Abstract
Aims:
Hydroxychloroquine and chloroquine ([hydroxy]chloroquine) are drugs used to treat malaria and rheumatological disorders and were recently suggested as beneficial for prevention and treatment of patients with coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 infection. However, longitudinal studies to assess the electrocardiographic and cardiotoxic effects of these drugs are limited. In this study, we aimed to investigate the effect of these drugs on QTc-interval and incidence of sudden cardiac death (SCD).
Methods
We designed a longitudinal follow-up study of individuals within the prospective population-based Rotterdam Study. Eligible individuals had available data on medication and repeated ECG measurements. The study period was between 1 January 1991 and 1 January 2014. We studied on current and past use of [hydroxy]chloroquine as a time-varying exposure; high versus low daily dose of [hydroxy]chloroquine. QTc-interval duration, and the occurrence of SCD were the main outcomes. SCD was defined as an unexpected and sudden death due to cardiac arrhythmia within one hour of the onset of acute symptoms, and in patients without cardiac symptoms within 24 hours before death.
Results
Among the study population of 14 594 individuals (58.8% women) with an average age of 65 years, 346 patients used [hydroxy]chloroquine at any time during follow-up. The total number of SCD cases was 609. In a multiple linear mixed model analysis, the current use of [hydroxy]chloroquine was associated with a significantly increased duration of the QTc-interval of 8.1 ms (95% CI: 3.6; 12.6) compared with non-users. The association was stronger among current-high daily dosage [15.3 (95%CI: 7.0; 23.6)] compared with current-low daily dosage [5.5 (95%CI: 0.4; 10.7)] users. In a Cox proportional hazard regression analysis, the risk of SCD was significantly higher in participants who were current users of [hydroxy]chloroquine than in non-users [adjusted hazard ratio; 3.7 (95%CI: 1.1; 12.6)].
Conclusions
In this longitudinal study, persons who received [hydroxy]chloroquine had an increased QTc-interval duration and the association was dose-dependent. [Hydroxy]chloroquine was associated with a significantly increased risk of SCD. As long as their activity against COVID-19 is controversial, cardiotoxicity is a strong argument against using these drugs to treat COVID-19 infections.
Keywords: Hydroxychloroquine, Chloroquine, Sudden cardiac death, QT/QTc-interval
Introduction
Hydroxychloroquine and chloroquine ([hydroxy]chloroquine) are antimalarial drugs that are also used to treat immune-mediated disorders such as rheumatoid arthritis and systemic lupus erythematosus (SLE).1 They were recently suggested as potential, but controversial, therapies for patients with coronavirus disease 2019 (COVID-19).2,3 Although myocardial toxicity is uncommon, cardiomyopathy related to [hydroxy]chloroquine therapy is a severe complication that often leads to death.4 However, given the effect of confounding factors such as heart failure attributed to autoimmune diseases and hypertension, a causal relationship with direct myocardial toxicity is difficult to assess.4
[Hydroxy]chloroquine inhibits voltage-gated sodium and potassium channels on heart muscle cells, which leads to prolongation of the QTc-interval. This reflects delayed cardiac repolarization which is a risk factor for sudden cardiac death (SCD). Despite that in an observational study involving patients with COVID-19 admitted to the hospital, hydroxychloroquine use was not associated with mortality,5 recent clinical trials that started treating COVID-19 patients with [hydroxy]chloroquine were halted due to increased risk of arrhythmia and mortality.6 In a recent randomized, controlled, open-label platform trial, in patients hospitalized with COVID-19, researchers randomly assigned 1561 patients to receive hydroxychloroquine and 3155 to receive usual care. The results of this study suggest that the mortality rate among patients in the hydroxychloroquine group was not lower than those who received usual care.7
Longitudinal studies to assess the electrocardiographic and cardiotoxic effects of [hydroxy]chloroquine are limited. Given that the risk of SCD associated with [hydroxy]chloroquine has never been studied on a population-based scale and because the value of these drugs against COVID-19 infections is still inconclusive, we investigated the association between [hydroxyl]chloroquine and QTc-interval duration and SCD in a population-based prospective cohort study.
