Abstract
Although fever is one of the main presenting symptoms of COVID-19 infection, little public attention has been given to fever as an evolved defense. Fever, the regulated increase in the body temperature, is part of the evolved systemic reaction to infection known as the acute phase response. The heat of fever augments the performance of immune cells, induces stress on pathogens and infected cells directly, and combines with other stressors to provide a nonspecific immune defense. Observational trials in humans suggest a survival benefit from fever, and randomized trials published before COVID-19 do not support fever reduction in patients with infection. Like public health measures that seem burdensome and excessive, fevers involve costly trade-offs but they can prevent infection from getting out of control. For infections with novel SARS-CoV-2, the precautionary principle applies: unless evidence suggests otherwise, we advise that fever should be allowed to run its course.
Lay summary: For COVID-19, many public health organizations have advised treating fever with medicines such as acetaminophen or ibuprofen. Even though this is a common practice, lowering body temperature has not improved survival in laboratory animals or in patients with infections. Blocking fever can be harmful because fever, along with other sickness symptoms, evolved as a defense against infection. Fever works by causing more damage to pathogens and infected cells than it does to healthy cells in the body. During pandemic COVID-19, the benefits of allowing fever to occur probably outweigh its harms, for individuals and for the public at large.
Keywords: fever, antipyretic, acetaminophen, host defense, COVID-19, sickness behavior
INTRODUCTION
Although fever appears many times during our lives, its utility is often ignored. This is reflected in recent observations concerning COVID-19, caused by the SARS-CoV-2 virus. Several authors have raised and discussed the concern that certain drugs that upregulate ACE2 receptors, such as ACE inhibitors and non-steroidal anti-inflammatory drugs (NSAIDs), might make the infection worse [1–3]. Less attention has been given to the possibility that fever suppression by antipyretics itself carries risks in the setting of an acute infectious illness, such as COVID-19 [4, 5]. Fever is one of the most common presenting symptoms of COVID-19, occurring in approximately 80% of cases [6]. Major health organizations have issued inconsistent and sometimes contradictory guidance for fever treatment during COVID-19. Guidelines have variously advised taking antipyretics for fever reduction [7, 8], recommended antipyretics, e.g. acetaminophen, ‘to feel better’ without mentioning fever [9], provided reassurance that antipyretics, e.g. NSAIDS, are not harmful [7, 10, 11], and recommended antipyretics for fever and other symptoms, but not solely to reduce body temperature [12]. Against this backdrop, a large literature exists on fever and associated symptoms as a defense against infections [13–20], and previous researchers have warned against the routine use of antipyretics during infections [21–27]. Now we find that this concern must be revisited for COVID-19, an often-lethal disease for which we currently have no cure or vaccine.
In this review, we outline the role of fever during infections, and we consider whether fever is beneficial, harmful or neutral to the host and to the pathogen. We review the evidence for and against interventions that lower or raise an infected patient’s temperature. Additionally, we discuss how population-level approaches to fever might affect public health. In three text boxes, we provide more detailed context from an evolutionary medicine viewpoint.
FEVER AND SICKNESS SYMPTOMS
Fever is part of a systemic response to infection known as the acute phase response (Box 1). Fever itself is the controlled elevation of body temperature due to the upward resetting of the hypothalamic thermostat. This distinguishes fever from hyperthermia, e.g. heat stroke, which involves overheating of the body while the temperature set-point remains normal. More rarely, especially in very severe infections, there may be a controlled depression of body temperature, termed anapyrexia [28], to be distinguished from unregulated hypothermia. Fever is perceived as alarming and uncomfortable in early stages since the individual feels cold and may shiver. And fever is uncomfortable when it breaks, since the individual feels uncomfortably warm and may sweat. As part of the acute phase response, fever is almost invariably accompanied by uncomfortable sickness symptoms and behaviors, notably lethargy, depression and aches. Although fever and sickness symptoms/behaviors are regulated in different regions of the brain, both are inhibited by antipyretic drugs (e.g. NSAIDs and acetaminophen) [29, 30]. The NSAIDs, particularly ibuprofen, are considered to have more anti-inflammatory properties than acetaminophen and may carry additional risks of promoting infection [31].
Box 1.
The acute phase response
In addition to the local inflammatory response to infection, the systemic defensive responses to infection are known as the acute phase response [13, 89]. Besides fever, other components include mobilization of leukocytes; production of a variety of protective proteins (acute phase proteins); reduced blood levels of iron, zinc, and manganese; reduced erythrocyte production (beyond simple iron deficiency); reduced appetite (anorexia); breakdown of muscle protein and fat (cachexia or hypercatabolism); and the uncomfortable, motivation-sapping sickness symptoms and behaviors we associate with infection, including lethargy, depression and aches. The acute phase response is induced and regulated by the infected individual’s own pro-inflammatory cytokines and other mediators acting on specific cell receptors. Because these responses are initiated by the host, not by the pathogen, and because they are evolutionarily conserved—appearing in all vertebrates and many invertebrates [32, 33]—the acute phase response is considered an adaptive non-specific response to infection.
While some components of the acute phase response are generally accepted to be beneficial, other acute phase responses—lassitude, anorexia and cachexia—can seem more harmful than beneficial and their function has been debated [16, 18, 90–92]. Each of the components of the acute phase response involves either self-harm or the expenditure of limited resources. This includes manufacturing acute phase proteins and supporting an increased metabolic rate. Indeed, in humans, a 2°C higher febrile temperature uses about 20% more energy than that used at normal temperature [17, 93].
