans, Marjolein Wolvers, Mirte Simmelink, Patricia Peters-Sanders, Petra Theissen (on behalf of the Radboudumc Pulmonary Function Lab), Roderick Kriekaart, Sigrid Amstelveen-Bokkerink, Sonja Roelofs-Willems, Stephan Keijmel, Susan Lassche, Tiny Fasotti-Dumont, Tjerk Berens, Willem Jan van der Woude, and Wolter Snijders for participation in the COVID-19 aftercare facility; Anouk Stoffels, Arthur Lemson, Bram Geurts, Dennis Bosboom, Liesbeth Peters-Bax, Miranda Snoeren, Olette Berger-Hartog, and Zjala Ebadi for data collection; Astrid van den Beld, Nicole Tijhuis, Noortje van der Burg- van Elk, and Ria Janssen for organizational support; Mariska Klaassen, Petra Koning-Boezeman, and Rienk Elzinga for managerial support.
Potential conflicts of interest. M. B. reports grants and speaker bureau fees from Canon Medical Systems, outside the submitted work. M. P. reports personal fees from Bracco, personal fees from Bayer, personal fees from Siemens Healthineers, personal fees from Canon Medical Systems, grants from Siemens Healthineers, and grants from Canon Medical Systems, outside the submitted work.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 5 November 2020.
- 2.Prescott HC, Girard TD. Recovery from severe COVID-19: leveraging the lessons of survival from sepsis. JAMA 2020; 324:739–40. [DOI] [PubMed] [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40:502–9. [DOI] [PubMed] [Google Scholar]
- 4.Peiris JS, Chu CM, Cheng VC, et al. ; HKU/UCH SARS Study Group . Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003; 361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limonard GJ, Peters JB, Besselink R, et al. Persistence of impaired health status of Q fever patients 4 years after the first Dutch outbreak. Epidemiol Infect 2016; 144:1142–7. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Clinical management of COVID-19. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 12 August 2020.
- 7.Revel MP, Parkar AP, Prosch H, et al. COVID-19 patients and the radiology department—advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol 2020; 30:4903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200:e70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49:1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 10.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26:511–22. [DOI] [PubMed] [Google Scholar]
- 11.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93:580–6. [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–7. [DOI] [PubMed] [Google Scholar]
- 14.Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003; 123:1401–7. [DOI] [PubMed] [Google Scholar]
- 15.Franssen FM, Rutten EP, Groenen MT, Vanfleteren LE, Wouters EF, Spruit MA. New reference values for body composition by bioelectrical impedance analysis in the general population: results from the UK Biobank. J Am Med Dir Assoc 2014; 15:448.e1–6. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–70. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg E, Ruis C, Biessels GJ, Kappelle LJ, van Zandvoort MJ. The Telephone Interview for Cognitive Status (modified): relation with a comprehensive neuropsychological assessment. J Clin Exp Neuropsychol 2012; 34:598–605. [DOI] [PubMed] [Google Scholar]
- 18.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982; 21:1–16. [DOI] [PubMed] [Google Scholar]
- 19.Boeschoten MA, Bakker A, Jongedijk RA, Olff M.. PTSD checklist for DSM-5 and life events checklist for DSM-5 with extended A criterion—Nederlandstalige versie. Diemen, Netherlands: Arq Psychotrauma Expert Group, 2014. [Google Scholar]
- 20.Weis DSM, Marmar CR.. The impact of event scale—revised. In: Wilson JP, TMK, eds. Assessing psychological trauma and PTSD. New York: Guilford Press, 1997: 399–411. [Google Scholar]
- 21.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998; 51:1055–68. [DOI] [PubMed] [Google Scholar]
- 22.Peters JB, Daudey L, Heijdra YF, Molema J, Dekhuijzen PN, Vercoulen JH. Development of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: the Nijmegen Clinical Screening Instrument. Qual Life Res 2009; 18:901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vercoulen JH, Daudey L, Molema J, et al. An integral assessment framework of health status in chronic obstructive pulmonary disease (COPD). Int J Behav Med 2008; 15:263–79. [DOI] [PubMed] [Google Scholar]
- 24.Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 1994; 38:383–92. [DOI] [PubMed] [Google Scholar]
- 25.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19:787–805. [DOI] [PubMed] [Google Scholar]
- 26.Maillé AR, Koning CJ, Zwinderman AH, Willems LN, Dijkman JH, Kaptein AA. The development of the “Quality-of-life for Respiratory Illness Questionnaire (QOL-RIQ)”: a disease-specific quality-of-life questionnaire for patients with mild to moderate chronic non-specific lung disease. Respir Med 1997; 91:297–309. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther 1997; 35:785–91. [DOI] [PubMed] [Google Scholar]
- 28.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess 1985; 49:71–5. [DOI] [PubMed] [Google Scholar]
- 29.Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005; 60:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herridge MS, Cheung AM, Tansey CM, et al. ; Canadian Critical Care Trials Group . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003; 348:683–93. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020; 21:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Ye L, Xia R, et al. Chest CT and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014; 370:1626–35. [DOI] [PubMed] [Google Scholar]
- 35.Belli S, Balbi B, Prince I, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived the hospitalisation. Eur Respir J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 37.Mazza MG, De Lorenzo R, Conte C, et al. ; COVID-19 BioB Outpatient Clinic Study Group . Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 2020; 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol 2020; 39:2085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singhania N, Bansal S, Singhania G. An atypical presentation of novel coronavirus disease 2019 (COVID-19). Am J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.