Methods
Setting
This study is embedded within the framework of the Rotterdam Study, a prospective population-based cohort study among people ≥40 years of age living in the well-defined Ommoord district of Rotterdam, the Netherlands. Initially, in 1990, all inhabitants aged 55 years or over (n = 10 215) were invited to participate of whom 78% agreed. In 2000, out of 4472 invitees, 3011 participants who had reached the age of 55 years were invited to participate in the second cohort. In 2006, a third cohort included 3932 (out of 6057 invited) inhabitants aged 45 years and older with the total study population being 14 926 individuals by the end of 2008 (overall participation 72%).
The participants were all extensively examined at study entry i.e. baseline and subsequent follow-up visits that take place every 3–6 years. They were interviewed at home and then underwent an extensive set of examinations e.g. echocardiogram, echocardiography, computed tomography-scanning, and magnetic resonance imaging with an emphasis on imaging (of heart, blood vessels, eyes, skeleton, and later brain) and on collecting biospecimens that enabled further in-depth molecular and genetic analyses. The participants in the Rotterdam Study are followed for a variety of diseases that are frequent in the elderly, which include coronary heart disease, heart failure, and stroke, dementia, but also several other chronic diseases. Almost all the participants provided written informed consent to participate in this study. The Rotterdam study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare, and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The complete design of the Rotterdam Study has been described in a separate publication.8
Study population
This study included participants from the first examinations of the first (1989–92), the second (2000–01), and the third (2006–08) cohorts. We included participants if they had information on medication data with at least one baseline interview or clinical examination. We excluded participants with ECGs recorded while on other QTc-prolonging medication use9 (n = 1183). Participants who later withdrew informed consent for the collection of follow-up data (n = 313) were also excluded from the analyses. The population for SCD assessment in this study consisted of 14 594 participants from the three cohorts. Data on repeated ECG measurements were available in 11 936 subjects. Figure 1 shows the flowchart of the study population.
Figure 1.
Flow chart of the study population selection.
Study design
We performed two longitudinal analyses with a cohort design: First, we studied the associations between the use of [hydroxy]chloroquine and QTc-interval in up to five serial ECGs, and second, we studied the associations between the use of [hydroxy]chloroquine and the risk of SCD.
Baseline measurements
At baseline, information on individuals' characteristics, medical and medication history, and lifestyle factors was obtained. Information on body mass index (BMI), hypertension, type 2 diabetes mellitus (T2D), myocardial infarction, heart failure, smoking behaviour, and lipid-lowering drugs was gathered. We calculated BMI as weight (kg) divided by height (m2). Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or a prescription for an antihypertensive agent. Type 2 diabetes mellitus was defined as the baseline measurement of fasting blood glucose (>7.0 mmol/L), or non-fasting blood glucose >11.0 mmol/L values or glucose-lowering medication use. Myocardial infarction was defined as cases with pathology outcomes of an acute myocardial infarction confirmed by a medical specialist within 28 days of death or a rise/fall in cardiac biomarkers values and or changes in objective indicative ECG and the presence of cardiac signs e.g. pain and cardiogenic shock. Heart failure was defined according to the guidelines of the European Society of Cardiology (ESC) as the combination of typical symptoms and signs, confirmed by objective evidence of cardiac dysfunction or a positive response to therapy.10,11 Smoking status was categorized as current, past, and never smokers through structured interviews.
Follow-up measurements
Follow-up data on [hydroxy]chloroquine dispensing data and the outcomes for all individuals included in this study were available. Outpatient clinic reports, hospital discharge letters, electrocardiograms, and imaging data were collected from general practitioner records and hospital records. Dispensing data on [hydroxy]chloroquine use, including dispensing date, Anatomical Therapeutic Chemical-code, prescribed daily dose, and the amount prescribed was obtained from all pharmacies in the study district. The prescription duration was calculated as the number of dispensed tablets, divided by the prescribed daily number. Each subject was considered as a current [hydroxy]chloroquine user (filled prescription ≤100 days after the day of the last intake). Past [hydroxy]chloroquine users were individuals with discontinuation for more than 100 days after the last intake. This cut-off was chosen because the biological elimination half-life of these drugs is 40–50 days.12,13
Follow-up started at baseline and individuals were followed until the occurrence of SCD, other types of death, removal, or the end of follow-up on 1 January 2014, whichever came first.
