Abstract
We report the first surgical series of patients developing pleural empyema after severe bilateral interstitial lung disease in confirmed severe acute respiratory syndrome coronavirus 2 infection. The empyema results in a complex medical challenge that requires combination of medical therapies, mechanical ventilation and surgery. The chest drainage approach was not successful to relieve the symptomatology and to drain the excess fluid. After multidisciplinary discussion, a surgical approach was recommended. Even though decortication and pleurectomy are high-risk procedures, they must be considered as an option for pleural effusion in Coronavirus disease-positive patients. This is a life-treating condition, which can worsen the coronavirus disease manifestation and should be treated immediately to improve patient’s status and chance of recovery.
Keywords: Coronary disease 2019, Pleural empyema, Surgery
Since December 2019, several cases of unknown-origin pneumonia started to be diagnosed in Wuhan, China.
INTRODUCTION
Since December 2019, several cases of unknown-origin pneumonia started to be diagnosed in Wuhan, China. One month later, The World Health Organization [1] recognized the responsible pathogen as the 2019 novel coronavirus 2. The associated respiratory manifestation was later named as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the outbreak was named as coronavirus disease 2019 (COVID-19). The disorder became rapidly a worldwide pandemic emergency and, at the end of May 2020, there were more than 5 million cases worldwide and 346.000 deaths [1].
The most common symptoms reported were fever, dry cough and tiredness. About 20% of patients became seriously ill, requiring hospitalization for difficulty in breathing.
With growing global concerns about the COVID-19 outbreak, still many disease-related information and guidelines for clinical management are missing. We report 3 cases of pleural empyema after bilateral interstitial COVID-19 pneumonia, presenting a complex medical challenge. The treatment required the combination of medical and surgical therapy.
PATIENTS AND METHODS
Case 1
A 60-year-old male, non-smoker with a history of chronic cardiac disease, hypertension and obstructive sleep apnoea syndrome was referred to the emergency room for desaturation and dyspnoea during COVID-19 emergency. He was prescribed with 3 g/day of paracetamol for diffuse myalgia without fever.
Blood tests showed elevated level of C-reactive protein (150 mg/l), Serum Glutamic Pyruvic Transaminase of 87 Unit/l and a decreased lymphocyte count (0.60 × 109/l). Physical examination revealed bilateral wheezing with a heart rate of 119 bpm, respiratory rate of 24 breaths/min and oxygen saturation in the range of 70–80% under oxygen mask at 15 l/min.
Chest X-ray and thorax ultrasound showed bilateral ground‐glass opacities and consolidations. Arterial blood gas analysis revealed partial respiratory failure with respiratory alkalosis, pH of 7.49, an oxygen partial pressure of 7.0 kPa, carbon dioxide partial pressure of 4.39 kPa and bicarbonates concentration of 25 mmol/l.
The reverse transcription polymerase chain reaction for COVID-19 test was positive. The patient was transferred to the general intensive care unit, intubated and ventilated in controlled volume. Hydroxychloroquine was administered for 4 days with Tocilizumab 800 mg. Due to the development of acute kidney injury, hemofiltration was required for 2 days until normalization of kidney function.
In the next few days, after Meropenem and Linezolid therapy, we observed clinical and radiological improvement and the return of spontaneous breathing.
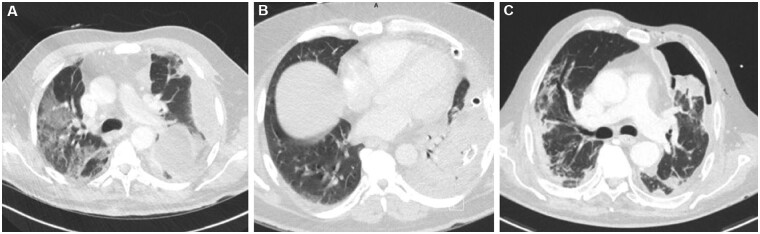
However, 3 days after discharge, the patient was readmitted because of dyspnoea, desaturation and need of high-flow oxygen therapy. Chest X‐ray and computed tomography scan (CT scan) showed left pleural effusions, bilateral ground‐glass opacities and consolidation in the left lower lobe (Fig. 1A). He immediately started on Piperacillin/Tazobactam therapy, which was later changed to Vancomicin. A chest tube insertion was required, showing evidence of purulent material.
Figure 1:
Computed tomography scan showing (A) bilateral ground‐glass opacities and consolidation, (B) coronavirus disease 2019 bilateral pneumonia manifestation and (C) persistent pneumothorax.
Due to an increase of the inflammation indexes and the absence of clinical recovery, a left thoracotomy was indicated to achieve decortication and pleurectomy. The aim of the procedure was to clean the pleural cavity from the presence of grossly purulent pleural fluid, which turned out negative at microbiological evaluation. The procedure was successfully performed without any perioperative complication.