The most widely-cited explanation for these elements of the acute phase response was proposed by Hart [16] and extended by Straub et al. [92]. This hypothesis centers around the need to conserve resources and to reallocate energy resources towards supporting an effective immune defense. Resources are conserved by restricting less essential activities and not foraging for food. Another hypothesis is that replicating pathogens can be especially vulnerable to many of the harmful components of the acute phase response, so that the harm involved is directed more to pathogens than to the host [18, 49]. In this view, reduced appetite is a nutritional strategy that disproportionately starves pathogens of energy and micronutrients. A recently proposed additional hypothesis views sickness behavior as an evolved defense that primarily benefits close relatives. In this view, termed the ‘inclusive behavioral immune system’, social withdrawal and self-isolation prevent infection from spreading to relatives who share genes with an infected individual [91, 94].
HOW FEVER WORKS—EXPERIMENTS AND THEORY
Fever is widespread throughout the animal kingdom. In addition to the internally generated fevers of mammals and birds, many invertebrates and poikilothermic vertebrates have been observed to raise their body temperature behaviorally, although baseline and febrile temperatures vary substantially depending on the species [32, 33]. Likewise, pathogens are expected to be adapted to the temperature of their host [20], and it has been noted that most fungi are precluded from infecting mammals and birds due to high endothermic temperatures [34]. The subsequent discussion of specific temperatures is primarily directed at mammalian fever with the recognition that many of the principles could apply more widely to other species and their pathogens.
Fever’s ability to protect against infection has been well established through numerous in vitro and in vivo experiments and has been extensively reviewed [13, 14, 16, 17, 23]. A variety of infection-fighting mechanisms have been proposed for fever, though the relative importance of each mechanism remains to be established. Febrile temperatures enhance a variety of immune cells functions, reviewed in [13] and [14]. These include motility, phagocytosis and reactive oxygen species production by neutrophils and monocytes, as well as enhanced function of natural killer cells, dendritic cells, T-helper cells and antibody-producing cells. Febrile temperatures increase type I interferon responses [35], notable here because interferons have antiviral activities, and reduced type I interferon activity is associated with severe COVID-19 disease [36]. In addition, fever can induce heat shock proteins in both pathogens and host cells, resulting in downstream induction of adaptive and innate components of the host immune response [17, 37]. Fever also increases the vulnerability of rapidly dividing pathogens to destruction, acting in concert with other stresses such as iron deprivation [38] and the effects of antibiotics [39]. Very elevated temperatures (e.g. 42°C) are synergistic with reduced pH and hypoxia in killing of mammalian cells [40]. The combined actions of heat-enhanced performance of immune cells plus heat-induced stress of pathogens (including infected cells), along with other stressors, highlight fever’s multilayered defense likely being greater than the sum of its parts.
Clint and Fessler [20] pointed out another theoretical benefit to fever. They note that ‘pathogen strains that are more fever-tolerant are consistently outcompeted by strains that are better optimized for normal body temperature and are thus more successful at infecting non-febrile hosts’. They propose that fever is beneficial in defense by widening the differences between the pathogen life-history trade-offs between growth in infected hosts versus transmission to new hosts. Because fever-adapted pathogens pay a fitness cost at normal host body temperature, between-strain competition may explain the wide phylogenetic distribution of fever and its evolutionary persistence as a host strategy.
WHY PEOPLE TAKE ANTIPYRETICS
Antipyretics are taken by some people in order to treat sickness symptoms, such as headache, malaise and muscle aches. Others take antipyretics out of concern that the high temperature itself is harmful. Patients can misunderstand the source of fever, believing that it is caused directly by the infection, instead of the body’s response to infection. Some patients and medical professionals consider fever to be dangerous and take or prescribe antipyretics to return the body temperature to normal. The excessive fear of fever has been termed fever phobia [41, 42]. Parents in particular are often anxious that their children’s high temperature will cause a seizure. Febrile seizures are frightening events that occur mostly in children younger than 6 years of age. Fortunately, most common febrile seizures are harmless and leave no neurological sequelae [43, 44]. In addition, there is no evidence that taking an antipyretic will prevent the occurrence of a febrile seizure [43].
Because of the ubiquity of prescribed and over-the-counter NSAIDs and acetaminophen, these drugs may be assumed to be benign. In many cases, blocking fever and sickness symptoms is inconsequential because most infections are self-limiting, as is typical for the common cold. For potentially lethal infections like COVID-19, interfering with evolved defenses may be more problematic.
THE BENEFITS AND COSTS OF FEVER
Historically, fever has been used therapeutically in ways that highlight its potential benefits and costs. Before the advent of antibiotics, severe neurosyphilis was successfully treated by infecting patients with Plasmodium vivix malaria to induce fever [45, 46]. In similar fashion, there are reports of certain cases of cancer treated successfully with live or killed bacteria that stimulate fever, a therapy once known as Coley’s toxin [47, 48]. These therapies carry risks, and like many interventions, they can be dangerous. Evolved host defenses like fever involve costs and risks also, but those risks must be taken in context.
Somewhat counterintuitively, the harms of fever may be central to its success as a defensive strategy [49]. Analogous to the risks of chemotherapy against rapidly replicating cancer cells, the body induces harm to both self and pathogen, gambling that it can endure those costs more than the pathogens (or pathogen-containing cells). This gamble, termed ‘immune brinksmanship’, [18, 49] has typically paid off during evolution for humans and other animals, supported by the ubiquity of fever and the acute phase response across much of the animal kingdom. This viewpoint implies that treatments that reduce fever and sickness symptoms might make a patient feel better at the cost of long-evolved defense and potentially worsen outcomes during infections.