Assessment of the outcomes
Assessment of QTc-interval
All 12-lead ECGs from individuals with available medication information were included and were obtained using an ACTA electrocardiograph (ESAOTE, Florence, Italy) stored digitally at a sampling frequency of 500 Hz. ECG measurements including QT, QRS, and RR-interval durations were obtained by digital processing of ECGs using a modular ECG analysis system (MEANS).14,15 The corrected QT (QTc)-interval from the start of the QRS complex until the end of the T wave was estimated using the Bazett formula (QTc = QT/√RR) to adjust for the heart rate.16 For each subject, up to a maximum of five QTc measurements were recorded during the regular examination cycles. We excluded the ECGs with left or right bundle branch block.
Sudden cardiac death
Sudden cardiac death was defined as an unexpected and sudden death due to cardiac arrhythmia that occurs within 1 h of the onset of acute symptoms, and in patients within 24 h of not being symptomatic. The adjudication of SCD cases in the Rotterdam Study was performed by two physicians and ascertained by a cardiologist as described in detail previously.17
Statistical analysis
Descriptive analyses were performed by reporting mean [standard deviation (SD)] or median [interquartile range (IQR)] for continuous variables and numbers (with percentage) for categorical variables.
We assessed the ECG parameters in patients on current, and past [hydroxy]chloroquine use and compared the results with those in non-users. Since each subject had up to a maximum of five ECGs recorded, which are correlated in the same person, we performed a repeated measure analysis applying a linear mixed model. The analyses were also stratified by sex; given different cut-off points of prolonged QTc in women and men (in women the cut-off points of ≤450 ms) as standard, 451–470 ms as borderline, and >470 ms as prolonged, and in men, ≤430 ms as standard, 431–450 ms as borderline, and >450 ms as prolonged.18
We also studied the association between [hydroxy]chloroquine use dose categories {high [>0.3 defined daily dosages (DDD)] vs. low dosages (≤0.3 DDD)} and the mean QTc-interval duration. The DDD for hydroxychloroquine is 516 mg; for chloroquine, the DDD is 500 mg. The 0.3 DDD cut-off is based on the usual maintenance dose in hydroxychloroquine of 200 mg daily.
To study the longitudinal association between current and past [hydroxy]chloroquine use and the risk of SCD, we used a Cox proportional hazard model. In all analyses, [hydroxy]chloroquine was analysed as a time-dependent variable,19 and past use (discontinuation) was a separate exposure category. In this way, every participant's total follow-up time was distinguished into non-use, current use, and past use.
Because the elimination half-life can be very long due to lysosomal storage, we performed a sensitivity analysis with [hydroxy]chloroquine use with a cut-off of 250 days (five times the half-life in blood, in which the total amount of [hydroxy]chloroquine would normally be reduced by 97%).
In a sensitivity analysis, the association was further adjusted for baseline QTc-interval above the cut-off of >450 ms in men and >470 ms in women.
All analyses were adjusted for age and sex, baseline measurements of BMI, T2D, heart failure, myocardial infarction, hypertension, smoking behaviour, lipid-lowering drugs, and the average number of prescriptions until the occurrence of the outcomes.
We checked the proportional hazards assumption by plotting partial residuals. A two-sided P-value <0.05 was considered statistically significant. Imputation was performed using expectation-maximization, a single imputation method to impute the missing values. Data were analysed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of the study population (n = 14 594) are shown in Table 1. In total, 346 patients used [hydroxy]chloroquine at any time during the study period. Mean age and median BMI were significantly higher among non-users (65.3 and 26.3) compared with [hydroxy]chloroquine users (62.1 and 26.0), respectively.
Table 1.