After the procedure, we observed a progressive clinical and radiological improvement with reduction in the inflammation indices that allowed discharge. He was tested negative after 47 days of COVID-19 infection. At the last follow-up, the patient was in good conditions without any further complication.
Case 2
A 50-year-old male, with a history of hypertension and type-2 diabetes was referred to our Department for cough, desaturation, dyspnoea, dysphagia and high fever during the previous 4 days.
Blood tests showed a C-reactive protein of 161 mg/l, Serum Glutamic Pyruvic Transaminase of 87 U/l and a decreased lymphocyte count to 0.40 × 109/l. Chest X-ray evidenced bilateral peripheral infiltrates. Arterial blood gas analysis revealed partial respiratory failure with 7.45 pH, oxygen partial pressure 8.0 kpa, carbon dioxide partial pressure 4.14 kpa and bicarbonates concentration of 21 mmol/l.
Vital signs were heart rate 119 bpm, respiratory rate of 24 breaths/min and oxygen saturation in the range of 70–80% under oxygen mask at 15 l/min. Physical examination revealed bilateral wheezing. He was immediately tested and was positive for 2019 novel coronavirus.
Hydroxychloroquine and lopinavir/ritonavir were administered for 6 days together with Tazobactam.
At the end of the treatment, imaging exams showed left pleural empyema; therapy with Imipenem/Cilastatine was immediately administered. Bacteriological examination and polymerase chain reaction result were negative, and a chest tube was positioned; however, inflammation indices continued to raise without any symptom relief.
CT scan revealed left pleural effusions and a consolidation in the left lower lobe (Fig. 1B). After multidisciplinary discussion, the patient was candidate to left thoracotomy for pleural decortication.
In the postoperative days, the bacteriological examination revealed the presence of Pseudomonas aeruginosa and Actinomyces odontolyticus resistant to Tazocin, Ceftazidime and Cefepime. Moreover, the blood cultures were positive for Finegoldia magna species.
The patient was treated with Meropenem 2 g/3 times/day until the infection resolved; he was later discharged to a rehabilitation centre. At the last follow-up, the patient had recovered from SARS-CoV-2 infection (total positivity 45 days) without any further complication.
Case 3
A 72-year-old male was referred to our Department after 14 days of fever and cough. The X-ray examinations revealed bilateral peripheral infiltrates. The blood examinations showed C-reactive protein 68 mg/l and 1.03 × 109/l lymphocyte. The arterial blood gas analysis evidenced respiratory insufficiency with an oxygen partial pressure of 8.2 kPa. Moreover, an acute kidney injury was revealed by renal ultrasound.
He was tested for 2019 novel coronavirus and the result was positive with bilateral pneumonia manifestation. Due to worsening clinical conditions, he was immediately started on Co-Amoxicillin treatment without any improvement. An invasive intubation with Venturi mask was required (at 60% level) and later, an orotracheal intubation and invasive mechanical ventilation.
In the next few days, the manifestation was worsening and a culture from the bronchial aspirate was performed. The pleural puncture showed the presence of P. aeruginosa, for which Tobramycin, Colistin and Ciprofloxacin were administered.
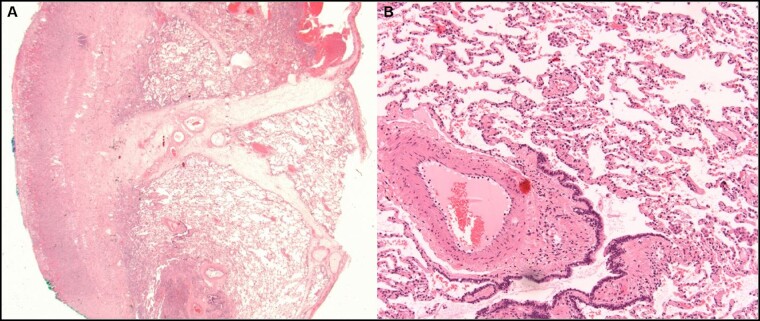
Imaging investigations showed a persistent pneumothorax requiring chest tube placement (Fig. 1C). Missed resolution of pneumothorax was possibly the expression of a pleural fistula in a context of necrotic lung due to pleural empyema. Thus, the patient underwent left thoracoscopy with pleural decortication and lingula wedge resection. Histology showed severe acute and fibrinous pleuritis with granulation tissue and oedema of the intersegmental septa (Fig. 2A); the subpleural lung parenchyma was normal (Fig. 2B).
Figure 2:
(A) Wedge excision of the lung showing severe acute and fibrinous pleuritis with granulation tissue and oedema of the intersegmental septa (original magnification ×15). (B) Subpleural lung parenchyma without significant histological changes, in particular without signs of diffuse alveolar damage (original magnification ×200).
The postoperative days were normal and with progressive improvement of both symptomatology and follow-up imaging. Nowadays, the patient’s saturation is normal, without the need of oxygen support. He became negative for SARS-CoV-2 infection after 70 days.