ASSOCIATION OF FEVER WITH FAVORABLE OUTCOMES—OBSERVATIONAL STUDIES
Building on previous theoretical and basic scientific research on fever, recent observational trials in humans have examined the use of antipyretics and fever on disease outcomes. Inhibition of fever with acetaminophen has been linked with delayed recovery, including from chickenpox and malaria [50, 51]. The use of NSAIDs has been linked with complications, including empyema and prolonged hospitalization, in children and adults with lower respiratory tract infections, reviewed in [52]. Potentially beneficial effects of fever have also been reported in observational trials. In a prospective trial that examined the effect of mitochondrial genetic variants in patients with sepsis, the mitochondrial haplotype H was linked with higher febrile temperatures and improved survival; overall, the best survival occurred in those with the highest core temperature within the first 24 h [53]. In two studies of sepsis and severe infection in Sweden and Denmark, each involving more than 2000 patients, fever was associated with lower mortality, and those with the highest body temperatures had the best survival [54, 55]. Young et al. [56] showed that lower temperatures were associated with higher mortality among a cohort of 269,078 ICU patients with infection in New Zealand and Australia, and the same result was seen in a cohort numbering 366, 973 in the UK. In a recent observational study of patients with COVID-19 pneumonia, it was found that having a fever (≥39°C) was associated with better survival [57], although another study involving COVID-19 showed a positive association between fever and more severe cases [58].
FEVER SUPPRESSION DOES NOT IMPROVE OUTCOMES—HUMAN INTERVENTIONAL STUDIES
Evidence from randomized controlled trials suggests that intervening to reduce fever does not improve patients’ survival. In the HEAT trial, Young et al. [59] randomized critically ill patients to fever suppression with acetaminophen versus a group with fever but not receiving acetaminophen. No improvement in survival was seen in the acetaminophen group, but no clear harm was seen either. In a randomized controlled trial in ICU patients, ibuprofen did not improve survival [60]. Some human trials have shown harm from reducing the body temperature lower than normal in critically ill patients with infection. In 2013, a randomized, although not blinded, trial using chilled intravenous saline to induce hypothermia in patients with meningitis was stopped early after an interim review revealed increased mortality in the intravenous cooling group [61]. A more recent trial studied mechanical cooling of critically ill patients with septic shock. This trial randomized patients to induced hypothermia using external chilled circulating water, compared to those receiving usual care, which included some medications such as acetaminophen. The trial was also terminated early after enrolling 436 of a planned 560 patients; interim analysis pointed to higher mortality in the mechanical cooling group [62]. A meta-analysis of randomized controlled trials comparing aggressive treatment of fever versus usual care found no survival benefit from aggressive fever reduction [63]. Even medically fragile patients, including those with heart disease and limited physiological reserves, did not benefit from intensive efforts to reduce body temperature [63]. Taken together, these studies suggest that survival outcomes of patients with infection are not improved by interventions used to lower body temperature and suppress fever. On the contrary, randomized trials of body temperature reduction using intravenous or mechanical cooling have shown a signal of harm. These results are in line with increased mortality seen in observational trials [56] and suggest that interfering with the physiologic set point for body temperature can have unintended consequences.
THE EFFECT OF ANTIPYRETICS ON ANTIBODY RESPONSES TO INFECTION OR VACCINES
When given with vaccinations, antipyretics have been observed to decrease post-vaccination muscle soreness and fever. Concerns have been raised that antipyretics might simultaneously reduce the production of antibodies after vaccinations and might have a similar deleterious effect during infections. In a small study of rhinovirus-infected volunteers, aspirin and acetaminophen both suppressed antibody responses [64]. Several large vaccine trials have reported statistically significant reductions in antibody levels when antipyretics were used at the time of vaccination [65–68]. In contrast, several studies have reported no effect of antipyretics on antibody production [69–71]. When it occurs, the mechanism for inhibited antibody responses involves the enzyme cyclooxygenase-2, which is upregulated by antibody-producing B cells [72] and inhibited by NSAIDs and acetaminophen [73]. The potential for NSAIDs and acetaminophen to reduce protective antibodies will remain a concern for COVID-19 infection and for any future vaccines for this disease.
PUBLIC HEALTH CONSIDERATIONS
We see three important public health considerations relating to inhibition of fever and sickness symptoms. First, fever is used to monitor and restrict the spread of epidemics in the population (e.g. screening for entry into buildings) and for monitoring recovery status of patients. By masking high temperatures, antipyretics reduce the effectiveness of these public health measures. Second, if people feel fine after taking antipyretics while still shedding virus, they are a threat for spreading the infection [74]. In discussing the possibility of asymptomatic transmission in COVID-19, Han and Yang [75] noted a published report of asymptomatic spread of the virus from what was thought to be the index case in Germany. In fact, the index case had taken an antipyretic for a fever, highlighting the potential for these medications to make the disease more difficult to detect. Third and most important, there is real concern about patient morbidity and mortality due to abrogation of host defenses, which remain inadequately understood. The protective value of fever may be slight but crucial for individual patients. In a pandemic involving millions, even a modest protective effect can affect large numbers of patients.
EARLY DEFENSE TO ‘FLATTEN THE CURVE’ IN THE INFECTED INDIVIDUAL
Analogous to epidemics affecting populations, early-stage infections within individuals exhibit exponential growth of pathogens and/or pathogen-infected cells (Table 1). As we have recently learned, timing is everything when introducing control measures in a pandemic. Early interventions have a disproportionate effect on exponential growth and outcomes. Early initiation of host defenses in individuals is similarly important. At the early part of the pathogen growth curve, the benefit of control measures may not be apparent to the observer and the costs may seem excessive. Just as with an epidemic, the question arises for an individual’s immune defenses: ‘how much of a threat exists and how vigorous and costly should the response be?’ While there are costs in overreacting, an absent or insufficient immune response to infection can be far more costly to the individual [76].