Baseline characteristics of the study populations
| Total (14 594) | [hydroxy]chloroquine users (n = 346) | [hydroxy]chloroquine non-users (n = 14 248) | P-value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 65.3 (10.3) | 62.1 (7.1) | 65.3 (10.4) | <0.001 |
| Sex, women, n (%) | 8580 (58.8) | 209 (60.4) | 8371 (58.8) | 0.54 |
| BMI (kg/m2), median (IQR) | 26.3 (24.4–28.8) | 26.0 (24.0–28.3) | 26.3 (24.4–28.2) | 0.02 |
| Hypertension, n (%) | 7194 (49.3) | 173 (50.0) | 7021 (49.3) | 0.79 |
| Total cholesterol (mmol/L), median (IQR) | 5.7 (5.0–6.4) | 5.6 (5.03–6.2) | 5.7 (5.0–6.4) | 0.08 |
| HDL cholesterol (mmol/L), median (IQR) | 1.3 (1.1–1.6) | 1.3 (1.1 -1.6) | 1.3 (1.1 -1.6) | 0.94 |
| Lipid-lowering medication, n (%) | 5167 (35.4) | 94 (27.2) | 5073 (35.6) | 0.001 |
| Glucose (mmol/L), median (IQR) | 5.50 (5.1–6.0) | 5.5 (5.1–6.0) | 5.5 (5.1–6.0) | 0.55 |
| Type 2 diabetes, n (%) | 1411 (9.6) | 34 (9.8) | 1377 (9.7) | 0.46 |
| Myocardial infarction, n (%) | 473 (3.2) | 8 (2.3) | 465 (3.3) | 0.18 |
| Heart failure, n (%) | 267 (1.8) | 5 (1.4) | 262 (1.8) | 0.41 |
| Smoking status, ever, n (%) | 7685 (52.6) | 217 (62.7) | 7468 (52.4) | 0.15 |
| QTc-interval (ms), median (IQR) | 431.7 (418.9–423.3) | 430.2 (417.8–440.0) | 431.7 (418.9–443.3) | 0.07 |
| Follow-up (years), median (IQR) | 10.4 (6.3-15.4) | 13.1 (7.2-20.7) | 10.2 (6.3-15.3) | <0.001 |
CVD, cardiovascular disease; HDL, high-density lipoprotein; IQR, interquartile range; SD, standard deviation.
A total of 26 974 ECGs in 82% of individuals (n = 11 936) were recorded. Over a median (IQR) follow-up time of 10.4 (6.3–15.4) years, the number of SCD cases was 609 with a cumulative incidence rate of 4.2%.
The association of [hydroxy]chloroquine use and the risk of increased QTc-interval duration
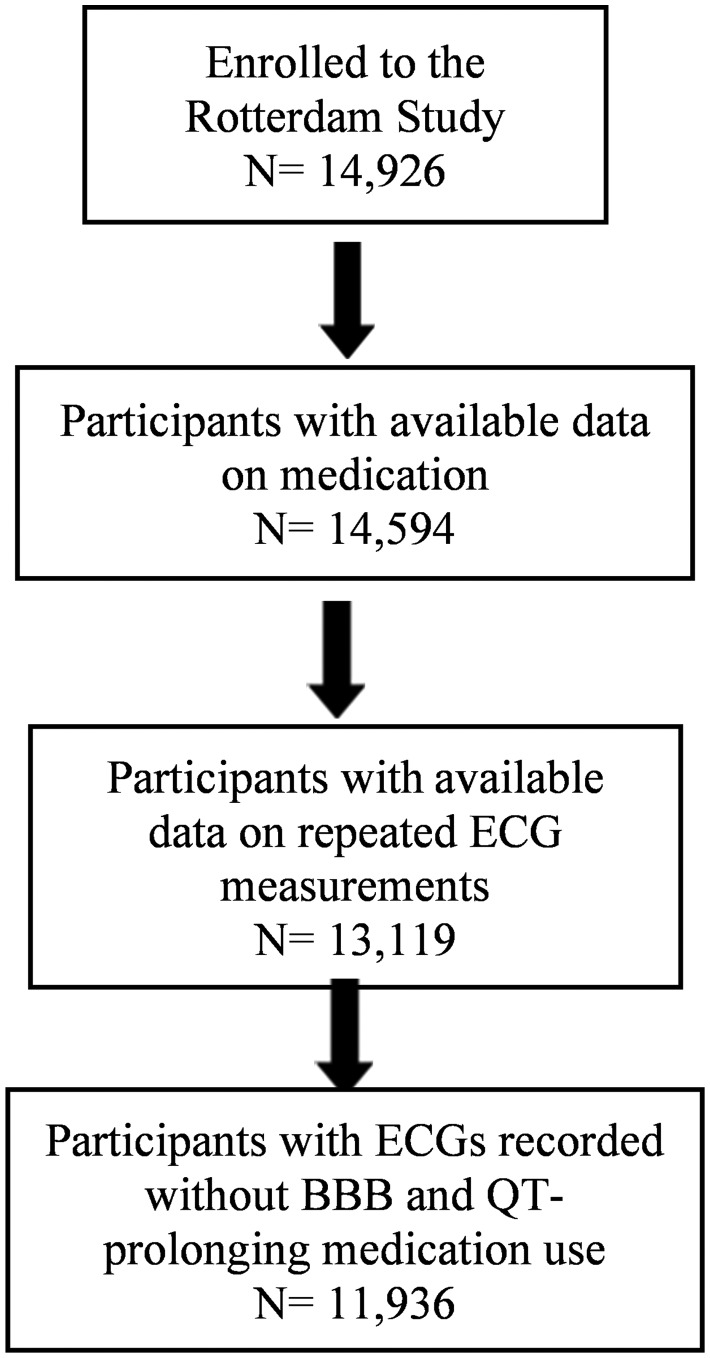