DISCUSSION
Since February 2020, during COVID-19 outbreak, the Ticino area has recorded a total of 3561 patients who were positive for SARS-CoV-2 infection, among whom 160 required intensive care. The total number of patients discharged as still positive for the infection was 929; instead, 350 died due to COVID-19 [2].
Pleural effusion is an uncommon complication of Covid-19 infection [3]. Zhang et al. [4] examined 34 consecutive COVID-19 patients and reported only 1 case with pleural effusion. Another study [5] reported the absence of pleural empyema at the beginning of the symptomatology, but an increase of the cases during the clinical course of the disease. Guidelines for the treatment of pleural empyema in COVID-19 patients are still absent. In case of lower respiratory tract infection, the virus generates a strong inflammatory response with pro-inflammatory cytokines release, oxidant stress and damage of alveolar epithelium. In addition, viral vasculitis, microvascular pulmonary thrombosis and embolism have been described [6]. The combination of severe inflammatory syndrome with the onset of reactive pleural effusion, and the exposure to severe risk factors as the prolonged intubation, might have facilitated the bacterial superinfection and the consequent developing of pleural empyema.
Currently, surgical treatment of pleural empyema in COVID-19 patients is not yet described in literature due to the reduced number of cases and possibly due to the risk grade of the procedure. Moreover, for the acute Middle East Respiratory Syndrome, which has similar manifestation to SARS‐CoV‐2, they represent a poor prognostic indicator [7].
Thoracic empyema has been considered a surgical disease, with open decortication as the most definitive method of treatment [8] and non-operative management being associated with higher risk of mortality compared with surgical decortication [9]. In our series, all patients were intubated and operated on a mean of 7 cmH2O peak pressure of ventilation and a mean of 70% FiO2. All had invasive ventilation in intensive care unit which is associated with ventilator-associated pneumonia [10]. The mean length of ICU and hospital stay was 11 and 26 days, respectively. All patients had within 4 weeks of their admission a new test in order to confirm negative result. All pleural fluids and/or biopsies sent for microbiology tests were negative for SARS-CoV-2 infection.
This is the first case series of COVID-19 patients with surgically resolved pleural empyema. Considering that this is a rare but possible complication, we want to underline the importance of clinical and radiological surveillance and laboratory testing in individuals with recent diagnosis of SARS-CoV-2. Moreover, these results suggest that it is worth investing in surgical approach considering pleural effusion, together with SARS‐CoV‐2 manifestation, is a life-threatening condition.
ACKNOWLEDGEMENT
The authors thank Professor Luca Mazzucchelli for his constructive comments on the manuscript.
Conflict of interest: none declared.
Author contributions
Adele Tessitore: Conceptualization; Data curation; Writing—original draft; Writing—review & editing. Miriam Patella: Data curation; Writing—review & editing. Mauro Giuliani: Formal analysis; Resources; Writing—review & editing. Thomas Theologou: Formal analysis; Resources; Writing—review & editing. Stefania Freguia: Formal analysis; Writing—review & editing. Eleonora Maddalena Minerva: Formal analysis; Resources; Writing—review & editing. Gregor Rugel: Data curation; Formal analysis; Writing—review & editing. Stefano Cafarotti: Conceptualization; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Clemens Aigne, Alper Toker, and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
REFERENCES
- 1. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (9 September 2020, date last accessed).
- 2. https://github.com/openZH/covid_19 (9 September 2020, date last accessed).
- 3. Bao C, Liu X, Zhang H, Li Y, Liu J.. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol 2020;17:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang L, Kong X, Li X, Zhu J, Liu S, Li W. et al. CT imaging features of 34 patients infected with COVID-19. Clin Imaging 2020;68:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan C-S, Xie R-M, Lv Z-B, Yan S, Zhang Z-X, Chen B-D.. CT findings of COVID-19 in follow-up: comparison between progression and recovery. Diagn Interv Radiol 2020;26:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das KM, Lee EY, Al Jawder SE, Enani MA, Singh R, Skakni L. et al. Acute middle east respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. Am J Roentgenol 2015;205:W267–74. [DOI] [PubMed] [Google Scholar]
- 8. Rakesh HR, Gelzinis TA.. The updated ATS/STS/STR clinical practice guidelines on the management of malignant pleural effusions: what is new in 2018? J Cardiothorac Vasc Anesth 2019;33:1181–6. [DOI] [PubMed] [Google Scholar]
- 9. Nayak R, Brogly SB, Lajkosz K, Lougheed MD, Petsikas D.. Outcomes of operative and nonoperative treatment of thoracic empyema: a population-based Study. Ann Thorac Surg 2019;108:1456–63. [DOI] [PubMed] [Google Scholar]
- 10. Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J 2017;50:1700582. [DOI] [PubMed] [Google Scholar]