Table 1.
Similarities between epidemics and individual infections
| Features in common |
|---|
| Exponential increase of cases (epidemics) and of pathogens (infections) |
| Early intervention is best |
| Early control efforts can seem excessive and unwarranted |
| Failure of early control greatly increases costs (both due to the infections/pathogens themselves and due to the control measures) |
| Terminating control efforts early leads to setbacks |
| Complete eradication of the disease can be costly |
The current COVID-19 pandemic has increased awareness of basic epidemiologic principles and the concept of ‘flattening’ the exponential growth curve. By slowing the spread of infection, the increasingly and overwhelmingly rapid spread is avoided, potentially ending the epidemic or at least gaining valuable time to develop sophisticated defenses and vaccines. Likewise for flattening the curve in newly infected individuals. Defenses like fever that are deployed early have the potential to favorably alter the trajectory of infection, providing time until adaptive immune defenses (e.g. cytotoxic T lymphocytes and antibodies) can respond. Much attention has been devoted to the difficulty of treating late-stage COVID-19 patients. In these patients, early-stage immune defenses may have been inadequate to control the infection [36], resulting in later defenses that can be harmful themselves [77, 78]. With COVID-19, Shi et al. [79] suggest that stronger early immune efforts are key to avoiding overwhelming late-stage disease. Similarly, fever and the acute phase response may prevent late sequelae of infection.
PERMISSIVE APPROACHES TO ABNORMAL PATIENT PARAMETERS
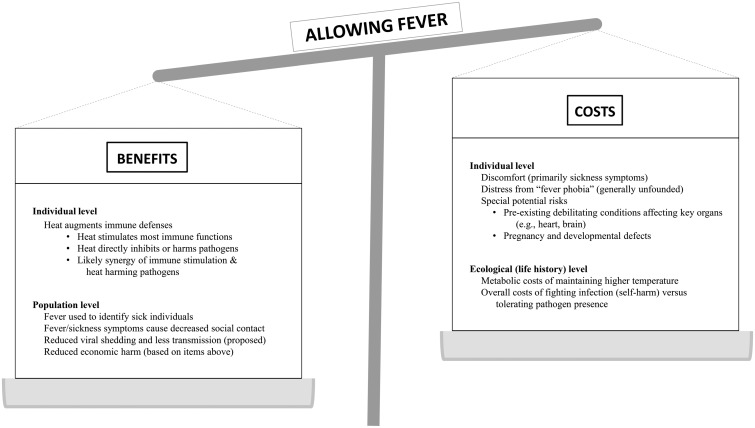
We have discussed the benefits and costs of allowing fever to occur (Fig. 1). We note that with technological advances it is possible and therapeutically tempting to ‘correct’ abnormal patient parameters all types. Several decades of intensive care experience have shown that many aggressive interventions aimed at restoring homeostasis do not improve outcomes [80, 81]. Reduced intensity of treatment has produced equivalent or better patient outcomes in a variety of clinical settings, including mechanically ventilated patients receiving lower than normal tidal volumes [82], avoiding supplemental oxygen in patients with acute myocardial infarctions [83], less aggressive nutritional supplementation in patients receiving intensive care [84], and abandoning early goal-directed therapy in sepsis [85]. The risks of enhancing infection by restoring blood iron levels to ‘normal’ when the levels have been reduced by the anemia of inflammation has long been known [86]. Similarly, aggressive measures to normalize body temperature, compared to usual care, have not improved outcomes in febrile patients [63]. Even usual care, the typical treatment of fever with NSAIDs and acetaminophen, is not evidence-based, raising questions for infection outcomes that could be answered by new research. Whether to raise temperatures therapeutically is another unanswered question. Subnormal temperatures of severely septic patients are often therapeutically raised towards ‘normal’, but often anapyrexia is self-limiting, and it is possible that transient anapyrexia may function to mitigate the downstream costs of a vigorous immune response [87].
Figure 1.
Letting fever run its course—benefits and risks
CONCLUSIONS
People with infections have recurring concerns and seek medical guidance for fever, and also for aches and pains, depression, fatigue, and reduced appetite. We note the curious situation that biomedical science can promptly determine the exact genotype of infectious agents through advances in molecular biology, but we are still in the dark regarding our most ancient evolved responses to infection. Since there is no currently accepted understanding of how the numerous seemingly harmful components of the acute phase response function together in defense, this should be a long-term research priority. More urgently, this pandemic is an opportunity to undertake a randomized controlled study on the effect of antipyretics on COVID-19 outcomes. Until that time, medical decision making should be guided by the precautionary principle to take reasonable steps to reduce risk to patients [88]. Practitioners should consider the likely protective role of fever, weighed against the need to treat pain and discomfort. Especially with COVID-19, in which we are almost exclusively relying on intrinsic host immune defenses to resolve the infection, we propose erring on the side of not intervening with anti-pathogen defenses like fever. In the absence of evidence definitively showing their harm in specific situations, it would be prudent in a life-threatening infection to take advantage of all of our evolved defenses.
Box 2.
What temperature do pathogens actually experience?
Many potential pathogens can survive and function over a wide range of temperatures cooler than their optimum. Temperatures that are slightly higher than the optimum can damage proteins (including enzyme function), membrane lipids, and RNA and disrupt DNA synthesis in the cells of both hosts and pathogens [20, 95, 96]. For cultured mammalian cells without preconditioning, a sharp increase in lethality occurs above 43°C, with mitosis and the S-phase of the cell cycle being most sensitive [96, 97].