Seven out of 77 ECGs (9.1%) recorded in 66 patients currently treated with [hydroxy]chloroquine had an increased QTc-interval duration; three were women with QTc-interval duration of 470 ms, or greater, and four were men with QTc-interval duration of 450 ms or greater. The mean QTc-interval duration for the reference group was 428.469 ms. The association of current [hydroxy]chloroquine use with increased QTc-interval duration in the total population was statistically significant [mean change: 8.1 (95% CI: 3.6; 12.6 ms)] after adjustment for age, sex, BMI, hypertension, T2D, myocardial infarction, heart failure, smoking behaviour, and lipid-lowering drugs. However, this association was not statistically significant in past users (Figure 2).
Figure 2.
Association between [hydroxy]chloroquine and the mean QTc-interval duration. ECG, Electrocardiogram measurements; LCI, lower confidence interval; UCL, upper confidence interval. Adjusted for age, sex, body mass index, hypertension, type 2 diabetes, myocardial infarction, heart failure, smoking behaviour, and lipid-lowering drugs.
In a sex-stratified analysis, women [mean change: 9.1 (95% CI: 3.9; 14.3 ms)] but not men [mean change: 5.8 (95% CI: −2.9; 14.3 ms)] who were using [hydroxy]chloroquine had a longer QTc-interval. This association was not statistically significant in past users, neither in women nor in men (data not shown).
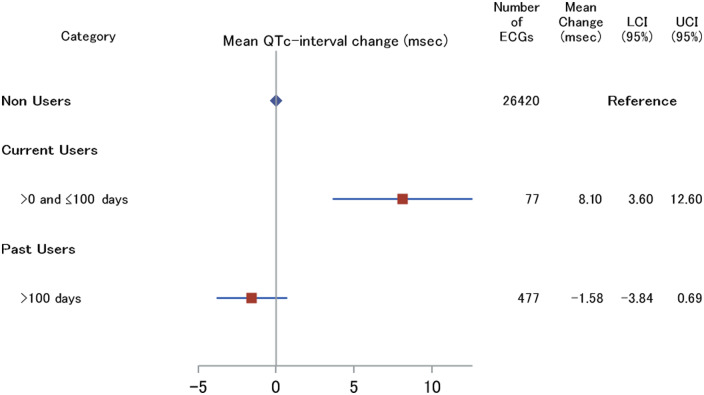
When we compared the associations of current-high and current-low daily dosages of [hydroxy]chloroquine and QTc-interval duration, our results showed a clear trend towards increased QTc-interval duration in current-high daily dosage [mean change: 15.3 (95% CI: 7.0; 23.6 ms)] compared with current-low daily dosage [mean change: 5.5 (95% CI: 0.4; 10.7 ms)] after adjustment for confounders (Figure 3).
Figure 3.
Association between [hydroxy]chloroquine use dose categories and the mean QTc-interval duration. Low dosage: ≤0.3 DDD. High dosage: >0.3 DDD. Hydroxychloroquine DDD = 520 mg. Chloroquine DDD = 500 mg. ECG, electrocardiogram measurements; LCI, lower confidence interval; UCL, upper confidence interval. Adjusted for age, sex, body mass index, hypertension, type 2 diabetes, myocardial infarction, heart failure, smoking behaviour, and lipid-lowering drugs.
