To the Editor:
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) has been widely used to detect SARS-CoV-2 viral RNA genomes from upper respiratory samples. During the first months of the COVID-19 pandemic, testing focused on patients who were presumed positive in the context of triage and epidemiological monitoring. After the first wave of infection, molecular testing has expanded to a population with a high true-negative rate—clearing asymptomatic individuals for return to work, school, and society based on absence of detectable virus. Minimizing analytical false negatives for COVID-19 testing is a public health imperative.
Multiple RT-qPCR test designs for detecting SARS-CoV-2 have been developed and given emergency use authorization by the US Food and Drug Administration (FDA). As of July 2020, nearly half of the FDA-authorized designs use qPCR primers and probes specified by the US Centers for Disease Control and Prevention (CDC). The CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel is comprised of 3 qPCR assays: N1 and N2 generate SARS-CoV-2-specific amplicons from reverse transcribed viral RNA; RP is a human specimen and extraction control that targets a single exon within the human RPP30 gene. Failure of this control is intended to indicate potential loss of RNA or RNA degradation (1). In practice, this design generates a misleading control-positive signal in scenarios that preclude RNA virus detection.
The CDC RP control is capable of detecting reverse transcribed RNA; it also detects human genomic DNA. Indeed, the CDC uses the same control design for RT-qPCR and qPCR panels targeting viral respiratory pathogens with RNA and DNA genomes, respectively (personal communication with CDC). When human DNA is present, intact RNA and reverse transcription are unnecessary to generate a positive specimen and extraction control signal.
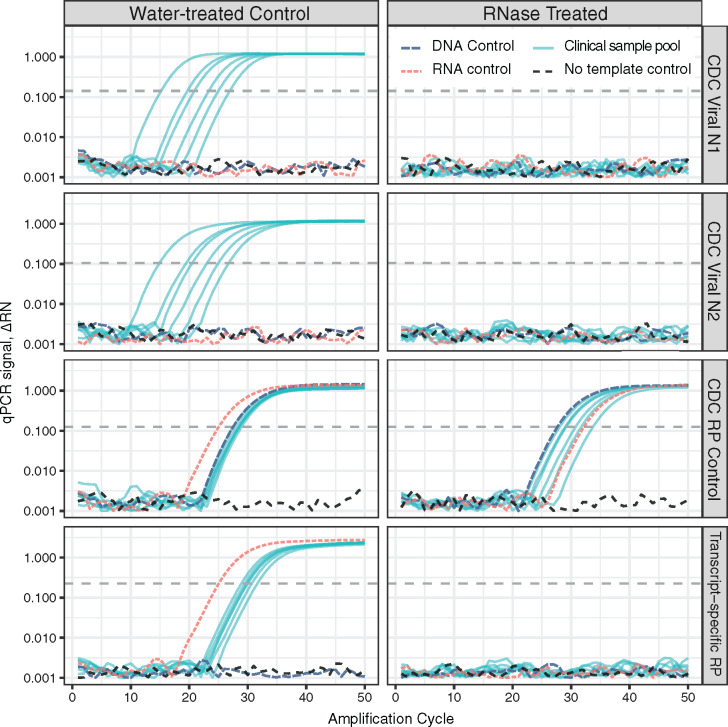
Single-digit copies of genomic DNA are sufficient to generate a strong control signal using the CDC design (data not shown). DNA is copurified by solid phase and liquid–liquid extraction procedures used for isolation of RNA from clinical specimens. qPCR-only (no-RT) reanalysis of RNA samples extracted from COVID-19 case nasopharyngeal swabs yielded strong control signals from all specimens tested (data not shown). More worryingly, DNA cross-reactivity leads to analytical false negatives from true-positive patient samples where RNA has been degraded (Fig. 1).
Fig. 1.
The de facto standard COVID-19 RT-qPCR test design includes a specimen and extraction control that is DNA cross-reactive and generates analytical false-negative results following loss of RNA integrity. Purified nucleic acid samples from COVID-19 positive cases were pooled, aliquoted, and treated with either nuclease-free water or ribonuclease A (RNase). Commercial human RNA, DNA, and no-template controls were treated in parallel. Samples were analyzed by RT-qPCR with Viral One-step master mix using the CDC-specified Viral N1, Viral N2, and human specimen and extraction control (RP) assays, plus a transcript-specific control targeting spliced exons 1–3 of RPP30 (Hs.PT.58.2854947, idtdna.com). Strong viral and specimen control signals are generated from each infected-patient pool after mock treatment (water, left). Purified DNA is sufficient template for the CDC RP control. Loss of RNA integrity precludes detection of viral genomes, while the cross-reactive specimen control continues to generate strong signal from copurified DNA (RNase, right). A transcript-specific RP control properly reflects the loss of sample integrity and returns no signal from samples containing degraded RNA.