However, one of the concerns about the efficacy of fever in harming pathogens is that febrile temperature (i.e. core body temperature) rarely exceeds 40–41°C in humans, if that. So how can the heat of fever be expected to harm or even kill most host-adapted pathogens [95]? And furthermore, why should we expect that the heat will harm the pathogens (including virally infected cells) more than the host [18]?
The temperature to which pathogens at the infected site are actually exposed is currently unknown [18, 98]. However, it is almost certainly higher than that of the blood entering the infected site since heat is generated at inflammatory foci. For example, studies assessing the temperature of inflamed atherosclerotic plaques have found temperatures up to 2°C higher than core temperature [99, 100]. It has been proposed that one source of this heat is from the macrophages in these inflamed plaques that have upregulated levels of mitochondrial uncoupling protein 2, which generates heat rather than ATP [101]. Neutrophils activated to undergo the respiratory burst (as with phagocytosis) generate substantial heat [102–104], as expected from the oxidative reactions that produce reactive oxygen species. Likewise, blood mononuclear cells activated by interleukin-2 or interferon-γ rapidly generate heat [105]. That high temperatures can be generated very locally was revealed when mitochondrial temperatures were found to be as high as 50°C as measured by temperature-sensitive mitochondrial dyes [106].
LeGrand and Day [18] proposed that since growth and replication are universally sensitive to disruption by stressors of any kind, replicating pathogens (and infected cells generating pathogens) localized at the infected site would tend to be more vulnerable to heat stress than non-replicating host cells (stromal cells or infiltrating effector immune cells). Immune cells recruited to the site of infection (e.g. neutrophils, macrophages and lymphocytes) are routinely exposed to the high temperatures at infected sites (and themselves participate in heat generation). Therefore, it is not surprising that the optimal functional temperature of activated leukocytes is higher than normal core body temperature. In this view, fever provides a crucial temperature boost to locally warmed tissues at infected sites, elevating the temperature to the level that damages pathogens. Additionally, the systemic febrile temperature may impair replication of pathogens that have spread. This is analogous to iron deprivation as a host defense during infection: systemically iron is mildly restricted, but locally at the infected site it is much more restricted, and limited even further within phagolysosomes [18].
Box 3.
The trade-offs of immune brinksmanship
All immune defenses involve important costs and benefits. The term ‘immune brinksmanship’ was coined as a metaphor for the trade-off that the host faces between self-directed costs of immunity and harm directed towards pathogenic microbes and infected host cells [49]. The benefits of the acute phase response typically outweigh its costs, because fever and other nonspecific stressors exploit the vulnerabilities of rapidly dividing pathogens [49]. As a result, costs are preferentially imposed on pathogens instead of healthy host cells. Some pathogens have counteradaptations that protect against acute phase response stresses, but these impose trade-offs themselves for pathogens. Microbe-derived heat shock proteins, for instance, impair pathogen replication and trigger additional immune responses from the host [37].
LeGrand and Alcock [49] identified a number of conditions where immune brinksmanship may be a losing strategy for an infected host. One example is having insufficient metabolic, nutritional or physiologic reserves needed to survive the stress. Having comorbidities, such as heart failure or impaired lung function, also reduce the potential payoff. Other threats to the host, such as having co-infection with another pathogen or a recent previous infection, also decrease the odds of success. Other costs specific to fever include harming tissues with rapid growth, such as during spermatogenesis in the testes [107] and embryonic and fetal development [108]. Additionally, some specialized pathogens may be relatively resistant to stresses imposed by the host.
Old age is a risk factor for respiratory failure and death in COVID-19, and in some of these cases, the costs of the immune response may exceed its benefits [109]. Proximate explanations include attenuated immune responses in the aged (immunosenescence) [110] or excessive innate immune activation (inflammaging) [111]. We note that impaired infection control may impose additional immune costs, taking the patient to the brink with potentially lethal self-harm. Also, because SARS-CoV-2 is a novel virus, some lethal cases may occur because humans have had insufficient time to evolve optimal immune responses to it.
FUNDING
This work was supported by the Polish Minister of Science and Higher Education under the program “Regional Initiative of Excellence” in 2019–2022 (grant no. 008/RID/2018/19).
Conflict of interest: None declared.
REFERENCES
- 1. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michaud V, Deodhar M, Arwood M. et al. ACE2 as a therapeutic target for COVID-19; its role in infectious processes and regulation by modulators of the RAAS system. J Clin Med 2020;9:2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Datta PK, Liu F, Fischer T. et al. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics 2020;10:7448–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmitt BD, Offit PA.. Could fever improve COVID-19 outcomes? Contemp Pediatrics 2020;37:8–9. [Google Scholar]
- 5. Steiner AA. Should We Let Fever Run Its Course in the Early Stages of COVID-19? https://deepblue.lib.umich.edu/handle/2027.42/154746 (12 August 2020, date last accessed). [DOI] [PMC free article] [PubMed]
- 6. Zhu J, Ji P, Pang J. et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol 2020;92:1902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Clinical Management of Severe Acute Respiratory Infection When COVID-19 is Suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (17 October 2020, date last accessed).
- 8. National Health Service. Fever in Adults. 2020. https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/fever-in-adults (17 October 2020, date last accessed).
- 9. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Discontinuation of Transmission-Based Precautions and Disposition of Patients with COVID-19 in Healthcare Settings (Interim Guidance). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html (17 October 2020, date last accessed).
- 10. European Medicines Agency. EMA Gives Advice on the Use of Non-Steroidal Anti-Inflammatories for COVID-19. 2020. https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19 (17 October 2020, date last accessed).
- 11. Food and Drug Administration. FDA Advises Patients on Use of Non-Steroidal anti-Inflammatory Drugs (NSAIDs) for COVID-19. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19 (17 October 2020, date last accessed).