The association of [hydroxy]chloroquine use and the risk of sudden cardiac death
The analysis results for the association of [hydroxy]chloroquine use both current and past use and the risk of SCD in the total population are shown in Figure 4. All currently exposed cases of SCD had used hydroxychloroquine, not chloroquine. Current use of hydroxychloroquine resulted in a significantly increased risk of SCD even after adjustment for age, sex, BMI, T2D, heart failure, myocardial infarction, hypertension, smoking behaviour, lipid-lowering drugs, and the average number of prescriptions until the occurrence of SCD [multivariable model adjusted hazard ratio (HR), 3.7 (95% CI: 1.1; 12.6)] but not in past users [1.7 (95% CI: 0.97; 2.9)]. After adjustment for baseline QTc-interval above the cut-off, the HR did not change; HR of 3.8 (95% CI: 1.1; 12.8).
Figure 4.
Association between [hydroxy]chloroquine and risk of sudden cardiac death. SCD, sudden cardiac death; HR, hazard ratio; CI, confidence interval. Adjusted for age, sex, body mass index, hypertension, type 2 diabetes, myocardial infarction, heart failure, smoking behaviour, and lipid-lowering drugs, the average number of prescriptions until the occurrence of sudden cardiac death.
When choosing the cut-off of 250 days, the association between current hydroxychloroquine (n = 5) and the risk of SCD was slightly stronger; HR of 4.0 (95% CI: 1.3; 11.8).
Discussion
In this large prospective and population-based cohort study, current use of [hydroxy]chloroquine was associated with a significantly increased duration of the QTc-interval while this was not observed in past users. More importantly, the association was dose-dependent in which the higher dose, the higher the mean QTc-interval. Similarly, there was a significantly increased risk of SCD in current users but not in past users. Although the majority of participants used [hydroxy]chloroquine because of rheumatoid arthritis—a potential risk factor for cardiovascular disease20—this suggests that [hydroxyl]chloroquine played at least a modifying role. A clinically relevant QTc-interval prolongation, a well-known risk factor for SCD, was observed in almost 9% of ECGs recorded in patients currently treated with [hydroxy]chloroquine. Also, several case-reports of cardiomyopathy attributed to [hydroxyl]chloroquine itself have been published.21–23
So far, [hydroxy]chloroquine has been given to thousands of individuals to prevent or treat the COVID-19 pandemic worldwide, although the efficacy is controversial. In an animal study, both in vitro and SARS-CoV-2-infected animals, evaluating the antiviral activity of hydroxychloroquine alone or in combination with azithromycin compared with the placebo, no significant difference was shown on viral load levels.24 Moreover, this study revealed no preventive effect of [hydroxy]chloroquine, possibly because [hydroxy]chloroquine targets a pathway that is not operative in lung cells.25 According to the FDA's most updated review comments, hydroxychloroquine and chloroquine are potential causes of cardiac toxicities, including QTc prolongation, ventricular arrhythmias, torsade de Pointes (TdP), and conduction disorders.
Both hydroxychloroquine and chloroquine have a long elimination half-life of 40–50 days.12,13 Long-term treatment with [hydroxy]chloroquine increases lysosomal dysfunction that impairs intracellular degradation processes and eventually accumulates glycogen and phospholipids as metabolic products.13,26–28 Toxicity associated with [hydroxy]chloroquine could occur within the recommended daily dosages, but plasma levels do not help in understanding the underlying mechanism.28,29 The structure of [hydroxy]chloroquine is similar to the class IA antiarrhythmic quinidine that inhibits voltage-gated sodium and potassium channels. Several different risk factors are known to induce drug-associated QT/QTc prolongation, such as female gender, heart disease, electrolyte disturbances, diabetes, concomitant use of QT/QTc-interval-prolonging medications, and genetic factors that cause QTc-interval prolongation and affect myocardial depolarization and repolarization.30 Given the risk of cardiac adverse effects, these drugs should be used with caution in individuals with known risk factors such as heart disease, a family history of SCD, and notably in patients who are already taking QT/QTc-interval-prolonging medications.