Pooling multiple samples prior to analysis is being used to increase throughput and reduce testing cost (2–4). The problems caused by a DNA-reactive control are magnified by pooling: one RNase-containing sample can render an entire pool virus RNA negative, while a few cells’ worth of DNA from a single patient are sufficient to generate a specimen and extraction control signal. A DNA-reactive control opens the door to silent assay failures and false-negative reporting of individuals who were COVID-19 positive from whom virus was successfully collected and whose samples were intact prior to pooling with dominant negative samples.
The absence of viral signal is insufficient for clinical interpretation. Controls must demonstrate that the test worked as intended and would have found virus had it been present. The current goal of testing is not just to find needles in haystacks—it is to conclusively state that individual haystacks contained no needles.
This widely used design has high analytical sensitivity for detecting the SARS-CoV-2 virus, but incorrectly reports “assay success, no virus found” when faced with degraded specimens. A specimen and extraction control that specifically detects human RNA (Fig. 1) eliminates this preventable class of false-negative results and can improve negative predictive value. A redesigned control will properly return “don’t know” instead of incorrectly reporting “no.” This distinction enables focused retesting and conservative clinical management rather than prematurely giving an all-clear.
Acknowledgments
The author acknowledges Amy A. Caudy (Maple Flavored Solutions, LLC) for technical discussion, A. Bruce Futcher (Stony Brook University) for initial discussions, and Richard Kew and Karen Bai (Stony Brook Medicine) for assistance accessing clinical samples. The author would like to acknowledge the support of the Stony Brook Medicine Biobank for providing de-identified purified RNA from patient samples and Maple Flavored Solutions LLC for access to instruments and donation of supplies and reagents.
Author Declaration: A version of this paper was previously posted as a preprint on bioRxiv as https://doi.org/10.1101/2020.05.13.094839.
Data and Materials Availability: all data are available, without restriction, by written request to the author.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
A.P. Rosebrock conceived, designed, executed, analyzed, and wrote this manuscript.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: A.P. Rosebrock, Maple Flavored Solutions LLC.
Consultant or Advisory Role: None declared.
Stock Ownership: A.P. Rosebrock, Maple Flavored Solutions LLC.
Honoraria: None declared.
Research Funding: Startup funds from Stony Brook University and NIGMS R01GM132238. Stony Brook Medicine Biobank provided de-identified purified RNA from patient samples. Maple Flavored Solutions LLC provided access to instruments and donated supplies and reagents.
Expert Testimony: None declared.
Patents: None declared.
Nonstandard Abbreviations:
- RNA
ribonucleic acid
- SARS-CoV-2
severe acute respiratory syndrome-associated coronavirus 2
- cDNA
complementary DNA
- COVID-19
Coronavirus disease 2019
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- qPCR
quantitative polymerase chain reaction
- RNase
ribonuclease
- CDC
US Centers for Disease Control and Prevention
- DNA
deoxyribonucleic acid
- EUA
emergency use authorization
- FDA
US Food and Drug Administration
- N1, N2
CDC SARS-CoV-2viral detection amplicons 1 and 2 (CDC abbreviation)
- RP
RPP30 specimen and extraction control (CDC abbreviation)
Human Genes
RPP30 ribonuclease P/MRP subunit p30 (deprecated name TSG15).
References
- 1.U.S. Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel–Instructions for Use. https://www.fda.gov/media/134922/download (Accessed May 2020).
- 2.U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: Facilitating Diagnostic Test Availability for Asymptomatic Testing and Sample Pooling. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-facilitating-diagnostic-test-availability-asymptomatic-testing-and (Accessed July 2020).
- 3. Lohse S Pfuhl T Berkó-Göttel B Rissland J Geißler T Gärtner B, et al. . Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect Dis 2020;20:1231–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yelin I Aharony N Tamar E S Argoetti A Messer E Berenbaum D, et al. . Evaluation of COVID-19 RT-qPCR Test in Multi sample Pools. Clin Infect Dis 2020;71:2073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]