- 12. National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing Symptoms (Including at the End of Life) in the Community. 2020. https://www.nice.org.uk/guidance/ng163 (17 October 2020, date last accessed). [PubMed]
- 13. Blatteis CM. Fever: pathological or physiological, injurious or beneficial? J Therm Biol 2003;28:1–13. [Google Scholar]
- 14. Evans SS, Repasky EA, Fisher DT.. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015;15:335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harden LM, Kent S, Pittman QJ. et al. Fever and sickness behavior: friend or foe? Brain Behav Immun 2015;50:322–33. [DOI] [PubMed] [Google Scholar]
- 16. Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 1988;12:123–37. [DOI] [PubMed] [Google Scholar]
- 17. Hasday JD, Thompson C, Singh IS.. Fever, immunity, and molecular adaptations. Compr Physiol 2014;4:109–48. [DOI] [PubMed] [Google Scholar]
- 18. LeGrand EK, Day JD.. Self-harm to preferentially harm the pathogens within: non-specific stressors in innate immunity. Proc R Soc B 2016;283:20160266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shephard AM, Bharwani A, Durisko Z. et al. Reverse engineering the febrile system. Q Rev Biol 2016;91:419–57. [DOI] [PubMed] [Google Scholar]
- 20. Clint E, Fessler DMT.. Insurmountable heat: the evolution and persistence of defensive hyperthermia. Q Rev Biol 2016;91:25–46. [DOI] [PubMed] [Google Scholar]
- 21. Berlim MT, Abeche AM.. Evolutionary approach to medicine. South Med J 2001;94:26–32. [PubMed] [Google Scholar]
- 22. Cunha BA. Fever myths and misconceptions: the beneficial effects of fever as a critical component of host defenses against infection. Heart Lung 2012;41:99–101; author reply 99. [DOI] [PubMed] [Google Scholar]
- 23. Kluger MJ, Kozak W, Conn CA. et al. The adaptive value of fever. Infect Dis Clin North Am 1996;10:1–20. [DOI] [PubMed] [Google Scholar]
- 24. Knoebel EE, Narang AS, Ey JL.. Fever: to treat or not to treat. Clin Pediatr (Phila) 2002;41:9–16. [DOI] [PubMed] [Google Scholar]
- 25. Purssell E. Symptoms in the host: infection and treatment model. J Clin Nurs 2005;14:555–61. [DOI] [PubMed] [Google Scholar]
- 26. Wrotek S, Kamecki K, Kwiatkowski S. et al. Cancer patients report a history of fewer fevers during infections than healthy controls. J Pre Clin Clin Res 2009;3:31–5. [Google Scholar]
- 27. Young PJ, Saxena MK, Beasley RW.. Fever and antipyresis in infection. Med J Aust 2011;195:458–9. [DOI] [PubMed] [Google Scholar]
- 28. Mercer JB. The glossary of terms for thermal physiology. Third edition. Revised by the commission for thermal physiology of the International Union of Physiological Sciences (IUPS thermal commission). Jpn J Physiol 2001;51:245–88. [PubMed] [Google Scholar]
- 29. Corrard F, Copin C, Wollner A. et al. Sickness behavior in feverish children is independent of the severity of fever. An observational, multicenter study. PLoS One 2017;12:e0171670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev 2018;98:477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Micallef J, Soeiro T, Jonville-Béra A-P.. Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie 2020;75:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gray DA, Marais M, Maloney SK.. A review of the physiology of fever in birds. J Comp Physiol B Biochem Syst Environ Physiol 2013;183:297–312. [DOI] [PubMed] [Google Scholar]
- 33. Żbikowska E, Wrotek S, Cichy A. et al. Thermal preferences of wintering snails Planorbarius corneus (L.) exposed to lipopolysaccharide and Zymosan. J Invertebr Pathol 2013;112:57–61. [DOI] [PubMed] [Google Scholar]
- 34. Robert VA, Casadevall A.. Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis 2009;200:1623–6. [DOI] [PubMed] [Google Scholar]
- 35. Lane WC, Dunn MD, Gardner CL. et al. The efficacy of the interferon alpha/beta response versus arboviruses is temperature dependent. mBio 2018;9:e00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hadjadj J, Yatim N, Barnabei L. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020;369:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2002;2:185–94. [DOI] [PubMed] [Google Scholar]
- 38. Kluger MJ, Rothenburg BA.. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science 1979;203:374–6. [DOI] [PubMed] [Google Scholar]
- 39. Mackowiak PA, Marling-Cason M, Cohen RL.. Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis 1982;145:550–3. [DOI] [PubMed] [Google Scholar]
- 40. Gerweck LE, Nygaard TG, Burlett M.. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res 1979;39:966–72. [PubMed] [Google Scholar]
- 41. Bertille N, Purssell E, Corrard F. et al. Fever phobia 35 years later: did we fail? Acta Paediatr 2016;105:9–10. [DOI] [PubMed] [Google Scholar]
- 42. Clericetti CM, Milani GP, Bianchetti MG. et al. Systematic review finds that fever phobia is a worldwide issue among caregivers and healthcare providers. Acta Paediatr 2019;108:1393–7. [DOI] [PubMed] [Google Scholar]
- 43. Offringa M, Newton R, Cozijnsen MA. et al. Prophylactic drug management for febrile seizures in children. Cochrane Database Syst Rev 2017;2:CD003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verity CM. Do seizures damage the brain? The epidemiological evidence. Arch Dis Child 1998;78:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Freitas DRC, Santos JB, Castro CN.. Healing with malaria: a brief historical review of malariotherapy for neurosyphilis, mental disorders and other infectious diseases. Rev Soc Bras Med Trop 2014;47:260–1. [DOI] [PubMed] [Google Scholar]
- 46. Vogel G. Malaria as lifesaving therapy. Science 2013;342:686. [DOI] [PubMed] [Google Scholar]