Although the association between QT-prolongation and SCD in population-based studies gave conflicting results,31,32 prolonged QTc-interval is considered a potential mediating factor in triggering TdP. Torsade de Pointes is a potentially life-threatening tachyarrhythmia which often leads to ventricular fibrillation and SCD. However, the effect of QTc-interval prolongation on TdP and eventually SCD is not straightforward. A QT/QTc-interval above 500 ms has been associated with a higher risk of TdP and SCD. However, SCD can also occur in individuals with QT/QTc-intervals within the normal range. Nevertheless, QT/QTc-interval prolongation is still considered a surrogate marker of increased risk of SCD.30,33
Strengths and limitations
Our study's strength is its prospective cohort design and long follow-up and the fact that we had precise and detailed pharmacy-based filling data available at the time of ECG or death, as well as access to up to five recorded ECGs per individual over a relatively long follow-up. This enabled us to obtain more precise ECG measures along with [hydroxy]chloroquine use. Furthermore, the risk of selection or information bias is unlikely as the SCD cases were ascertained without prior knowledge of this study hypothesis. However, our study also has some limitations, such as the small number of cases of SCD currently exposed to [hydroxy]chloroquine. A second limitation is that we did not have data on the indication for use in the currently exposed cases of SCD. However, during the repeated drug interviews, almost all participants stated that they used these drugs for a rheumatic disorder or SLE. Confounding by indication cannot be ruled out, but the fact that past users no longer had an increased risk argues against confounding by indication.
Conclusions
Patients who received [hydroxy]chloroquine during a follow-up time of almost 10 years experienced increased QTc-interval duration, and the risk of SCD was higher in this population. Although further longitudinal studies may be warranted to confirm our results, it seems that the widespread use of [hydroxy]chloroquine to treat COVID-19 infections with a high burden of cardiovascular disease34—as propagated by some—should be discouraged until unequivocal proof of the drug efficacy is delivered.
Funding
This project has received funding from the Innovative Medicines initiative 2 Joint Undertaking (grant agreement No 116030). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Data sharing statement
We are not planning to disseminate our results to the study participants.
Conflict of interest: all authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol 2011;7:718–729. [DOI] [PubMed] [Google Scholar]
- 2. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li X, Wang Y, Agostinis P, Rabson A, Melino G, Carafoli E, Shi Y, Sun E. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis 2020;11:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013;2:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020;382:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahase E. Covid-19: WHO halts hydroxychloroquine trial to review links with increased mortality risk. BMJ 2020;369:m2126. [DOI] [PubMed] [Google Scholar]
- 7. Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020; Oct 8:NEJMoa2022926. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020;Oct 8:NEJMoa2022926. [Google Scholar]
- 8. Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, Kieboom BCT, Klaver CCW, de Knegt RJ, Luik AI, Nijsten TEC, Peeters RP, van Rooij FJA, Stricker BH, Uitterlinden AG, Vernooij MW, Voortman T. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol 2020;35:483–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woosley RL, Black K, Heise CW, Romero K. CredibleMeds.org: what does it offer? Trends Cardiovasc Med 2018;28:94–99. [DOI] [PubMed] [Google Scholar]
- 10. Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol 2012;27:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bleumink G, Knetsch A, Sturkenboom M, Straus S, Hofman A, Deckers J, Witteman J, Stricker B. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 2004;25:1614–1619. [DOI] [PubMed] [Google Scholar]
- 12. Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol 1989;27:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins KP, Jackson KM, Gustafson DL. Hydroxychloroquine: a physiologically-based pharmacokinetic model in the context of cancer-related autophagy modulation. J Pharmacol Exp Ther 2018;365:447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Bemmel JH, Kors JA, Van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med 1990;29:346–353. [PubMed] [Google Scholar]