- 47. Vernon LF. William Bradley Coley, MD, and the phenomenon of spontaneous regression. Immunotargets Ther 2018;7:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wrotek S, Brycht Ł, Wrotek W. et al. Fever as a factor contributing to long-term survival in a patient with metastatic melanoma: a case report. Complement Ther Med 2018;38:7–10. [DOI] [PubMed] [Google Scholar]
- 49. LeGrand EK, Alcock J.. Turning up the heat: immune brinksmanship in the acute-phase response. Q Rev Biol 2012;87:3–18. [DOI] [PubMed] [Google Scholar]
- 50. Brandts CH, Ndjavé M, Graninger W. et al. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet 1997;350:704–9. [DOI] [PubMed] [Google Scholar]
- 51. Doran TF, De Angelis C, Baumgardner RA. et al. Acetaminophen: more harm than good for chickenpox? J Pediatr 1989;114:1045–8. [DOI] [PubMed] [Google Scholar]
- 52. Voiriot G, Philippot Q, Elabbadi A. et al. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J Clin Med 2019;8:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baudouin SV, Saunders D, Tiangyou W. et al. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet 2005;366:2118–21. [DOI] [PubMed] [Google Scholar]
- 54. Henriksen DP, Havshøj U, Pedersen PB. et al. Hospitalized acute patients with fever and severe infection have lower mortality than patients with hypo- or normothermia: a follow-up study. QJM-Mon J Assoc Phys 2016;109:473–9. [DOI] [PubMed] [Google Scholar]
- 55. Sundén-Cullberg J, Rylance R, Svefors J. et al. Fever in the emergency department predicts survival of patients with severe sepsis and septic shock admitted to the ICU. Crit Care Med 2017;45:591–9. [DOI] [PubMed] [Google Scholar]
- 56. Young PJ, Saxena M, Beasley R. et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med 2012;38:437–44. [DOI] [PubMed] [Google Scholar]
- 57. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang G, Zhang Q, Wu C. et al. Clinical characteristics of adult fevered COVID-19 patients and predictors for developing severe events. Front Med 2020;7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Young P, Saxena M, Bellomo R. et al. Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med 2015;373:2215–24. [DOI] [PubMed] [Google Scholar]
- 60. Bernard GR, Wheeler AP, Russell JA. et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The ibuprofen in Sepsis Study Group. N Engl J Med 1997;336:912–8. [DOI] [PubMed] [Google Scholar]
- 61. Mourvillier B, Tubach F, van de Beek D. et al. Induced hypothermia in severe bacterial meningitis: a randomized clinical trial. JAMA 2013;310:2174–83. [DOI] [PubMed] [Google Scholar]
- 62. Itenov TS, Johansen ME, Bestle M. et al. Induced hypothermia in patients with septic shock and respiratory failure (CASS): a randomised, controlled, open-label trial. Lancet Respir Med 2018;6:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Young PJ, Bellomo R, Bernard GR. et al. Fever control in critically ill adults. An individual patient data meta-analysis of randomised controlled trials. Intensive Care Med 2019;45:468–76. [DOI] [PubMed] [Google Scholar]
- 64. Graham NM, Burrell CJ, Douglas RM. et al. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis 1990;162:1277–82. [DOI] [PubMed] [Google Scholar]
- 65. Doedée AMCM, Boland GJ, Pennings JLA. et al. Effects of prophylactic and therapeutic paracetamol treatment during vaccination on hepatitis B antibody levels in adults: two open-label, randomized controlled trials. PLoS One 2014;9:e98175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li-Kim-Moy J, Wood N, Jones C. et al. Impact of fever and antipyretic use on influenza vaccine immune reponses in children. Pediatr Infect Dis J 2018;37:971–5. [DOI] [PubMed] [Google Scholar]
- 67. Prymula R, Siegrist C-A, Chlibek R. et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 2009;374:1339–50. [DOI] [PubMed] [Google Scholar]
- 68. Wysocki J, Center KJ, Brzostek J. et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine 2017;35:1926–35. [DOI] [PubMed] [Google Scholar]
- 69. Prymula R, Esposito S, Zuccotti GV. et al. A phase 2 randomized controlled trial of a multicomponent meningococcal serogroup B vaccine (I). Hum Vaccin Immunother 2014;10:1993–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sil A, Ravi MD, Patnaik BN. et al. Effect of prophylactic or therapeutic administration of paracetamol on immune response to DTwP-HepB-Hib combination vaccine in Indian infants. Vaccine 2017;35:2999–3006. [DOI] [PubMed] [Google Scholar]
- 71. Walter EB, Hornik CP, Grohskopf L. et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine 2017;35:6664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ryan EP, Pollack SJ, Murant TI. et al. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol 2005;174:2619–26. [DOI] [PubMed] [Google Scholar]
- 73. Bancos S, Bernard MP, Topham DJ. et al. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell Immunol 2009;258:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Earn DJD, Andrews PW, Bolker BM.. Population-level effects of suppressing fever. Proc R Soc B 2014;281:20132570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Han Y, Yang H.. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): A Chinese perspective. J Med Virol 2020;92:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nesse RM. The smoke detector principle. Natural selection and the regulation of defensive responses. Ann N Y Acad Sci 2001;935:75–85. [PubMed] [Google Scholar]