- 15. Willems JL, Abreu-Lima C, Arnaud P, van Bemmel JH, Brohet C, Degani R, Denis B, Gehring J, Graham I, van Herpen G, Machado H, Macfarlane PW, Michaelis J, Moulopoulos SD, Rubel P, Zywietz C. The diagnostic performance of computer programs for the interpretation of electrocardiograms. N Engl J Med 1991;325:1767–1773. [DOI] [PubMed] [Google Scholar]
- 16. Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920;7:353–370. [Google Scholar]
- 17. Niemeijer MN, van den Berg ME, Leening MJG, Hofman A, Franco OH, Deckers JW, Heeringa J, Rijnbeek PR, Stricker BH, Eijgelsheim M. Declining incidence of sudden cardiac death from 1990–2010 in a general middle-aged and elderly population: The Rotterdam Study. Heart Rhythm 2015;12:123–129. [DOI] [PubMed] [Google Scholar]
- 18. Straus SMJM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MCJM, Stricker BHC, Witteman JCM. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006;47:362–367. [DOI] [PubMed] [Google Scholar]
- 19. Xu S, Shetterly S, Raebel MA, Ho PM, Tsai TT, Magid D. Estimating the effects of time-varying exposures in observational studies using Cox models with stabilized weights adjustment. Pharmacoepidemiol Drug Saf 2014;23:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Løgstrup BB, Olesen KKW, Masic D, Gyldenkerne C, Thrane PG, Ellingsen T, Bøtker HE, Maeng M. Impact of rheumatoid arthritis on major cardiovascular events in patients with and without coronary artery disease. Ann Rheum Dis 2020;79:1182–1188. [DOI] [PubMed] [Google Scholar]
- 21. Yogasundaram H, Putko BN, Tien J, Paterson DI, Cujec B, Ringrose J, Oudit GY. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 2014;30:1706–1715. [DOI] [PubMed] [Google Scholar]
- 22. Dogar MU, Shah NN, Ishtiaq S, Shah PN, Shah P, Mathew S, Vittorio TJ. Hydroxychloroquine-induced restrictive cardiomyopathy: a case report. Postgrad Med J 2018;94:185–186. [DOI] [PubMed] [Google Scholar]
- 23. Tselios K, Gladman DD, Harvey P, Mak S, Chantal M, Butany J, Urowitz MB. Hydroxychloroquine-induced cardiomyopathy in systemic lupus erythematosus. J Clin Rheumatol 2016;22:287–288. [DOI] [PubMed] [Google Scholar]
- 24. Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, Naninck T, Pizzorno A, Lemaitre J, Gonçalves A, Kahlaoui N, Terrier O, Fang RHT, Enouf V, Dereuddre-Bosquet N, Brisebarre A, Touret F, Chapon C, Hoen B, Lina B, Calatrava MR, van der Werf S, de Lamballerie X, Le Grand R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature 2020;585:584–587. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann M, Mösbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Krüger N, Gassen NC, Müller MA, Drosten C, Pöhlmann S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 2020;585:588–590. [DOI] [PubMed] [Google Scholar]
- 26. Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti-malarial action of chloroquine. Nature 1972;235:50–52. [DOI] [PubMed] [Google Scholar]
- 27. Thomé R, Lopes SC, Costa FT, Verinaud L. Chloroquine: modes of action of an undervalued drug. Immunol Lett 2013;153:50–57. [DOI] [PubMed] [Google Scholar]
- 28. Mehra MR, Desai SS, Kuy SRam, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382:2582–2582. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Nguyen LS, Dolladille C, Drici M-D, Fenioux C, Alexandre J, Mira J-P, Moslehi JJ, Roden DM, Funck-Brentano C, Salem J-E. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization pharmacovigilance database. Circulation 2020;142:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The cardiotoxicity of antimalarials. WHO Evidence Review Group Meeting, 13–14 October 2016 Varembé Conference Centre, Geneva, Switzerland.
- 31. Goldberg RJ, Bengtson J, Chen ZY, Anderson KM, Locati E, Levy D. Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience). Am J Cardiol 1991;67:55–58. [DOI] [PubMed] [Google Scholar]
- 32. Okin PM, Devereux RB, Howard BV, Fabsitz RR, Lee ET, Welty TK. Assessment of QT interval and QT dispersion for prediction of all-cause and cardiovascular mortality in American Indians: the Strong Heart Study. Circulation 2000;101:61–66. [DOI] [PubMed] [Google Scholar]
- 33. Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, Kovacs RJ. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013;6:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal MZ, Lavie CJ, Fonarow GC. Cardiovascular disease in hospitalizations with a diagnosis of coronavirus from pre-COVID-19 era in United States: national analysis from 2016-2017 [published online ahead of print, 2020 Sep 22]. Mayo Clin Proc 2020; doi:10.1016/j.mayocp.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]