- 77. Broggi A, Ghosh S, Sposito B. et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020;369:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Major J, Crotta S, Llorian M. et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 2020;369:712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shi Y, Wang Y, Shao C. et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020;27:1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kox M, Pickkers P.. “Less is more” in critically ill patients: not too intensive. JAMA Intern Med 2013;173:1369. [DOI] [PubMed] [Google Scholar]
- 81. Vincent J-L, Singer M, Marini JJ. et al. Thirty years of critical care medicine. Crit Care 2010;14:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Determann RM, Royakkers A, Wolthuis EK. et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 2010;14:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stub D, Smith K, Bernard S. et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 2015;131:2143–50. [DOI] [PubMed] [Google Scholar]
- 84. Arabi YM, Reintam Blaser A, Preiser J-C.. Less is more in nutrition: critically ill patients are starving but not hungry. Intensive Care Med 2019;45:1629–31. [DOI] [PubMed] [Google Scholar]
- 85. Rowan KM, Angus DC, Bailey M, PRISM Investigators et al. Early, goal-directed therapy for septic shock - A patient-level meta-analysis. N Engl J Med 2017;376:2223–34. [DOI] [PubMed] [Google Scholar]
- 86. Weinberg ED. Iron and susceptibility to infectious disease. Science 1974;184:952–6. [DOI] [PubMed] [Google Scholar]
- 87. Fonseca MT, Rodrigues AC, Cezar LC. et al. Spontaneous hypothermia in human sepsis is a transient, self-limiting, and nonterminal response. J Appl Physiol 2016;120:1394–401. [DOI] [PubMed] [Google Scholar]
- 88. Goldstein BD. The precautionary principle also applies to public health actions. Am J Public Health 2001;91:1358–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gabay C, Kushner I.. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 90. Schrock JM, Snodgrass JJ, Sugiyama LS.. Lassitude: the emotion of being sick. Evol Hum Behav 2020;41:44–57. [Google Scholar]
- 91. Shakhar K. The Inclusive Behavioral immune system. Front Psychol 2019;10:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Straub RH, Cutolo M, Buttgereit F. et al. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med 2010;267:543–60. [DOI] [PubMed] [Google Scholar]
- 93. Manthous CA, Hall JB, Olson D. et al. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med 1995;151:10–4. [DOI] [PubMed] [Google Scholar]
- 94. Shakhar K, Shakhar G.. Why do we feel sick when infected–can altruism play a role? PLoS Biol 2015;13:e1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. González Plaza JJ, Hulak N, Zhumadilov Z. et al. Fever as an important resource for infectious diseases research. Intractable Rare Dis Res 2016;5:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hildebrandt B, Wust P, Ahlers O. et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33–56. [DOI] [PubMed] [Google Scholar]
- 97. Dewey WC, Hopwood LE, Sapareto SA. et al. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977;123:463–74. [DOI] [PubMed] [Google Scholar]
- 98. Stankov S. Definition of inflammation, causes of inflammation and possible anti-inflammatory strategies. Open Inflamm J 2012;5:1–9. [Google Scholar]
- 99. Casscells W, Hathorn B, David M. et al. Thermal detection of cellular infiltrates in living atherosclerotic plaques: possible implications for plaque rupture and thrombosis. Lancet 1996;347:1447–51. [DOI] [PubMed] [Google Scholar]
- 100. Madjid M, Willerson JT, Casscells SW.. Intracoronary thermography for detection of high-risk vulnerable plaques. J Am Coll Cardiol 2006;47:C80–85. [DOI] [PubMed] [Google Scholar]
- 101. Van De Parre TJL, Martinet W, Verheye S. et al. Mitochondrial uncoupling protein 2 mediates temperature heterogeneity in atherosclerotic plaques. Cardiovasc Res 2007;77:425–31. [DOI] [PubMed] [Google Scholar]
- 102. Eftimiadi C, Rialdi G.. Increased energy expenditure by granulocytes during phagocytosis of Staphylococcus aureus compared with other staphylococci. J Infect Dis 1984;150:366–71. [DOI] [PubMed] [Google Scholar]
- 103. Ljunggren L, Monti M, Rialdi G.. A comparison of the calorimetric analysis of granulocyte activation by flow and batch systems. Thermochimica Acta 1992;207:23–8. [Google Scholar]
- 104. Tan AM, Huang YQ, Qu SS.. Determination of the respiratory burst of polymorphonuclear leukocytes by microcalorimetry. J Biochem Biophys Methods 1998;37:91–4. [DOI] [PubMed] [Google Scholar]
- 105. Nogueira-Machado JA, Mares-Guia ML, Lima e Silva FC. et al. Calorimetry: a highly sensitive technique for evaluating the effect of IL-2, IFN-γ and IL-10 on the response of peripheral blood mononuclear cells. Thermochimica Acta 1999;327:57–62. [Google Scholar]
- 106. Chrétien D, Bénit P, Ha H-H. et al. Mitochondria are physiologically maintained at close to 50°C. PLoS Biol 2018;16:e2003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Carlsen E, Andersson A-M, Petersen JH. et al. History of febrile illness and variation in semen quality. Hum Reprod 2003;18:2089–92. [DOI] [PubMed] [Google Scholar]
- 108. Edwards MJ. Review: hyperthermia and fever during pregnancy. Birth Defects Res Part A Clin Mol Teratol 2006;76:507–16. [DOI] [PubMed] [Google Scholar]
- 109. Group RC, Horby P, Lim WS. et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med 2020:NEJMoa2021436. [Google Scholar]
- 110. Gomez CR, Boehmer ED, Kovacs EJ.. The aging innate immune system. Curr Opin Immunol 2005;17:457–62. [DOI] [PubMed] [Google Scholar]
- 111. Cunha LL, Perazzio SF, Azzi J. et al. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol 2020;11:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]